Abstract
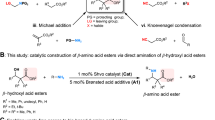
This protocol describes a procedure for the synthesis of α, β-branched-b-amino aldehydes via Proline-catalyzed asymmetric Mannich reaction of aldehydes with N-tert–butoxycarbonyl-imines. The crystalline β-amino aldehydes are formed in good yields and extremely high levels of diastereo- and enantioselectivities without the need for chromatographic purification and are readily oxidized to the corresponding β-amino acids. The protocol can be completed in approximately 14 h on small scales or up to 30 h on larger scales.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Juaristi, E. & Soloshonok, V.A (eds.) Enantioselective Synthesis of β-Amino Acids (Wiley-VCH, New York, 2005).
Kobayashi, S. & Ueno, M. Mannich reaction. In Comprehensive Asymmetric Catalysis (eds. Jacobsen, E.N., Pfaltz, A. & Yamamoto, H.) Suppl 1, Chapter 29.5, 143–159 (Springer, Berlin, 2004).
Kobayashi, S. & Ishitani, H. Catalytic enantioselective addition to imines. Chem. Rev. 99, 1069–1094 (1999).
Hart, D.J. & Ha, D.C. The ester enolate-imine condensation route to β-lactams. Chem. Rev. 89, 1447–1465 (1989).
Ishitani, H., Ueno, M. & Kobayashi, S. Catalytic enantioselective Mannich-type reactions using a novel chiral zirconium catalyst. J. Am. Chem. Soc. 119, 7153–7154 (1997).
Yamasaki, S., Iida, T. & Shibasaki, M. Direct catalytic asymmetric Mannich-type reaction of unmodified ketones utilizing the cooperation of an AlLibis (binaphthoxide) complex and La(OTf)3·nH2O. Tetrahedron Lett. 40, 307–310 (1999).
List, B. The direct catalytic asymmetric three-component Mannich reaction. J. Am. Chem. Soc. 122, 9336–9337 (2000).
Córdova, A., Watanabe, S., Tanaka, F., Notz, W. & Barbas, C.F. A highly enantioselective route to either enantiomer of both α- and β-amino acid derivatives. J. Am. Chem. Soc. 124, 1866–1867 (2002).
Hayashi, Y. et al. The direct and enantioselective, one-pot, three-component, cross-Mannich reaction of aldehydes. Angew. Chem. Int. Ed. Engl. 42, 3677–3680 (2003).
Haak, E., Bytschkov, I. & Doye, S. A one-pot procedure for the synthesis of α-amino phosphonates from alkynes. Eur. J. Org. Chem. 3, 457–463 (2002).
Yang, J.W., Stadler, M. & List, B. Proline-catalyzed Mannich reaction of aldehydes with N-Boc-imines. Angew. Chem. Int. Ed. Engl. 46, 609–611 (2007).
Enders, D. & Vrettou, M. Asymmetric synthesis of (+)-polyoxamic acid via an efficient organocatalytic Mannich reaction as the key step. Synthesis 13, 2155–2158 (2006).
Enders, D., Grondal, C. & Vrettou, M. Efficient entry to amino sugars and derivatives via asymmetric organocatalytic Mannich reactions. Synthesis 21, 3597–3604 (2006).
Chowdari, N.S., Suri, J.T. & Barbas, C.F. Asymmetric synthesis of quaternary α- and β-amino acids and β-lactams via proline-catalyzed mannich reactions with branched aldehyde donors. Org. Lett. 6, 2507–2510 (2004).
Kanazawa, A.M., Denis, J.-N. & Greene, A.E. Highly stereocontrolled and efficient preparation of the protected, esterification-ready docetaxel (taxotere) side chain. J. Org. Chem. 59, 1238–1240 (1994).
Wenzel, A.G. & Jacobsen, E.N. Asymmetric catalytic Mannich reactions catalyzed by urea derivatives: enantioselective synthesis of β-aryl-β-amino acids. J. Am. Chem. Soc. 124, 12964–12965 (2002).
Song, J., Wang, Y. & Deng, L. The Mannich reaction of malonates with simple imines catalyzed by bifunctional cinchona alkaloids: enantioselective synthesis of β-amino acids. J. Am. Chem. Soc. 128, 6048–6049 (2006).
Acknowledgements
Generous support by the Max-Planck-Society, the Fonds der Chemischen Industrie (Silver Award to B.L.), and by Novartis (Young Investigator Award to B.L.) is gratefully acknowledged. We also thank Merck, Saltigo, and Wacker for support, and BASF and Degussa for donating chemicals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yang, J., Stadler, M. & List, B. Practical Proline-catalyzed asymmetric Mannich reaction of aldehydes with N-Boc-imines. Nat Protoc 2, 1937–1942 (2007). https://doi.org/10.1038/nprot.2007.272
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.272
This article is cited by
-
Modular synthesis of chiral 1,2-dihydropyridines via Mannich/Wittig/cycloisomerization sequence that internally reuses waste
Nature Communications (2021)
-
Synthesis of the 3′-thio-nucleosides and subsequent automated synthesis of oligodeoxynucleotides containing a 3′-S-phosphorothiolate linkage
Nature Protocols (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.