Abstract
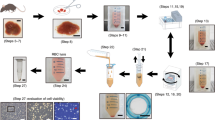
We describe a protocol for easy isolation and culture of human umbilical vein endothelial cells (HUVECs) to supply every researcher with a method that can be applied in cell biology laboratories with minimum equipment. Endothelial cells (ECs) are isolated from umbilical vein vascular wall by a collagenase treatment, then seeded on fibronectin-coated plates and cultured in a medium with Earles' salts and fetal calf serum (FCS), but without growth factor supplementation, for 7 days in a 37 °C–5% CO2 incubator. Cell confluency can be monitored by phase-contrast microscopy; ECs can be characterized using cell surface or intracellular markers and checked for contamination. Various protocols can be applied to HUVECs, from simple harvesting to a particular solubilization of proteins for proteomic analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ryan, J.W. & Ryan, U.S. Endothelial surface enzymes and the dynamic processing of plasma substrates. Int. Rev. Exp. Pathol. 26, 1–43 (1984).
Kaiser, L. & Sparks, H.V. Jr . Endothelial cells. Not just a cellophane wrapper. Arch. Intern. Med. 147, 569–573 (1987).
Vane, J.R., Anggard, E.E . & Botting, R.M. Regulatory functions of the vascular endothelium. N. Engl. J. Med. 323, 27–36 (1990).
Lazo, J.S. Endothelial injury caused by antineoplastic agents. Biochem. Pharmacol. 35, 1919–1923 (1986).
Baudin, B. Toxicité endothéliale des chimiothérapies anticancéreuses. Sang Thrombose et Vaisseaux 7, 175–183 (1995).
Baudin, B., Bénéteau-Burnat, B. & Giboudeau, J. Cytotoxicity of amiodarone in cultured human endothelial cells. Cardiovasc. Drugs Ther. 10, 557–560 (1996).
Mailloux, A. et al. Anticancer drugs induce necrosis of human endothelial cells involving both oncosis and apoptosis. Eur. J. Cell Biol. 80, 442–449 (2001).
Mailloux, A., Deslandes, B., Vaubourdolle, M. & Baudin, B. Captopril and enalaprilat decrease antioxidant defences in human endothelial cells and are unable to protect against apoptosis. Cell Biol. Intern. 27, 825–830 (2003).
Mailloux, A., Bruneel, A., Vaubourdolle, M. & Baudin, B. Cyclosporin A but not estradiol can protect endothelial cells from etoposide-induced apoptosis. Endothelium 11, 141–149 (2004).
Bruneel, A. et al. Proteomic study of human umbilical vein endothelial cells in culture. Proteomics 3, 714–723 (2003).
Bruneel, A. et al. Proteomics of human umbilical vein endothelial cells applied to etoposide-induced apoptosis. Proteomics 5, 3876–3884 (2005).
Pernet, P., Bruneel, A., Baudin, B. & Vaubourdolle, M. PHProteomicDB: a module for two-dimensional gel electrophoresis database creation on personal Web sites. Genomics Proteomics Bioinformatics 4, 134–136 (2006).
Jaffe, E.A., Nachman, R.L., Becker, C.G. & Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52, 2745–2756 (1973).
Ryan, U.S. & Ryan, J.W. Vital and functional activities of endothelial cells. in Pathobiology of the Endothelial Cells (eds. Nossel, H.L. & Vogel, H.J.) 455–469 (1982).
Goldsmith, J.C., McCormick, J.J. & Yen, A. Endothelial cell cycle kinetics. Changes in culture and correlation with endothelial properties. Lab. Invest. 51, 643–647 (1984).
Noveral, J.P., Mueller, S.N. & Levine, E.M. Release of angiotensin I-converting enzyme by endothelial cells in vitro. J. Cell. Physiol. 131, 1–5 (1987).
Dimmeler, S. & Zeiher, A.M. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ. Res. 87, 434–439 (2000).
Sawada, S. et al. Prostacyclin generation by cultured human vascular endothelial cells with reference to angiotensin I-converting enzyme. Jap. Circ. J. 50, 242–247 (1986).
Okabe, T. et al. Induction by fibroblast growth factor of angiotensin-converting enzyme in vascular endothelial cells in vitro . Biochem. Biophys. Res. Commun. 145, 1211–1216 (1987).
Reinders, J.H., Vervoorn, R.C., Verweij, C.L., Van Mourik, J.A. & De Groot, P.G. Perturbation of cultured human vascular endothelial cells by phorbol ester or thrombin alters the cellular von Willebrand factor distribution. J. Cell. Physiol. 133, 79–87 (1987).
Nakache, M., Gaub, H.E., Schreiber, A.B. & Mac Connel, H.M. Topological and modulated distribution of surface markers on endothelial cells. Proc. Natl. Acad. Sci. USA 83, 2874–2878 (1986).
Wu, Q.Y. et al. Differential distribution of von Willebrand factor in endothelial cells. Comparison between normal pigs and pigs with von Willebrand disease. Arteriosclerosis 7, 47–54 (1987).
Baudin, B., Bérard, M., Carrier, J.L., Legrand, Y. & Drouet, L. Vascular origin determines angiotensin I-converting enzyme expression in endothelial cells. Endothelium 5, 73–84 (1997).
Miettinen, M., Holthofer, H., Lehto, V.-P., Miettinen, A. & Virtanen, I. Ulex europaeus I lectin as a marker for tumors derived from endothelial cells. Am. J. Clin. Pathol. 79, 32–36 (1983).
Carley, W.W., Niedbala, M.J. & Gerritsen, M.E. Isolation, cultivation, and partial characterization of microvascular endothelium derived from human lung. Am. J. Resp. Cell Mol. Biol. 7, 620–630 (1992).
Gorman, L., Mercer, L.-P. & Hennig, B. Growth requirements of endothelial cells in culture: variations in serum and amino acid concentrations. Nutrition 12, 266–270 (1996).
Seeger, J.M. & Klingman, N. Improved endothelial cell seeding with cultured cells and fibronectin-coated grafts. J. Surg. Res. 38, 641–647 (1985).
Balconi, G., Pietra, A., Busacca, M., De Gaetano, G. & Dejana, E. Success rate of primary human endothelial cell culture from umbilical cords is influenced by maternal and fetal factors and interval from delivery. In Vitro 19, 807–810 (1983).
Mano, Y., Sawasaki, Y., Takahashi, K. & Goto, T. Cultivation of arterial endothelial cells from human umbilical cord. Experientia 39, 1144–1146 (1983).
Hirschberg, H., Bergh, O.J. & Thorsby, E. Antigen-presenting properties of human vascular endothelial cells. J. Exp. Med. 152, 249–255 (1980).
Schor, A.M., Schor, S.L. & Allen, T.D. The synthesis of sub-endothelial matrix by bovine aortic endothelial cells in culture. Tissue Cell 16, 677–691 (1984).
Ishisaki, A., Hayashi, H., Li, A.-J. & Imamura, T. Human umbilical vein endothelium-derived cells retain potential to differentiate into smooth muscle-like cells. J. Biol. Chem. 278, 1303–1309 (2003).
Baudin, B., Bénéteau-Burnat, B., Baumann, F.C. & Giboudeau, J. A reliable radiometric assay for the determination of angiotensin I-converting enzyme activity in urines. J. Clin. Chem. Clin. Biochem. 28, 857–861 (1990).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Baudin, B., Bruneel, A., Bosselut, N. et al. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc 2, 481–485 (2007). https://doi.org/10.1038/nprot.2007.54
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.54
This article is cited by
-
Vascular and pulmonary effects of ibuprofen on neonatal lung development
Respiratory Research (2023)
-
Metrnl deficiency retards skin wound healing in mice by inhibiting AKT/eNOS signaling and angiogenesis
Acta Pharmacologica Sinica (2023)
-
Tankyrase inhibition interferes with junction remodeling, induces leakiness, and disturbs YAP1/TAZ signaling in the endothelium
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Generation of individualized immunocompatible endothelial cells from HLA-I-matched human pluripotent stem cells
Stem Cell Research & Therapy (2022)
-
Human placental mesenchymal stromal cells are ciliated and their ciliation is compromised in preeclampsia
BMC Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.