Key Points
-
The p53-dependent apoptotic pathway is central to tumour prevention and is consistently disrupted in malignancy; disruption can occur in p53 itself or in any of its cofactors or target genes.
-
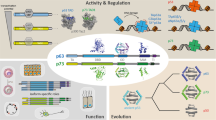
The members of the ASPP (ankyrin-repeat-, SH3-domain- and proline-rich-region-containing protein) family have been identified as specific regulators of p53-, p63- and p73-mediated apoptosis. The family comprises three members of which two, ASPP1 and ASPP2, are pro-apoptotic and the third, inhibitory iASPP (iASPP), is anti-apoptotic. The importance of the ASPP family in regulating p53 function is supported by the genetic evidence that C. elegans iASPP is an inhibitor of p53 and can also inhibit the function of human p53 in human cells as effectively as humn iASPP.
-
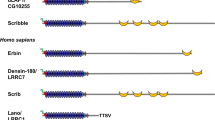
The ASPP family is characterized by a highly conserved carboxyl terminus — ankryin repeats, SH3 (Src homology 3) domain and proline-rich region — which is the preferred binding site for its partners, including the tumour suppressor p53, BCL2, RELA/p65, protein phosphatase 1, YES-associated protein and adenomatosis polyposis coli 2. The amino terminus is conserved only in the pro-apoptotic members, ASPP1 and ASPP2.
-
ASPP expression levels correlate with cellular sensitivity (ASPP1 and ASPP2 increase sensitivity) and resistance (iASPP increases resistance) to apoptosis. Deregulation of ASPP expression has been reported in several different cancers, underlining their importance in tumour development. The members of the ASPP family might be new molecular targets for cancer therapy.
Abstract
One of the most frequently mutated genes in human cancers, tumour suppressor p53 (TP53), can induce cell-cycle arrest and apoptosis. The apoptotic function of p53 is tightly linked to its tumour-suppression function and the efficacy of many cancer therapies depends on this. The identification of a new family of proteins, known as ASPPs (ankyrin-repeat-, SH3-domain- and proline-rich-region-containing proteins), has led to the discovery of a novel mechanism that selectively regulates the apoptotic function, but not the cell-cycle-arrest function, of p53, and gives an insight into how p53 responds to different stress signals. ASPPs might be new molecular targets for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Haupt, S., Berger, M., Goldberg, Z. & Haupt, Y. Apoptosis — the p53 network. J. Cell Sci. 116, 4077–4085 (2003).
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
Leu, J. I., Dumont, P., Hafey, M., Murphy, M. E. & George, D. L. Mitochondrial p53 activates Bak and causes disruption of a Bak–Mcl1 complex. Nature Cell Biol. 6, 443–450 (2004).
Chipuk, J. E. et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, 1010–1014 (2004).
Hainaut, P. & Hollstein, M. p53 and human cancer: the first ten thousand mutations. Adv. Cancer Res. 77, 81–137 (2000).
Soussi, T., Kato, S., Levy, P. P. & Ishioka, C. Reassessment of the TP53 mutation database in human disease by data mining with a library of TP53 missense mutations. Hum. Mutat. 25, 6–17 (2005).
Ryan, K. M. & Vousden, K. H. Characterization of structural p53 mutants which show selective defects in apoptosis but not cell cycle arrest. Mol. Cell. Biol. 18, 3692–3698 (1998).
Ludwig, R. L., Bates, S. & Vousden, K. H. Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol. Cell. Biol. 16, 4952–4960 (1996).
Lu, X. p53: a heavily dictated dictator of life and death. Curr. Opin. Genet. Dev. 15, 27–33 (2005).
Di Stefano, V., Rinaldo, C., Sacchi, A., Soddu, S. & D'Orazi, G. Homeodomain-interacting protein kinase-2 activity and p53 phosphorylation are critical events for cisplatin-mediated apoptosis. Exp. Cell Res. 293, 311–320 (2004).
D'Orazi, G. et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nature Cell Biol. 4, 11–19 (2002).
Oda, K. et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102, 849–862 (2000).
Flores, E. R. et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416, 560–564 (2002).
Shikama, N. et al. A novel cofactor for p300 that regulates the p53 response. Mol. Cell 4, 365–376 (1999).
Samuels-Lev, Y. et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol. Cell 8, 781–794 (2001). The seminal paper on the ASPP family. This was the first paper to describe the biological importance of the p53–ASPP2 interaction. The identification and characerization of ASPP1 was also described in this paper. It was the first study to demonstrate that ASPP1 and ASPP2 belong to a family and the fundamental functions of ASPP1 and ASPP2 are also elucidated.
Iwabuchi, K., Bartel, P. L., Li, B., Marraccino, R. & Fields, S. Two cellular proteins that bind to wild-type but not mutant p53. Proc. Natl Acad. Sci. USA 91, 6098–6102 (1994). Iwabuchi's article first reported the discovery of 53BP1 and 53BP2 as binding partners of wild-type p53.
Joerger, A. C., Ang, H. C., Veprintsev, D. B., Blair, C. M. & Fersht, A. R. Structures of p53 cancer mutants and mechanism of rescue by second-site suppressor mutations. J. Biol. Chem. 280, 16030–16037 (2005).
Schultz, L. B., Chehab, N. H., Malikzay, A. & Halazonetis, T. D. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 151, 1381–1390 (2000).
Marston, N. J., Crook, T. & Vousden, K. H. Interaction of p53 with MDM2 is independent of E6 and does not mediate wild type transformation suppressor function. Oncogene 9, 2707–2716 (1994).
Scheffner, M., Takahashi, T., Huibregtse, J. M., Minna, J. D. & Howley, P. M. Interaction of the human papillomavirus type 16 E6 oncoprotein with wild-type and mutant human p53 proteins. J. Virol. 66, 5100–5105 (1992).
Thukral, S. K., Blain, G. C., Chang, K. K. & Fields, S. Distinct residues of human p53 implicated in binding to DNA, simian virus 40 large T antigen, 53BP1, and 53BP2. Mol. Cell. Biol. 14, 8315–8321 (1994).
Joo, W. S. et al. Structure of the 53BP1 BRCT region bound to p53 and its comparison to the Brca1 BRCT structure. Genes Dev. 16, 583–593 (2002).
Naumovski, L. & Cleary, M. L. The p53-binding protein 53BP2 also interacts with bcl2 and impedes cell cycle progression at G2/M. Mol. Cell. Biol. 16, 3884–3892 (1996). In this article Naumovski extended the coding sequence of 53BP2 to 1005 amino acids and characterized its binding to Bcl2, and hence renamed the protein Bbp.
Helps, N. R., Barker, H. M., Elledge, S. J. & Cohen, P. T. W. Protein phosphotase 1 interacts with p53BP2, a protein which binds to the tumour suppressor p53. FEBS 377, 295–300 (1995).
Egloff, M. P. et al. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 16, 1876–1887 (1997).
Yang, J. -P., Hori, M., Sanda, T. & Okamoto, T. Identification of a novel inhibitor of nuclear factor-κB, RelA-associated inhibitor. J. Biol. Chem. 274, 15662–15670 (1999). The original description of iASPP, in the short isoform RAI, as an inhibitor of RelA/p65 function.
Nakagawa, H., Koyama, K., Murata, Y., Akiyama, T. & Nakamura, Y. APCL, a central nervous system-specific homologue of adenomatous polyposis coli tumour suppressor binds to p53-binding protein 2 and translocates it to the perinucleus. Cancer Res. 60, 101–105 (2000).
Chen, Y., Liu, W., Naumovski, L. & Neve, R. L. ASPP2 inhibits APP–BP1-mediated NEDD8 conjugation to cullin-1 and decreases APP–BP1-induced cell proliferation and neuronal apoptosis. J. Neurochem. 85, 801–809 (2003).
Espanel, X. & Sudol, M. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. J. Biol. Chem. 276, 14514–14523 (2001).
Cao, Y., Hamada, T., Matsui, T., Date, T. & Iwabuchi, K. Hepatitis C virus core protein interacts with p53-binding protein, 53BP2/Bbp/ASPP2, and inhibits p53-mediated apoptosis. Biochem. Biophys. Res. Commun. 315, 788–795 (2004).
Nagase, T. et al. Prediction of the coding sequences of unidentified human genes. XII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 5, 355–364 (1998).
Slee, E. A. et al. The N-terminus of a novel isoform of human iASPP is required for its cytoplasmic localization. Oncogene 23, 9007–9016 (2004). This paper reports the cloning of full-length human iASPP and characterization of the protein.
Herron, B. J. et al. A mutation in NFκB interacting protein 1 results in cardiomyopathy and abnormal skin development in wa3 mice. Hum. Mol. Genet. 14, 667–677 (2005).
Bergamaschi, D. et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nature Genet. 33, 162–167 (2003). The seminal paper on iASPP, introducing iASPP as the third ASPP member. It also described the identification of the C. elegans iASPP and formally demonstrated the evolutionarily conserved function of human and C. elegans iASPPs in inhibiting p53-mediated apoptosis both in vitro and in vivo.
Takahashi, N. et al. Expression of 53BP2 and ASPP2 proteins from TP53BP2 gene by alternative splicing. Biochem. Biophys. Res. Commun. 315, 434–438 (2004).
Yang, J.-P. et al. NF-κB subunit p65 binds to 53BP2 and inhibits cell death induced by 53BP2. Oncogene 18, 5177–5186 (1999).
Stanelle, J., Stiewe, T., Theseling, C. C., Peter, M. & Putzer, B. M. Gene expression changes in response to E2F1 activation. Nucleic Acids Res. 30, 1859–1867 (2002).
Fogal, V. et al. ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death Differ. 12, 369–376 (2005).
Hershko, T., Chaussepied, M., Oren, M. & Ginsberg, D. Novel link between E2F and p53: proapoptotic cofactors of p53 are transcriptionally upregulated by E2F. Cell Death Differ. 12, 377–383 (2005).
Blattner, C., Sparks, A. & Lane, D. Transcription factor E2F-1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol. Cell. Biol. 19, 3704–3713 (1999).
Lopez, C. D. et al. Proapoptotic p53-interacting protein 53BP2 is induced by UV irradiation but suppressed by p53. Mol. Cell. Biol. 20, 8018–8025 (2000).
Zhu, Z. et al. Control of ASPP2/(53BP2L) protein levels by proteasomal degradation modulates p53 apoptotic function. J. Biol. Chem. 280, 34473–34480 (2005).
Gorina, S. & Pavletich, N. P. Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2. Science 274, 1001–1005 (1996). The crystal-structure study that describes the binding between ASPP2 and p53 and identifies the contact residues.
Bergamaschi, D. et al. ASPP1 and ASPP2: common activators of p53 family members. Mol. Cell. Biol. 24, 1341–1350 (2004). The first paper to show that ASPP1 and ASPP2 interact with and stimulate the apoptotic function of p63 and p73.
Scrable, H., Sasaki, T. & Maier, B. ΔNp53 or p44: priming the p53 pump. Int. J. Biochem. Cell Biol. 37, 913–919 (2005).
Moll, U. M. et al. Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage. Mol. Cell. Biol. 16, 1126–1137 (1996).
Park, B. S. et al. Phospho-ser 15-p53 translocates into mitochondria and interacts with Bcl-2 and Bcl-xL in eugenol-induced apoptosis. Apoptosis 10, 193–200 (2005).
Kobayashi, S. et al. 53BP2 induces apoptosis through the mitochondrial death pathway. Genes Cells 10, 253–260 (2005).
Sachdev, S., Hoffmann, A. & Hannink, M. Nuclear localization of IκB alpha is mediated by the second ankyrin repeat: the IκB alpha ankyrin repeats define a novel class of cis-acting nuclear import sequences. Mol. Cell. Biol. 18, 2524–2534 (1998).
Sutcliffe, J. E. & Brehm, A. Of flies and men; p53, a tumour suppressor. FEBS Lett. 567, 86–91 (2004).
Lettre, G. et al. Genome-wide RNAi identifies p53-dependent and -independent regulators of germ cell apoptosis in C. elegans. Cell Death Differ. 11, 1198–1203 (2004).
Maier, B. et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 18, 306–319 (2004).
Derry, W. B., Putzke, A. P. & Rothman, J. H. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294, 591–595 (2001).
Sogame, N., Kim, M. & Abrams, J. M. Drosophila p53 preserves genomic stability by regulating cell death. Proc. Natl Acad. Sci. USA 100, 4696–4701 (2003).
Jaklevic, B. R. & Su, T. T. Relative contribution of DNA repair, cell cycle checkpoints, and cell death to survival after DNA damage in Drosophila larvae. Curr. Biol. 14, 23–32 (2004).
Irwin, M. S. & Kaelin, W. G. p53 family update: p73 and p63 develop their own identities. Cell Growth Differ. 12, 337–349 (2001).
Courtois, S. et al. ΔN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene 21, 6722–6728 (2002).
Bourdon, J. C. et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 19, 2122–2137 (2005).
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Yang, A. et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999).
Yang, A. et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404, 99–103 (2000).
Mills, A. A. et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 (1999).
Yang, A., Kaghad, M., Caput, D. & McKeon, F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 18, 90–95 (2002).
Crook, T. et al. p53 mutation with frequent novel condons but not a mutator phenotype in BRCA1- and BRCA2-associated breast tumours. Oncogene 17, 1681–1689 (1998).
Mori, T., Okamoto, H., Takahashi, N., Ueda, R. & Okamoto, T. Aberrant overexpression of 53BP2 mRNA in lung cancer cell lines. FEBS Lett. 465, 124–128 (2000).
Ao, Y., Rohde, L. H. & Naumovski, L. p53-interacting protein 53BP2 inhibits clonogenic survival and sensitizes cells to doxorubicin but not paclitaxel-induced apoptosis. Oncogene 20, 2720–2725 (2001).
Sgroi, D. C. et al. In vivo gene expression profile analysis of human breast cancer progression. Cancer Res. 59, 5656–5661 (1999).
Mori, S. et al. p53 apoptotic pathway molecules are frequently and simultaneously altered in nonsmall cell lung carcinoma. Cancer 100, 1673–1682 (2004).
Liu, Z. J., Zhang, Y., Zhang, X. B. & Yang, X. Abnormal mRNA expression of ASPP members in leukemia cell lines. Leukemia 18, 880 (2004).
Agirre, X. et al. ASPP1, a common activator of TP53, is inactivated by aberrant methylation of its promoter in acute lymphoblastic leukemia. Oncogene 28 Nov 2005 (10.1038/sj.onc.1209236).
Liu, Z. J. et al. Downregulated mRNA expression of ASPP and the hypermethylation of the 5′-untranslated region in cancer cell lines retaining wild-type p53. FEBS Lett 579, 1587–1590 (2005).
Lossos, I. S., Natkunam, Y., Levy, R. & Lopez, C. D. Apoptosis stimulating protein of p53 (ASPP2) expression differs in diffuse large B-cell and follicular center lymphoma: correlation with clinical outcome. Leuk. Lymphoma 43, 2309–2317 (2002).
Friedler, A. et al. A peptide that binds and stabilizes p53 core domain: chaperone strategy for rescue of oncogenic mutants. Proc. Natl Acad. Sci. USA 99, 937–942 (2002).
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53–HDM-2 interaction and activates p53 function in tumors. Nature Med. 10, 1321–1328 (2004).
Takahashi, N. et al. Inhibition of the 53BP2S-mediated apoptosis by nuclear factor κB and Bcl-2 family proteins. Genes Cells 10, 803–811 (2005).
Takada, N. et al. RelA-associated inhibitor blocks transcription of human immunodeficiency virus type 1 by inhibiting NF-κB and Sp1 actions. J. Virol. 76, 8019–8030 (2002).
Acknowledgements
We would like to thank S. Barnsley for her critical reading of this manuscript and also all members of X.L.'s laboratory. This work is funded by the Ludwig Institute for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Ankyrin repeat
-
Short motif (about 30 amino acids) that is found in many proteins, usually in two or more repeats. Ankyrin repeats mediate protein interactions.
- SH3 domain
-
Conserved protein motifs of about 50 amino acids that mediate interactions with proline-rich regions on partner proteins.
- Proline-rich region
-
Protein motif that is characterized by the recurrence of the proline residue in the form PXXP where X is any amino acid. Proline-rich regions mediate protein–protein interactions, particularly to SH3 domains.
- Chromatin immunoprecipitation
-
A sensitive molecular-biology technique that is used to quantify the binding of protein to specific DNA sequences.
- Orthologues
-
Proteins from different organisms that share function and often sequence similarity, and are thought to be evolutionarily related.
- RNA interference
-
A gene-silencing technique that was originally discovered and used in C. elegans. When exposed to short double-stranded RNA sequences that have been taken from mRNAs, this organism ingests them and inactivates expression of the corresponding gene.
Rights and permissions
About this article
Cite this article
Trigiante, G., Lu, X. ASPPs and cancer. Nat Rev Cancer 6, 217–226 (2006). https://doi.org/10.1038/nrc1818
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc1818
This article is cited by
-
Targeting and regulation of autophagy in hepatocellular carcinoma: revisiting the molecular interactions and mechanisms for new therapy approaches
Cell Communication and Signaling (2023)
-
p53 inhibitor iASPP is an unexpected suppressor of KRAS and inflammation-driven pancreatic cancer
Cell Death & Differentiation (2023)
-
iASPP is essential for HIF-1α stabilization to promote angiogenesis and glycolysis via attenuating VHL-mediated protein degradation
Oncogene (2022)
-
Nuclear iASPP determines cell fate by selectively inhibiting either p53 or NF-κB
Cell Death Discovery (2021)
-
ASPP1 deficiency promotes epithelial-mesenchymal transition, invasion and metastasis in colorectal cancer
Cell Death & Disease (2020)