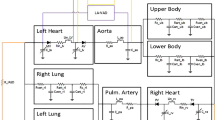
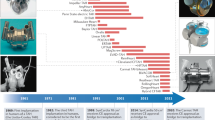
Abstract
Heart failure (HF) is a global phenomenon, and the overall incidence and prevalence of the condition are steadily increasing. Medical therapies have proven efficacious, but only a small number of pharmacological options are in development. When patients cease to respond adequately to optimal medical therapy, cardiac resynchronization therapy has been shown to improve symptoms, reduce hospitalizations, promote reverse remodelling, and decrease mortality. However, challenges remain in identifying the ideal recipients for this therapy. The field of mechanical circulatory support has seen immense growth since the early 2000s, and left ventricular assist devices (LVADs) have transitioned over the past decade from large, pulsatile devices to smaller, more-compact, continuous-flow devices. Infections and haematological issues are still important areas that need to be addressed. Whereas LVADs were once approved only for 'bridge to transplantation', these devices are now used as destination therapy for critically ill patients with HF, allowing these individuals to return to the community. A host of novel strategies, including cardiac contractility modulation, implantable haemodynamic-monitoring devices, and phrenic and vagus nerve stimulation, are under investigation and might have an impact on the future care of patients with chronic HF.
Key Points
-
Cardiac resynchronization therapy (CRT) has evolved as an effective therapy for many patients with chronic heart failure, especially those with left bundle branch block
-
CRT device optimization remains challenging, and is an area of intense investigation
-
Left ventricular assist devices can serve as a bridge to cardiac transplantation or destination therapy for critically ill patients with heart failure, and the use of the latest devices has increased patient survival
-
Physicians must be aware of various complex issues, including haematological and infectious concerns, when treating patients with chronic heart failure
-
Several novel, investigational devices for chronic heart failure are on the horizon and hold substantial promise to improve patient outcomes
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roger, V. L. et al. Heart disease and stroke statistics: 2012 update: a report from the American Heart Association. Circulation 125, e2–e220 (2012).
Mosterd, A. & Hoes, A. W. Clinical epidemiology of heart failure. Heart 93, 1137–1146 (2007).
Lloyd-Jones, D. M. et al. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation 106, 3068–3072 (2002).
The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N. Engl. J. Med. 327, 685–691 (1992).
The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 342, 145–153 (2000).
CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353, 9–13 (1999).
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353, 2001–2007 (1999).
Packer, M. et al. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 344, 1651–1658 (2001).
Poole-Wilson, P. et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 362, 7–13 (2003).
Pitt, B. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 341, 709–717 (1999).
Zannad, F. et al. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 364, 11–21 (2011).
Cohn, J. N. & Tognoni, G. for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 345, 1667–1675 (2001).
McMurray, J. J. V. et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 362, 767–771 (2003).
Ross, J. S. et al. Recent national trends in readmission rates after heart failure hospitalization. Circ. Heart Fail. 3, 97–103 (2010).
Chen, J., Normand, S. L., Wang, Y. & Krumholz, H. M. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA 306, 1669–1678 (2011).
Jencks, S. F., Williams, M. V. & Coleman, E. A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 360, 1418–1428 (2009).
Levy, D. et al. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 347, 1397–1402 (2002).
Smith, S. A. & Abraham, W. T. Device therapy in advanced heart failure: what to put in and what to turn off. Remote telemonitoring and implantable hemodynamic devices for advanced heart failure monitoring in the ambulatory setting and the evolving role of cardiac resynchronization therapy. Congest. Heart Fail. 17, 220–226 (2011).
Grines, C. L. et al. Functional abnormalities in isolated left bundle branch block: the effect of interventricular asynchrony. Circulation 79, 845–853 (1989).
Wilensky, R. L. et al. Serial electrocardiographic changes in idiopathic dilated cardiomyopathy confirmed at necropsy. Am. J. Cardiol. 62, 276–283 (1988).
Shamim, W. et al. Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int. J. Cardiol. 70, 171–178 (1999).
Cazeau, S. et al. Four chamber pacing in dilated cardiomyopathy. Pacing Clin. Electrophysiol. 17, 1974–1979 (1994).
Leclercq, C. et al. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J. Am. Coll. Cardiol. 32, 1825–1831 (1998).
Kass, D. A. et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 99, 1567–1573 (1999).
Auricchio, A. et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation 99, 2993–3001 (1999).
Daubert, J. C. et al. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin. Electrophysiol. 21, 239–245 (1998).
Cazeau, S. et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N. Engl. J. Med. 344, 873–880 (2001).
Abraham, W. T. et al. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 346, 1845–1853 (2002).
Yu, C. M. et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation 112, 1580–1586 (2005).
St John Sutton, M. G. et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 107, 1985–1990 (2003).
Moss, A. J. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 346, 877–883 (2002).
Young, J. B. et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. JAMA 289, 2685–2694 (2003).
Bristow, M. R. et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 350, 2140–2150 (2004).
Cleland, J. G. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 352, 1539–1549 (2005).
Vardas, P. E. et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology: developed in collaboration with the European Heart Rhythm Association. Europace 9, 959–998 (2007).
Steendijk, P. et al. Hemodynamic effects of long-term cardiac resynchronization therapy: analysis by pressure-volume loops. Circulation 113, 1295–1304 (2006).
Mullens, W. et al. Persistent hemodynamic benefits of cardiac resynchronization therapy with disease progression in advanced heart failure. J. Am. Coll. Cardiol. 53, 600–607 (2009).
Lindenfeld, J. et al. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation 115, 204–212 (2007).
Anand, I. S. et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison Of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation 119, 969–977 (2009).
van Bommel, R. J. et al. Effect of cardiac resynchronization therapy in patients with New York Heart Association functional class IV heart failure. Am. J. Cardiol. 106, 1146–1151 (2010).
Vidal, B. et al. Decreased likelihood of response to cardiac resynchronization in patients with severe heart failure. Eur. J. Heart Fail. 12, 283–287 (2010).
McAlister, F. A. et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 297, 2502–2514 (2007).
Abraham, W. T. et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation 110, 2864–2868 (2004).
Bleeker, G. B. et al. Cardiac resynchronization therapy in patients with systolic left ventricular dysfunction and symptoms of mild heart failure secondary to ischemic or nonischemic cardiomyopathy. Am. J. Cardiol. 98, 230–235 (2006).
Linde, C. et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J. Am. Coll. Cardiol. 52, 1834–1843 (2008).
Moss, A. J. et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 361, 1329–1338 (2009).
US Department of Health & Human Sciences: FDA. Summary of safety and effectiveness data (SSED) [online], (2010).
Zareba, W. et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial—Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 123, 1061–1072 (2011).
Sipahi, I., Carrigan, T. P., Rowland, D. Y., Stambler, B. S. & Fang, J. C. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch. Intern. Med. 171, 1454–1462 (2011).
Tang, A. S. et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 363, 2385–2395 (2010).
US Department of Health & Human Sciences: FDA. Summary of safety and effectiveness data (SSED) [online], (2012).
Goldenberg, I. et al. Reduction of the risk of recurring heart failure events with cardiac resynchronization therapy. J. Am. Coll. Cardiol. 58, 729–737 (2011).
Barsheshet, A. et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur. Heart J. 32, 1622–1630 (2011).
Versteeg, H. et al. Effect of cardiac resynchronization therapy-defibrillator implantation on health status in patients with mild versus moderate symptoms of heart failure. Am. J. Cardiol. 108, 1155–1159 (2011).
Adabag, S., Roukoz, H., Anand, I. S. & Moss, A. J. Cardiac resynchronization therapy in patients with minimal heart failure. J. Am. Coll. Cardiol. 58, 935–941 (2011).
Gold, M. R., Linde, C., Abraham, W. T., Gardiwal, A. & Daubert, J. C. The impact of cardiac resynchronization therapy on the incidence of ventricular arrhythmias in mild heart failure. Heart Rhythm 8, 679–684 (2011).
Al-Majed, N. S., McAlister, F. A., Bakal, J. A. & Ezekowitz, J. A. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann. Intern. Med. 154, 401–412 (2011).
Bank, A. J., Rischall, A., Gage, R. M., Burns, K. V. & Kubo, S. H. Comparison of cardiac resynchronization therapy outcomes in patients with New York Heart Association functional class I/II versus III/IV heart failure. J. Card. Fail. 18, 373–378 (2012).
Perry, R., De Pasquale, C. G., Chew, D. P., Aylward, P. E. & Joseph, M. X. QRS duration alone misses cardiac dyssynchrony in a substantial proportion of patients with chronic heart failure. J. Am. Soc. Echocardiogr. 19, 1257–1263 (2006).
Achilli, A. et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J. Am. Coll. Cardiol. 42, 2117–2124 (2003).
Yu, C. M. et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J. Am. Coll. Cardiol. 48, 2251–2257 (2006).
Bleeker, G. B. et al. Cardiac resynchronization therapy in patients with a narrow QRS complex. J. Am. Coll. Cardiol. 48, 2243–2250 (2006).
Beshai, J. F. et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N. Engl. J. Med. 357, 2461–2471 (2007).
Foley, P. W. et al. Cardiac resynchronisation therapy in patients with heart failure and a normal QRS duration: the RESPOND study. Heart 97, 1041–1047 (2011).
Williams, L. K. et al. Short-term hemodynamic effects of cardiac resynchronization therapy in patients with heart failure, a narrow QRS duration, and no dyssynchrony. Circulation 120, 1687–1694 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Auricchio, A. et al. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J. Am. Coll. Cardiol. 42, 2109–2116 (2003).
Byrne, M. J. et al. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J. Am. Coll. Cardiol. 50, 1484–1490 (2007).
Bilchick, K. C., Kamath, S., DiMarco, J. P. & Stukenborg, G. J. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation 122, 2022–2030 (2010).
Stevenson, W. G. et al. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America Guideline Committee. J. Card. Fail. 18, 94–106 (2012).
Patel, J. B. et al. Mitral regurgitation in patients with advanced systolic heart failure. J. Card. Fail. 10, 285–291 (2004).
Smith, S. A., Waggoner, A. D., de las Fuentes, L. & Davila-Roman, V. G. Role of serotoninergic pathways in drug-induced valvular heart disease and diagnostic features by echocardiography. J. Am. Soc. Echocardiogr. 22, 883–889 (2009).
Otsuji, Y. et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation 96, 1999–2008 (1997).
McKay, R. G. et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation 74, 693–702 (1986).
Yiu, S. F., Enriquez-Sarano, M., Tribouilloy, C., Seward, J. B. & Tajik, A. J. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: a quantitative clinical study. Circulation 102, 1400–1406 (2000).
Breithardt, O. A. et al. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J. Am. Coll. Cardiol. 41, 765–770 (2003).
Ypenburg, C. et al. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur. Heart J. 29, 757–765 (2008).
Vinereanu, D. et al. Mechanisms of reduction of mitral regurgitation by cardiac resynchronization therapy. J. Am. Soc. Echocardiogr. 20, 54–62 (2007).
Ypenburg, C. et al. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J. Am. Coll. Cardiol. 50, 2071–2077 (2007).
Boriani, G. et al. Impact of mitral regurgitation on the outcome of patients treated with CRT-D: data from the InSync ICD Italian Registry. Pacing Clin. Electrophysiol. 35, 146–154 (2012).
Verhaert, D. et al. Impact of mitral regurgitation on reverse remodeling and outcome in patients undergoing cardiac resynchronization therapy. Circ. Cardiovasc. Imaging 5, 21–26 (2012).
Ukkonen, H. et al. Effect of cardiac resynchronization on myocardial efficiency and regional oxidative metabolism. Circulation 107, 28–31 (2003).
Lindner, O. et al. Effect of cardiac resynchronization therapy on global and regional oxygen consumption and myocardial blood flow in patients with non-ischaemic and ischaemic cardiomyopathy. Eur. Heart J. 26, 70–76 (2005).
Lindner, O. et al. Global and regional myocardial oxygen consumption and blood flow in severe cardiomyopathy with left bundle branch block. Eur. J. Heart Fail. 7, 225–230 (2005).
Nowak, B. et al. Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J. Am. Coll. Cardiol. 41, 1523–1528 (2003).
Weber, K. T. & Brilla, C. G. Pathological hypertrophy and cardiac interstitium: fibrosis and renin–angiotensin–aldosterone system. Circulation 83, 1849–1865 (1991).
Weber, K. T. et al. Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press. 1, 75–85 (1992).
Weber, K. T. et al. Remodeling and reparation of the cardiovascular system. J. Am. Coll. Cardiol. 20, 3–16 (1992).
D'Ascia, C., Cittadini, A., Monti, M. G., Riccio, G. & Sacca, L. Effects of biventricular pacing on interstitial remodelling, tumor necrosis factor-α expression, and apoptotic death in failing human myocardium. Eur. Heart J. 27, 201–206 (2006).
Umar, S. et al. Myocardial collagen metabolism in failing hearts before and during cardiac resynchronization therapy. Eur. J. Heart Fail. 10, 878–883 (2008).
Garcia-Bolao, I. et al. Impact of collagen type I turnover on the long-term response to cardiac resynchronization therapy. Eur. Heart J. 29, 898–906 (2008).
Orrego, C. M. et al. Cellular evidence of reverse cardiac remodeling induced by cardiac resynchronization therapy. Congest. Heart Fail. 17, 140–146 (2011).
Iyengar, S. et al. Effect of cardiac resynchronization therapy on myocardial gene expression in patients with nonischemic dilated cardiomyopathy. J. Card. Fail. 13, 304–311 (2007).
Vanderheyden, M. et al. Endomyocardial upregulation of β1 adrenoreceptor gene expression and myocardial contractile reserve following cardiac resynchronization therapy. J. Card. Fail. 14, 172–178 (2008).
Vanderheyden, M. et al. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J. Am. Coll. Cardiol. 51, 129–136 (2008).
Barth, A. S. et al. Cardiac resynchronization therapy corrects dyssynchrony-induced regional gene expression changes on a genomic level. Circ. Cardiovasc. Genet. 2, 371–378 (2009).
Higgins, S. L. et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J. Am. Coll. Cardiol. 42, 1454–1459 (2003).
Bax, J. J. et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J. Am. Coll. Cardiol. 44, 1834–1840 (2004).
Chung, E. S. et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 117, 2608–2616 (2008).
Hsu, J. C. et al. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome. J. Am. Coll. Cardiol. 59, 2366–2373 (2012).
Arshad, A. et al. Cardiac resynchronization therapy is more effective in women than in men. J. Am. Coll. Cardiol. 57, 813–820 (2011).
van Bommel, R. J. et al. Site of latest activation in patients eligible for cardiac resynchronization therapy: patterns of dyssynchrony among different QRS configurations and impact of heart failure etiology. Am. Heart J. 161, 1060–1066 (2011).
Delgado, V. et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation 123, 70–78 (2011).
Singh, J. P. et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation 123, 1159–1166 (2011).
Taha, N. et al. Biventricular pacemaker optimization guided by comprehensive echocardiography—preliminary observations regarding the effects on systolic and diastolic ventricular function and third heart sound. J. Am. Soc. Echocardiogr. 23, 857–866 (2010).
Mullens, W. et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J. Am. Coll. Cardiol. 53, 765–773 (2009).
Hayes, D. L. et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm 8, 1469–1475 (2011).
Hunt, S. A. et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119, e391–e479 (2009).
Stehlik, J. et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth Adult Heart Transplant Report—2011. J. Heart Lung Transplant. 30, 1078–1094 (2011).
Dennis, C. et al. Clinical use of a cannula for left heart bypass without thoracotomy: experimental protection against fibrillation by left heart bypass. Ann. Surg. 156, 623–637 (1962).
DeVries, W. C. et al. Clinical use of the total artificial heart. N. Engl. J. Med. 310, 273–278 (1984).
Portner, P. M. et al. Implantable electrical left ventricular assist system: bridge to transplantation and the future. Ann. Thorac. Surg. 47, 142–150 (1989).
Frazier, O. H. et al. Multicenter clinical evaluation of the HeartMate 1000 IP left ventricular assist device. Ann. Thorac. Surg. 53, 1080–1090 (1992).
US Department of Health & Human Sciences: FDA. Medical devices: 1994 PMA approvals [online], (2010).
Oz, M. C., Goldstein, D. J. & Rose, E. A. Preperitoneal placement of ventricular assist devices: an illustrated stepwise approach. J. Card. Surg. 10, 288–294 (1995).
McCarthy, P. M. & Sabik, J. F. Implantable circulatory support devices as a bridge to heart transplantation. Semin. Thorac. Cardiovasc. Surg. 6, 174–180 (1994).
Goldstein, D. J., Oz, M. C. & Rose, E. A. Implantable left ventricular assist devices. N. Engl. J. Med. 339, 1522–1533 (1998).
Slater, J. P. et al. Low thromboembolic risk without anticoagulation using advanced-design left ventricular assist devices. Ann. Thorac. Surg. 62, 1321–1327 (1996).
Frazier, O. H. et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J. Thorac. Cardiovasc. Surg. 122, 1186–1195 (2001).
Rose, E. A. et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 345, 1435–1443 (2001).
Dembitsky, W. P. et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann. Thorac. Surg. 78, 2123–2129 (2004).
Dowling, R. D. et al. HeartMate VE LVAS design enhancements and its impact on device reliability. Eur. J. Cardiothorac. Surg. 25, 958–963 (2004).
Caccamo, M., Eckman, P. & John, R. Current state of ventricular assist devices. Curr. Heart Fail. Rep. 8, 91–98 (2011).
Asama, J., Shinshi, T., Hoshi, H., Takatani, S. & Shimokohbe, A. A compact highly efficient and low hemolytic centrifugal blood pump with a magnetically levitated impeller. Artif. Organs 30, 160–167 (2006).
Takatani, S. Progress of rotary blood pumps: presidential address, International Society for Rotary Blood Pumps 2006, Leuven, Belgium. Artif. Organs 31, 329–344 (2007).
Miller, L. W. et al. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 357, 885–896 (2007).
Slaughter, M. S. et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 361, 2241–2251 (2009).
Kirklin, J. K. et al. The fourth INTERMACS annual report: 4,000 implants and counting. J. Heart Lung Transplant. 31, 117–126 (2012).
Pagani, F. D. et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J. Am. Coll. Cardiol. 54, 312–321 (2009).
Kormos, R. L. et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 139, 1316–1324 (2010).
Lee, S. et al. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J. Heart Lung Transplant. 29, 209–215 (2010).
Klotz, S., Naka, Y., Oz, M. C. & Burkhoff, D. Biventricular assist device-induced right ventricular reverse structural and functional remodeling. J. Heart Lung Transplant. 24, 1195–1201 (2005).
Morgan, J. A., John, R., Lee, B. J., Oz, M. C. & Naka, Y. Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann. Thorac. Surg. 77, 859–863 (2004).
Rogers, J. G. et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J. Am. Coll. Cardiol. 55, 1826–1834 (2010).
Kirklin, J. K. et al. INTERMACS database for durable devices for circulatory support: first annual report. J. Heart Lung Transplant. 27, 1065–1072 (2008).
Kirklin, J. K. et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J. Heart Lung Transplant. 29, 1–10 (2010).
Wieselthaler, G. M. et al. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J. Heart Lung Transplant. 29, 1218–1225 (2010).
Strueber, M. et al. Multicenter evaluation of an intrapericardial left ventricular assist system. J. Am. Coll. Cardiol. 57, 1375–1382 (2011).
Aaronson, K. D. et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 125, 3191–3200 (2012).
Letsou, G. V. et al. Gastrointestinal bleeding from arteriovenous malformations in patients supported by the Jarvik 2000 axial-flow left ventricular assist device. J. Heart Lung Transplant. 24, 105–109 (2005).
Hetzer, R. et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur. J. Cardiothorac. Surg. 25, 964–970 (2004).
Morgan, J. A. et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J. Heart Lung Transplant. 31, 715–718 (2012).
Stern, D. R. et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J. Card. Surg. 25, 352–356 (2010).
Demirozu, Z. T. et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J. Heart Lung Transplant. 30, 849–853 (2011).
Uriel, N. et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J. Am. Coll. Cardiol. 56, 1207–1213 (2010).
Tsai, H. M., Sussman, I. I. & Nagel, R. L. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood 83, 2171–2179 (1994).
Klovaite, J., Gustafsson, F., Mortensen, S. A., Sander, K. & Nielsen, L. B. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J. Am. Coll. Cardiol. 53, 2162–2167 (2009).
Heilmann, C. et al. Acquired von Willebrand syndrome is an early-onset problem in ventricular assist device patients. Eur. J. Cardiothorac. Surg. 40, 1328–1333 (2011).
Vincentelli, A. et al. Acquired von Willebrand syndrome in aortic stenosis. N. Engl. J. Med. 349, 343–349 (2003).
John, R. et al. Low thromboembolic risk for patients with the HeartMate II left ventricular assist device. J. Thorac. Cardiovasc. Surg. 136, 1318–1323 (2008).
Boyle, A. J. et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J. Heart Lung Transplant. 28, 881–887 (2009).
Chaudhary, K. W. et al. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J. Am. Coll. Cardiol. 44, 837–845 (2004).
Ogletree, M. L. et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J. Heart Lung Transplant. 29, 554–561 (2010).
Heerdt, P. M. et al. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation 102, 2713–2719 (2000).
Hall, J. L. et al. Clinical, molecular, and genomic changes in response to a left ventricular assist device. J. Am. Coll. Cardiol. 57, 641–652 (2011).
Blaxall, B. C., Tschannen-Moran, B., Milano, C. A. & Koch, W. J. Differential gene expression and genomic patient stratification following left ventricular assist device support. J. Am. Coll. Cardiol. 41, 1096–1106 (2003).
Ambardekar, A. V. & Buttrick, P. M. Reverse remodeling with left ventricular assist devices: a review of clinical, cellular, and molecular effects. Circ. Heart Fail. 4, 224–233 (2011).
Maybaum, S. et al. Cardiac improvement during mechanical circulatory support. Circulation 115, 2497–2505 (2007).
Radovancevic, B. et al. End-organ function in patients on long-term circulatory support with continuous- or pulsatile-flow assist devices. J. Heart Lung Transplant. 26, 815–818 (2007).
Kamdar, F. et al. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J. Heart Lung Transplant. 28, 352–359 (2009).
Refaat, M. M. et al. Survival benefit of implantable cardioverter-defibrillators in left ventricular assist device-supported heart failure patients. J. Card. Fail. 18, 140–145 (2012).
Slaughter, M. S. et al. HeartWare miniature axial-flow ventricular assist device: design and initial feasibility test. Tex. Heart Inst. J. 36, 12–16 (2009).
John, R., Mantz, K., Eckman, P., Rose, A. & May-Newman, K. Aortic valve pathophysiology during left ventricular assist device support. J. Heart Lung Transplant. 29, 1321–1329 (2010).
Mancini, D. M. et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation 98, 2383–2389 (1998).
Birks, E. J. et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N. Engl. J. Med. 355, 1873–1884 (2006).
Birks, E. J. et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation 123, 381–390 (2011).
Lamarche, Y. et al. Successful weaning and explantation of the HeartMate II left ventricular assist device. Can. J. Cardiol. 27, 358–362 (2011).
Kadish, A. et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am. Heart J. 161, 329–337. e1–2 (2011).
Abraham, W. T. et al. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J. Card. Fail. 17, 710–717 (2011).
Mazzaferri, E. L. Jr et al. Percutaneous left ventricular partitioning in patients with chronic heart failure and a prior anterior myocardial infarction: results of the PercutAneous Ventricular RestorAtion in Chronic Heart failUre PaTiEnts Trial. Am. Heart J. 163, 812–820.e1 (2012).
Ponikowski, P. et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur. Heart J. 33, 889–894 (2012).
Hayward, C. S. et al. Chronic extra-aortic balloon counterpulsation: first-in-human pilot study in end-stage heart failure. J. Heart Lung Transplant. 29, 1427–1432 (2010).
Anker, S. D., Koehler, F. & Abraham, W. T. Telemedicine and remote management of patients with heart failure. Lancet 378, 731–739 (2011).
Dipla, K. et al. The sarcoplasmic reticulum and the Na+/Ca2+ exchanger both contribute to the Ca2+ transient of failing human ventricular myocytes. Circ. Res. 84, 435–444 (1999).
Burkoff, D. et al. Electric currents applied during the refractory period can modulate cardiac contractility in vitro and in vivo. Heart Fail. Rev. 6, 27–34 (2001).
Pappone, C. et al. First human chronic experience with cardiac contractility modulation by nonexcitatory electrical currents for treating systolic heart failure: mid-term safety and efficacy results from a multicenter study. J. Cardiovasc. Electrophysiol. 15, 418–427 (2004).
Mortara, A. et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications Circulation 96, 3450–3458 (1997).
De Ferrari, G. M. et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 32, 847–855 (2011).
Author information
Authors and Affiliations
Contributions
S. A. Smith researched data for the article. Both authors discussed its content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
W. T. Abraham is or has been a consultant for the following companies: Biotronik, Medtronic, and St Jude Medical. S. A. Smith declares no competing interests.
Rights and permissions
About this article
Cite this article
Abraham, W., Smith, S. Devices in the management of advanced, chronic heart failure. Nat Rev Cardiol 10, 98–110 (2013). https://doi.org/10.1038/nrcardio.2012.178
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2012.178
This article is cited by
-
Experimental and numerical investigation of different geometrical parameters in a centrifugal blood pump
Research on Biomedical Engineering (2022)
-
Functional capillary impairment in patients with ventricular assist devices
Scientific Reports (2019)
-
Progress in heart failure treatment in Germany
Clinical Research in Cardiology (2018)
-
Heart failure with reduced ejection fraction
Nature Reviews Disease Primers (2017)
-
Circulating microbial RNA and health
Scientific Reports (2015)