Key Points
-
Lysosomal storage disorders (LSDs) include over 70 inborn errors of metabolism that collectively affect 1 in 5,000 live births but individually are orphan diseases.
-
How the primary defect, usually in a lysosomal enzyme, leads to a complex pathogenic cascade and typically neurodegeneration is incompletely understood.
-
Some LSDs can be prevented by carrier screening of at-risk populations, but currently, most countries do not include these diseases on newborn screening panels, primarily because most are without effective treatment.
-
Several therapies have been developed for specific LSDs and include enzyme replacement therapy, substrate reduction therapy and chemical chaperone therapy with haematopoietic stem cell transplantation.
-
Experimental therapies at the stage of clinical trials include gene therapy, heat shock protein 70 (HSP70)-inducing chaperone therapy and other disease-specific therapies.
-
Currently, there is a great deal of commercial activity in this field, catalysed by the incentives arising from orphan drug legislation.
Abstract
Lysosomal storage disorders (LSDs) — designated as 'orphan' diseases — are inborn errors of metabolism caused by defects in genes that encode proteins involved in various aspects of lysosomal homeostasis. For many years, LSDs were viewed as unattractive targets for the development of therapies owing to their low prevalence. However, the development and success of the first commercial biologic therapy for an LSD — enzyme replacement therapy for type 1 Gaucher disease — coupled with regulatory incentives rapidly catalysed commercial interest in therapeutically targeting LSDs. Despite ongoing challenges, various therapeutic strategies for LSDs now exist, with many agents approved, undergoing clinical trials or in preclinical development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cox, T. M. & Cachon-Gonzalez, M. B. The cellular pathology of lysosomal diseases. J. Pathol. 226, 241–254 (2012).
Rapola, J. Lysosomal storage diseases in adults. Pathol. Res. Pract. 190, 759–766 (1994).
Wassif, C. A. et al. High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet. Med. 18, 41–48 (2016).
Maubert, A., Hanon, C. & Metton, J. P. Adult onset Niemann-Pick type C disease and psychosis: literature review [French]. Encephale 39, 315–319 (2013).
Wraith, J. E. Lysosomal disorders. Semin. Neonatol. 7, 75–83 (2002).
Parenti, G., Andria, G. & Ballabio, A. Lysosomal storage diseases: from pathophysiology to therapy. Annu. Rev. Med. 66, 471–486 (2015).
Ballabio, A. & Gieselmann, V. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta 1793, 684–696 (2009).
Pastores, G. M., Torres, P. A. & Zeng, B. J. Animal models for lysosomal storage disorders. Biochem. Biokhimiia 78, 721–725 (2013).
Cox, T. M. Competing for the treasure in exceptions. Am. J. Hematol. 88, 163–165 (2013).
Brady, R. O. Enzyme replacement for lysosomal diseases. Annu. Rev. Med. 57, 283–296 (2006). This is a review by the pioneer Brady describing the development of ERT for LSDs.
Meikle, P. J. et al. Newborn screening for lysosomal storage disorders. Mol. Genet. Metab. 88, 307–314 (2006).
Matern, D. et al. Newborn screening for lysosomal storage disorders. Semin. Perinatol. 39, 206–216 (2015).
Wasserstein, M. P. et al. Clinical outcomes of children with abnormal newborn screening results for Krabbe disease in New York State. Genet. Med. 18, 1235–1243 (2016). This study highlights the complexity of issues associated with newborn screening for LSDs.
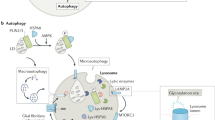
Settembre, C., Fraldi, A., Medina, D. L. & Ballabio, A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013). This is a review of the advances in our understanding of the complex signalling roles played by lysosomes.
Palmieri, M. et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 (2011).
Sardiello, M. & Ballabio, A. Lysosomal enhancement: a CLEAR answer to cellular degradative needs. Cell Cycle 8, 4021–4022 (2009).
Medina, D. L. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 (2015).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Mansueto, G. et al. Transcription factor EB controls metabolic flexibility during exercise. Cell. Metab. 25, 182–196 (2017).
Pastore, N. et al. TFE3 regulates whole-body energy metabolism in cooperation with TFEB. EMBO Mol. Med. 9, 605–621 (2017).
Castellano, B. M. et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 355, 1306–1311 (2017).
Schwake, M., Schroder, B. & Saftig, P. Lysosomal membrane proteins and their central role in physiology. Traffic 14, 739–748 (2013).
Chapel, A. et al. An extended proteome map of the lysosomal membrane reveals novel potential transporters. Mol. Cell. Proteom. 12, 1572–1588 (2013).
Yogalingam, G. et al. Neuraminidase 1 is a negative regulator of lysosomal exocytosis. Dev. Cell 15, 74–86 (2008).
Settembre, C. & Ballabio, A. Lysosomal adaptation: how the lysosome responds to external cues. Cold Spring Harb. Perspect. Biol. 6, a016907 (2014).
Toulmay, A. & Prinz, W. A. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr. Opin. Cell Biol. 23, 458–463 (2011).
Hariri, H., Ugrankar, R., Liu, Y. & Henne, W. M. Inter-organelle ER-endolysosomal contact sites in metabolism and disease across evolution. Commun. Integr. Biol. 9, e1156278 (2016).
Saftig, P. & Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 (2009).
Churchill, G. C. et al. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111, 703–708 (2002).
Morgan, A. J., Platt, F. M., Lloyd-Evans, E. & Galione, A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 439, 349–374 (2011).
Mrschtik, M. & Ryan, K. M. Lysosomal proteins in cell death and autophagy. FEBS J. 282, 1858–1870 (2015).
Ballabio, A. The awesome lysosome. EMBO Mol. Med. 8, 73–76 (2016).
ACOG Committee on Genetics. ACOG committee opinion. Number 298, August 2004. Prenatal and preconceptional carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet. Gynecol. 104, 425–428 (2004).
Langlois, S. & Wilson, R. D. Carrier screening for genetic disorders in individuals of Ashkenazi Jewish descent. J. Obstetr. Gynaecol. 28, 324–343 (2006).
Winchester, B. in Lysosomal Disorders of the Brain (eds Platt, F. M. & Walkley, S. U.) 81–130 (Oxford Univ. Press, 2004).
Bagshaw, R. D., Mahuran, D. J. & Callahan, J. W. A proteomic analysis of lysosomal integral membrane proteins reveals the diverse composition of the organelle. Mol. Cell. Proteom. 4, 133–143 (2005).
von Figura, K. Molecular recognition and targeting of lysosomal proteins. Curr. Opin. Cell Biol. 3, 642–646 (1991).
Sandhoff, K. Neuronal sphingolipidoses: membrane lipids and sphingolipid activator proteins regulate lysosomal sphingolipid catabolism. Biochimie 130, 146–151 (2016).
Platt, F. M., Boland, B. & van der Spoel, A. C. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 199, 723–734 (2012).
Walkley, S. U. Secondary accumulation of gangliosides in lysosomal storage disorders. Semin. Cell Dev. Biol. 15, 433–444 (2004).
Platt, F. M. & Walkley, S. U. in Lysosomal Disorders of the Brain (eds Platt, F. M. & Walkley, S. U.) 32–49 (Oxford Univ. Press, 2004).
Biegstraaten, M. et al. A monozygotic twin pair with highly discordant Gaucher phenotypes. Blood Cells Mol. Dis. 46, 39–41 (2011).
Lachmann, R. H., Grant, I. R., Halsall, D. & Cox, T. M. Twin pairs showing discordance of phenotype in adult Gaucher's disease. QJM 97, 199–204 (2004).
Auer, I. A. et al. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer's disease. Acta Neuropathol. 90, 547–551 (1995).
Carette, J. E. et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477, 340–343 (2011).
Becker, K. A. et al. Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am. J. Respir. Cell. Mol. Biol. 42, 716–724 (2010).
Kirkegaard, T. & Jaattela, M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta 1793, 746–754 (2009).
Platt, F. M. et al. Disorders of cholesterol metabolism and their unanticipated convergent mechanisms of disease. Annu. Rev. Genom. Hum. Genet. 15, 173–194 (2014).
Sidransky, E. & Lopez, G. The link between the GBA gene and parkinsonism. Lancet Neurol. 11, 986–998 (2012).
Aflaki, E., Westbroek, W. & Sidransky, E. The complicated relationship between Gaucher disease and parkinsonism: insights from a rare disease. Neuron 93, 737–746 (2017).
Eblan, M. J., Walker, J. M. & Sidransky, E. The glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 352, 728–731 (2005).
Bembi, B. et al. Gaucher's disease with Parkinson's disease: clinical and pathological aspects. Neurology 61, 99–101 (2003).
Lopez, G. & Sidransky, E. Autosomal recessive mutations in the development of Parkinson's disease. Biomarkers Med. 4, 713–721 (2010).
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Xu, M. et al. Disease models for the development of therapies for lysosomal storage diseases. Ann. NY Acad. Sci. 1371, 15–29 (2016).
Haskins, M. E., Giger, U. & Patterson, D. F. in Fabry Disease: Perspectives from 5 Years of FOS Ch. 6 (eds Mehta, A., Beck, M. & Sunder-Plassmann, G.) (Oxford PharmaGenesis, 2006).
Wraith, J. E. Mucopolysaccharidoses and mucolipidoses. Handb. Clin. Neurol. 113, 1723–1729 (2013).
Regier, D. S., Proia, R. L., D'Azzo, A. & Tifft, C. J. The GM1 and GM2 gangliosidoses: natural history and progress toward therapy. Pediatr. Endocrinol. Rev. 13 (Suppl. 1), 663–673 (2016).
Staretz-Chacham, O., Choi, J. H., Wakabayashi, K., Lopez, G. & Sidransky, E. Psychiatric and behavioral manifestations of lysosomal storage disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 1253–1265 (2010).
Wraith, J. E. et al. Recommendations on the diagnosis and management of Niemann-Pick disease type C. Mol. Genet. Metab. 98, 152–165 (2009).
Giugliani, R. et al. Current molecular genetics strategies for the diagnosis of lysosomal storage disorders. Expert Rev. Mol. Diagn. 16, 113–123 (2016). This is an overview of diagnostic strategies for LSDs, with a focus on molecular diagnostics.
Kingma, S. D., Bodamer, O. A. & Wijburg, F. A. Epidemiology and diagnosis of lysosomal storage disorders; challenges of screening. Best Pract. Res. Clin. Endocrinol. Metab. 29, 145–157 (2015).
Cismondi, I. A. et al. Guidelines for incorporating scientific knowledge and practice on rare diseases into higher education: neuronal ceroid lipofuscinoses as a model disorder. Biochim. Biophys. Acta 1852, 2316–2323 (2015).
Meikle, P. J., Fietz, M. J. & Hopwood, J. J. Diagnosis of lysosomal storage disorders: current techniques and future directions. Expert Rev. Mol. Diagn. 4, 677–691 (2004).
Fuller, M. et al. Screening patients referred to a metabolic clinic for lysosomal storage disorders. J. Med. Genet. 48, 422–425 (2011).
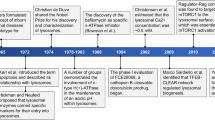
Hers, H. G. α-Glucosidase deficiency in generalised glycogen storage disease (Pompe's disease). Biochem. J. 86, 11–16 (1963). This is a landmark paper identifying a lysosomal hydrolase defect as the cause of an LSD.
de Duve, C. The lysosome turns fifty. Nat. Cell Biol. 7, 847–849 (2005). This is a retrospective review of what has been learned about the lysosome by de Duve, who was awarded the Nobel Prize in Physiology and Medicine in 1974 for his discovery of lysosomes and peroxisomes.
Kornfeld, S. Trafficking of lysosomal enzymes. FASEB J. 1, 462–468 (1987).
Fratantoni, J. C., Hall, C. W. & Neufeld, E. F. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science 162, 570–572 (1968).
Neufeld, E. F. From serendipity to therapy. Annu. Rev. Biochem. 80, 1–15 (2011). This is a definitive review by the pioneer Neufeld on the discovery of cross-correction and its application to LSD therapies.
Kornfeld, S. Trafficking of lysosomal enzymes in normal and disease states. J. Clin. Invest. 77, 1–6 (1986).
Dittmer, F. et al. Alternative mechanisms for trafficking of lysosomal enzymes in mannose 6-phosphate receptor-deficient mice are cell type-specific. J. Cell Sci. 112, 1591–1597 (1999).
Gonzalez, A., Valeiras, M., Sidransky, E. & Tayebi, N. Lysosomal integral membrane protein-2: A new player in lysosome-related pathology. Mol. Genet. Metab. 111, 84–91 (2014).
Johnson, W. G. et al. Intravenous injection of purified hexosaminidase A into a patient with Tay-Sachs disease. Birth Defects Orig Art. Ser. 9, 120–124 (1973).
Krivit, W. Stem cell bone marrow transplantation in patients with metabolic storage diseases. Adv. Pediatr. 49, 359–378 (2002).
Hobbs, J. R. et al. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 2, 709–712 (1981).
Walkley, S. U. & Dobrenis, K. Bone marrow transplantation for lysosomal diseases. Lancet 345, 1382–1383 (1995).
Krivit, W., Peters, C. & Shapiro, E. G. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Curr. Opin. Neurol. 12, 167–176 (1999). This article reviews the role of BMT as a disease-modifying therapy for some LSDs.
Bonney, D. K. et al. Successful allogeneic bone marrow transplant for Niemann-Pick disease type C2 is likely to be associated with a severe 'graft versus substrate' effect. J. Inherit Metab. Dis. 33 (Suppl. 3), S171–S173 (2010).
Breen, C. et al. Developmental outcome post allogenic bone marrow transplant for Niemann Pick Type C2. Mol. Genet. Metab. 108, 82–84 (2013).
Ho, A. L., Keshavarzi, S. & Levy, M. L. Exploitation of genetically modified neural stem cells for neurological disease. Adv. Exp. Med. Biol. 671, 74–92 (2010).
Jeyakumar, M. et al. Neural stem cell transplantation benefits a monogenic neurometabolic disorder during the symptomatic phase of disease. Stem Cells 27, 2362–2370 (2009).
Galli, R., Gritti, A., Bonfanti, L. & Vescovi, A. L. Neural stem cells: an overview. Circ. Res. 92, 598–608 (2003).
Nagabhushan Kalburgi, S., Khan, N. N. & Gray, S. J. Recent gene therapy advancements for neurological diseases. Discov. Med. 15, 111–119 (2013).
Yew, N. S. & Cheng, S. H. Gene therapy for lysosomal storage disorders. Pediatr. Endocrinol. Rev. 11 (Suppl. 1), 99–109 (2013).
Aronovich, E. L. & Hackett, P. B. Lysosomal storage disease: gene therapy on both sides of the blood-brain barrier. Mol. Genet. Metab. 114, 83–93 (2015).
Cheng, S. H. Gene therapy for the neurological manifestations in lysosomal storage disorders. J. Lipid Res. 55, 1827–1838 (2014).
Rastall, D. P. & Amalfitano, A. Recent advances in gene therapy for lysosomal storage disorders. Appl. Clin. Genet. 8, 157–169 (2015).
Biffi, A. Gene therapy for lysosomal storage disorders: a good start. Hum. Mol. Genet. 25, R65–R75 (2016). This is an overview of gene therapy approaches for LSDs and their current status.
Lim-Melia, E. R. & Kronn, D. F. Current enzyme replacement therapy for the treatment of lysosomal storage diseases. Pediatr. Ann. 38, 448–455 (2009).
Jolly, R. D. et al. Intracisternal enzyme replacement therapy in lysosomal storage diseases: dispersal pathways, regional enzyme concentrations and the effect of posttreatment posture. Neuropathol. Appl. Neurobiol. 39, 681–692 (2013).
Martin-Banderas, L. et al. Role of nanotechnology for enzyme replacement therapy in lysosomal diseases. a focus on Gaucher's disease. Curr. Med. Chem. 23, 929–952 (2016).
Desnick, R. J. & Schuchman, E. H. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Annu. Rev. Genom. Hum. Genet. 13, 307–335 (2012).
Lachmann, R. H. Enzyme replacement therapy for lysosomal storage diseases. Curr. Opin. Pediatr. 23, 588–593 (2011).
Foust, K. D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 (2009).
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009).
Conzelmann, E. & Sandhoff, K. Biochemical basis of late-onset neurolipidoses. Dev. Neurosci. 13, 197–204 (1991).
Kastelein, J. J., Ross, C. J. & Hayden, M. R. From mutation identification to therapy: discovery and origins of the first approved gene therapy in the Western world. Hum. Gene Ther. 24, 472–478 (2013).
Chandler, R. J. et al. Systemic AAV9 gene therapy improves the lifespan of mice with Niemann-Pick disease, type C1. Hum. Mol. Genet. 26, 52–64 (2017).
Keeling, K. M., Xue, X., Gunn, G. & Bedwell, D. M. Therapeutics based on stop codon readthrough. Annu. Rev. Genom. Hum. Genet. 15, 371–394 (2014).
Keeling, K. M. Nonsense suppression as an approach to treat lysosomal storage diseases. Diseases 4, 32 (2016).
Prakash, V., Moore, M. & Yanez-Munoz, R. J. Current progress in therapeutic gene editing for monogenic diseases. Mol. Ther. 24, 465–474 (2016).
Shim, G. et al. Therapeutic gene editing: delivery and regulatory perspectives. Acta Pharmacol. Sin. 38, 738–753 (2017).
Cox, D. B., Platt, R. J. & Zhang, F. Therapeutic genome editing: prospects and challenges. Nat. Med. 21, 121–131 (2015).
Brady, R. O. Enzyme replacement therapy: conception, chaos and culmination. Phil. Trans. R. Soc. Lond. B Biol. Sci. 358, 915–919 (2003).
Thurberg, B. L. et al. Clearance of hepatic sphingomyelin by olipudase alfa is associated with improvement in lipid profiles in acid sphingomyelinase deficiency. Am. J. Surg. Pathol. 40, 1232–1242 (2016).
Wasserstein, M. P. et al. Successful within-patient dose escalation of olipudase alfa in acid sphingomyelinase deficiency. Mol. Genet. Metab. 116, 88–97 (2015).
Aerts, J. M. & Cox, T. M. Roscoe O. Brady: Physician whose pioneering discoveries in lipid biochemistry revolutionized treatment and understanding of lysosomal diseases. Blood Cells Mol. Dis. http://dx.doi.org/10.1016/j.bcmd.2016.10.030 (2017).
Desnick, R. J. et al. Roscoe Owen Brady, MD: Remembrances of co-investigators and colleagues. Mol. Genet. Metab. 120, 1–7 (2017).
Futerman, A. H. & Platt, F. M. The metabolism of glucocerebrosides — From 1965 to the present. Mol. Genet. Metab. 120, 22–26 (2017).
Casey, D. Key strategic factors for stakeholders in the current global biosimilar market. Drug Discov. Today 21, 208–211 (2016).
van Gelder, C. M. et al. Enzyme therapy and immune response in relation to CRIM status: the Dutch experience in classic infantile Pompe disease. J. Inherit. Metab. Dis. 38, 305–314 (2015).
Angelini, C. & Semplicini, C. Enzyme replacement therapy for Pompe disease. Curr. Neurol. Neurosci. Rep. 12, 70–75 (2012).
Kazi, Z. B. et al. Sustained immune tolerance induction in enzyme replacement therapy-treated CRIM-negative patients with infantile Pompe disease. JCI Insight 2, e94328 (2017).
Hollak, C. E. & Linthorst, G. E. Immune response to enzyme replacement therapy in Fabry disease: impact on clinical outcome? Mol. Genet. Metab. 96, 1–3 (2009).
Ohashi, T. Enzyme replacement therapy for lysosomal storage diseases. Pediatr. Endocrinol. Rev. 10 (Suppl. 1), 26–34 (2012).
Ries, M. Enzyme replacement therapy and beyond— in memoriam Roscoe O. Brady, M.D. (1923–2016). J. Inherit Metab. Dis. 40, 343–356 (2017).
Fan, J. Q., Ishii, S., Asano, N. & Suzuki, Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 5, 112–115 (1999). This study shows the potential of chemical chaperone therapy that paved the way for the approval of migalastat for the treatment of Fabry disease in 2016.
Fan, J. Q. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol. Chem. 389, 1–11 (2008).
Parenti, G., Andria, G. & Valenzano, K. J. Pharmacological chaperone therapy: preclinical development, clinical translation, and prospects for the treatment of lysosomal storage disorders. Mol. Ther. 23, 1138–1148 (2015).
Butters, T. D., Dwek, R. A. & Platt, F. M. Imino sugar inhibitors for treating the lysosomal glycosphingolipidoses. Glycobiology 15, 43R–52R (2005).
Patnaik, S. et al. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. J. Med. Chem. 55, 5734–5748 (2012).
Marugan, J. J. et al. Non-iminosugar glucocerebrosidase small molecule chaperones. MedChemComm 3, 56–60 (2012).
Benito, J. M., Garcia Fernandez, J. M. & Ortiz Mellet, C. Pharmacological chaperone therapy for Gaucher disease: a patent review. Expert Opin. Ther. Patents 21, 885–903 (2011).
Narita, A. et al. Ambroxol chaperone therapy for neuronopathic Gaucher disease: a pilot study. Ann. Clin. Transl Neurol. 3, 200–215 (2016).
Hossain, M. A. et al. Chaperone therapy for Krabbe disease: potential for late-onset GALC mutations. J. Hum. Genet. 60, 539–545 (2015).
Parenti, G., Moracci, M., Fecarotta, S. & Andria, G. Pharmacological chaperone therapy for lysosomal storage diseases. Future Med. Chem. 6, 1031–1045 (2014).
Matsuda, J. et al. Chemical chaperone therapy for brain pathology in GM1-gangliosidosis. Proc. Natl Acad. Sci. USA 100, 15912–15917 (2003).
Germain, D. P. et al. Treatment of Fabry's disease with the pharmacologic chaperone migalastat. N. Engl. J. Med. 375, 545–555 (2016).
Giugliani, R. et al. A Phase 2 study of migalastat hydrochloride in females with Fabry disease: selection of population, safety and pharmacodynamic effects. Mol. Genet. Metab. 109, 86–92 (2013).
Hughes, D. A. et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J. Med. Genet. 54, 288–296 (2017).
Markham, A. Migalastat: first global approval. Drugs 76, 1147–1152 (2016).
Ishay, Y. et al. Combined β-glucosylceramide and ambroxol hydrochloride in patients with Gaucher related Parkinson disease: From clinical observations to drug development. Blood Cells Mol. Dis. http://dx.doi.org/10.1016/j.bcmd.2016.10.028 (2016).
Luan, Z. et al. The chaperone activity and toxicity of ambroxol on Gaucher cells and normal mice. Brain Dev. 35, 317–322 (2013).
Maegawa, G. H. et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J. Biol. Chem. 284, 23502–23516 (2009).
Pawlinski, L., Malecki, M. T. & Kiec-Wilk, B. The additive effect on the antiepileptic treatment of ambroxol in type 3 Gaucher patient. The early observation. Blood Cells Mol. Dis. http://dx.doi.org/10.1016/j.bcmd.2016.12.001 (2016).
Zimran, A., Altarescu, G. & Elstein, D. Pilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher disease. Blood Cells Mol. Dis. 50, 134–137 (2013).
Citro, V. et al. Identification of an allosteric binding site on human lysosomal α-galactosidase opens the way to new pharmacological chaperones for Fabry disease. PLoS ONE 11, e0165463 (2016).
Jung, O., Patnaik, S., Marugan, J., Sidransky, E. & Westbroek, W. Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease. Expert Rev. Proteom. 13, 471–479 (2016).
Aflaki, E. et al. A new glucocerebrosidase chaperone reduces α-synuclein and glycolipid levels in iPSC-derived dopaminergic neurons from patients with Gaucher disease and parkinsonism. J. Neurosci. 36, 7441–7452 (2016).
Haneef, S. A. & Doss, C. G. Personalized pharmacoperones for lysosomal storage disorder: approach for next-generation treatment. Adv. Protein Chem. Struct. Biol. 102, 225–265 (2016).
Platt, F. M. & Jeyakumar, M. Substrate reduction therapy. Acta Paediatr. Suppl. 97, 88–93 (2008).
Platt, F. M. et al. Prevention of lysosomal storage in Tay-Sachs mice treated with N-butyldeoxynojirimycin. Science 276, 428–431 (1997). This study provides in vivo evidence that SRT could reduce storage in peripheral organs and the CNS, paving the way for the approval of miglustat for the treatment of Gaucher disease and Niemann–Pick type C disease.
Platt, F. M. Sphingolipid lysosomal storage disorders. Nature 510, 68–75 (2014).
Coutinho, M. F., Santos, J. I. & Alves, S. Less is more: substrate reduction therapy for lysosomal storage disorders. Int. J. Mol. Sci. 17, 1065 (2016).
Radin, N. S. Treatment of Gaucher disease with an enzyme inhibitor. Glycoconj. J. 13, 153–157 (1996).
Sandhoff, K. & Kolter, T. Biochemistry of glycosphingolipid degradation. Clin. Chim. Acta 266, 51–61 (1997).
Belmatoug, N. et al. Management and monitoring recommendations for the use of eliglustat in adults with type 1 Gaucher disease in Europe. Eur. J. Intern. Med. 37, 25–32 (2017).
Patterson, M. C. et al. Oral miglustat in Niemann-Pick type C (NPC) disease. Rev. Neurol. 43, 8 (2006).
Judith Peterschmitt, M. et al. A pooled analysis of adverse events in 393 adults with Gaucher disease type 1 from four clinical trials of oral eliglustat: evaluation of frequency, timing, and duration. Blood Cells Mol. Dis. http://dx.doi.org/10.1016/j.bcmd.2017.01.006 (2017).
Becquemont, L. Type 1 Gaucher disease (CYP2D6-eliglustat). Therapie 72, 323–326 (2017).
Guerard, N., Zwingelstein, C. & Dingemanse, J. Lucerastat, an iminosugar for substrate reduction therapy: pharmacokinetics, tolerability, and safety in subjects with mild, moderate, and severe renal function impairment. J. Clin. Pharmacol. 57, 1425–1431 (2017).
Guérard, N., Morand, O. & Dingemanse, J. Lucerastat, an iminosugar with potential as substrate reduction therapy for glycolipid storage disorders: safety, tolerability, and pharmacokinetics in healthy subjects. Orphanet J. Rare Dis. 12, 9 (2017).
Guerard, N. et al. Lucerastat, an iminosugar for substrate reduction therapy: tolerability, pharmacodynamics, and pharmacokinetics in patients with Fabry disease on enzyme replacement. Clin. Pharmacol. Ther. http://dx.doi.org/10.1002/cpt.790 (2017).
Malinowska, M. et al. Genistein reduces lysosomal storage in peripheral tissues of mucopolysaccharide IIIB mice. Mol. Genet. Metab. 98, 235–242 (2009).
Moskot, M. et al. The phytoestrogen genistein modulates lysosomal metabolism and transcription factor EB (TFEB) activation. J. Biol. Chem. 289, 17054–17069 (2014).
Moskot, M. et al. Modulation of expression of genes involved in glycosaminoglycan metabolism and lysosome biogenesis by flavonoids. Sci. Rep. 5, 9378 (2015).
Clayton, N. P. et al. Antisense oligonucleotide-mediated suppression of muscle glycogen synthase 1 synthesis as an approach for substrate reduction therapy of Pompe disease. Mol. Ther. Nucleic Acids 3, e206 (2014).
Petersen, N. H. & Kirkegaard, T. HSP70 and lysosomal storage disorders: novel therapeutic opportunities. Biochem. Soc. Trans. 38, 1479–1483 (2010).
Kirkegaard, T. et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463, 549–553 (2010).
Kirkegaard, T. et al. Heat shock protein-based therapy as a potential candidate for treating the sphingolipidoses. Sci. Transl Med. 8, 355ra118 (2016). This study provides in vivo evidence in animal models that the HSP70 inducer arimoclomol has therapeutic benefit in multiple LSDs.
Kalmar, B., Lu, C. H. & Greensmith, L. The role of heat shock proteins in amyotrophic lateral sclerosis: the therapeutic potential of arimoclomol. Pharmacol. Ther. 141, 40–54 (2014).
Kieran, D. et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 10, 402–405 (2004).
Hargitai, J. et al. Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem. Biophys. Res. Commun. 307, 689–695 (2003).
Phukan, J. Arimoclomol, a coinducer of heat shock proteins for the potential treatment of amyotrophic lateral sclerosis. IDrugs 13, 482–496 (2010).
Ahmed, M. et al. Targeting protein homeostasis in sporadic inclusion body myositis. Sci. Transl Med. 8, 331ra41 (2016).
Deane, C. A. & Brown, I. R. Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol. Cell Stress Chaperones 21, 837–848 (2016).
Yang, C. et al. Celastrol increases glucocerebrosidase activity in Gaucher disease by modulating molecular chaperones. Proc. Natl Acad. Sci. USA 111, 249–254 (2014).
Kalmar, B. & Greensmith, L. Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects. Cell. Mol. Biol. Lett. 14, 319–335 (2009).
Wada, R., Tifft, C. J. & Proia, R. L. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is supressed by bone marrow transplantation. Proc. Natl Acad. Sci. USA 97, 10954–10959 (2000). This study reports the discovery that neuroinflammation actively contributes to disease progression, underscoring the potential of anti-inflammatory therapies for LSDs.
Jeyakumar, M. et al. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain 126, 974–987 (2003).
Jeyakumar, M. et al. NSAIDs increase survival in the Sandhoff disease mouse: synergy with N-butyldeoxynojirimycin. Ann. Neurol. 56, 642–649 (2004).
Williams, I. M. et al. Improved neuroprotection using miglustat, curcumin and ibuprofen as a triple combination therapy in Niemann-Pick disease type C1 mice. Neurobiol. Dis. 67, 9–17 (2014).
Pandey, M. K. et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature 543, 108–112 (2017).
Yamaguchi, A. et al. Possible role of autoantibodies in the pathophysiology of GM2 gangliosidoses. J. Clin. Invest. 113, 200–208 (2004).
Halstead, S. K. et al. Microarray screening of Guillain-Barre syndrome sera for antibodies to glycolipid complexes. Neurol. Neuroimmunol. Neuroinflamm. 3, e284 (2016).
Galban-Horcajo, F., Halstead, S. K., McGonigal, R. & Willison, H. J. The application of glycosphingolipid arrays to autoantibody detection in neuroimmunological disorders. Curr. Opin. Chem. Biol. 18, 78–86 (2014).
Rinaldi, S., Brennan, K. M. & Willison, H. J. Combinatorial glycoarray. Methods Mol. Biol. 808, 413–423 (2012).
Pereira, C. S., Ribeiro, H. & Macedo, M. F. From lysosomal storage diseases to NKT cell activation and back. Int. J. Mol. Sci. 18, 502 (2017).
Pereira, C. S., Sa-Miranda, C., De Libero, G., Mori, L. & Macedo, M. F. Globotriaosylceramide inhibits iNKT-cell activation in a CD1d-dependent manner. Eur. J. Immunol. 46, 147–153 (2016).
Speak, A. O. et al. Invariant natural killer T cells are not affected by lysosomal storage in patients with Niemann-Pick disease type C. Eur. J. Immunol. 42, 1886–1892 (2012).
Gadola, S. D. et al. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J. Exp. Med. 203, 2293–2303 (2006).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43 (2012).
Tyack, Z. et al. Predictors of health-related quality of life in people with a complex chronic disease including multimorbidity: a longitudinal cohort study. Qual. Life Res. 25, 2579–2592 (2016).
Mistry, P. K. et al. Gaucher disease: progress and ongoing challenges. Mol. Genet. Metab. 120, 8–21 (2017).
Cox, T. M. Gaucher disease: clinical profile and therapeutic developments. Biologics 4, 299–313 (2010).
Cox, T. M., Rosenbloom, B. E. & Barker, R. A. Gaucher disease and comorbidities: B-cell malignancy and parkinsonism. Am. J. Hematol. 90 (Suppl. 1), S25–S28 (2015).
Mechler, K., Mountford, W. K., Hoffmann, G. F. & Ries, M. Pressure for drug development in lysosomal storage disorders — a quantitative analysis thirty years beyond the US orphan drug act. Orphanet J. Rare Dis. 10, 46 (2015).
Grabrucker, A. M. et al. Nanoparticle transport across the blood brain barrier. Tissue Barriers 4, e1153568 (2016).
Luzuriaga, K. Early combination antiretroviral therapy limits HIV-1 persistence in children. Annu. Rev. Med. 67, 201–213 (2016).
Eng, C. M. et al. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N. Engl. J. Med. 345, 9–16 (2001).
Biegstraaten, M. et al. Management goals for type 1 Gaucher disease: an expert consensus document from the European working group on Gaucher disease. Blood Cells Mol. Dis. http://dx.doi.org/10.1016/j.bcmd.2016.10.008 (2016).
Macauley, S. L. Combination therapies for lysosomal storage diseases: a complex answer to a simple problem. Pediatr. Endocrinol. Rev. 13 (Suppl. 1), 639–648 (2016).
Haffner, M. E. History of orphan drug regulation — United States and Beyond. Clin. Pharmacol. Ther. 100, 342–343 (2016).
Haffner, M. E. Orphan drug product regulation — United States. Int. J. Clin. Pharmacol. Ther. 40, 84–88 (2002).
Haffner, M. E. & Maher, P. D. The impact of the Orphan Drug Act on drug discovery. Expert Opin. Drug Discov. 1, 521–524 (2006).
Guthrie, R. & Susi, A. A. Simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 32, 338–343 (1963).
Turgeon, C. T. et al. Measurement of psychosine in dried blood spots — a possible improvement to newborn screening programs for Krabbe disease. J. Inherit Metab. Dis. 38, 923–929, (2015).
Li, Y., Brockmann, K., Turecek, F., Scott, C. R. & Gelb, M. H. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: application to newborn screening for Krabbe disease. Clin. Chem. 50, 638–640 (2004).
Chamoles, N. A. et al. Retrospective diagnosis of GM1 gangliosidosis by use of a newborn-screening card. Clin. Chem. 47, 2068 (2001).
Chamoles, N. A., Blanco, M. B., Gaggioli, D. & Casentini, C. Hurler-like phenotype: enzymatic diagnosis in dried blood spots on filter paper. Clin. Chem. 47, 2098–2102 (2001).
Chamoles, N. A., Blanco, M. & Gaggioli, D. Diagnosis of α-L-iduronidase deficiency in dried blood spots on filter paper: the possibility of newborn diagnosis. Clin. Chem. 47, 780–781 (2001).
Chamoles, N. A., Blanco, M. & Gaggioli, D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin. Chim. Acta 308, 195–196 (2001).
Orsini, J. J. et al. Newborn screening for Krabbe disease in New York State: the first eight years' experience. Genet. Med. 18, 239–248 (2016).
Mazzacuva, F. et al. Identification of novel bile acids as biomarkers for the early diagnosis of Niemann-Pick C disease. FEBS Lett. 590, 1651–1662 (2016).
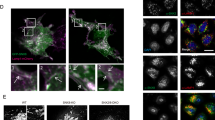
te Vruchte, D. et al. Relative acidic compartment volume as a lysosomal storage disorder-associated biomarker. J. Clin. Invest. 124, 1320–1328 (2014).
Cluzeau, C. V. et al. Microarray expression analysis and identification of serum biomarkers for Niemann-Pick disease, type C1. Hum. Mol. Genet. 21, 3632–3646 (2012).
Aerts, J. M. et al. Identification and use of biomarkers in Gaucher disease and other lysosomal storage diseases. Acta Paediatr. Suppl. 94, 43–48 (2005).
Fan, M. et al. Identification of Niemann-Pick C1 disease biomarkers through sphingolipid profiling. J. Lipid Res. 54, 2800–2814 (2013).
Porter, F. D. et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl Med. 2, 56ra81 (2011).
Greer, W. L. et al. The Nova Scotia (type D) form of Niemann-Pick disease is caused by a G3097-->T transversion in NPC1. Am. J. Hum. Genet. 63, 52–54 (1998).
Greer, W. L. et al. Linkage disequilibrium mapping of the Nova Scotia variant of Niemann-Pick disease. Clin. Genet. 55, 248–255 (1999).
Iselius, L., Hillborg, P. O. & Lindsten, J. The distribution of the gene for the juvenile type of Gaucher disease in Sweden. Acta Paediatr. Scand. 78, 592–596 (1989).
Lew, R. M. et al. Ashkenazi Jewish population screening for Tay-Sachs disease: the international and Australian experience. J. Paediatr. Child Health 51, 271–279 (2015). This is a study describing the benefits of carrier screening for the prevention of Tay–Sachs disease in the Ashkenazi Jewish community.
Gravel, R. A. et al. in The metabolic and molecular bases of inherited disease Vol. 3 (eds Scriver, C. R., Beadet, A. L., Valle, D. & Sly, W. S.) 3827–3876 (McGraw Hill, 2001).
Brady, R. O. Tay-Sachs disease: the search for the enzymatic defect. Adv. Genet. 44, 51–60 (2001).
Lightbody, J., Wiesmann, U., Hadorn, B. & Herschkowitz, N. I-Cell disease: multiple lysosomal-enzyme defect. Lancet 297, 451 (1971).
Kudo, M., Brem, M. S. & Canfield, W. M. Mucolipidosis II (I-cell disease) and mucolipidosis IIIA (classical pseudo-hurler polydystrophy) are caused by mutations in the GlcNAcphosphotransferase α/β -subunits precursor gene. Am. J. Hum. Genet. 78, 451–463 (2006).
Hopwood, J. J. & Ballabio, A. in The metabolic and molecular bases of inherited disease Vol. 3 (eds Scriver, C. R., Beadet, A. L., Valle, D. & Sly, W. S.) 3725–3732 (McGraw Hill, 2001).
Annunziata, I. & d'Azzo, A. Galactosialidosis: historic aspects and overview of investigated and emerging treatment options. Expert Opin. Orphan Drugs 5, 131–141 (2017).
Vanier, M. T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 5, 16 (2010).
Majer, F. et al. Danon disease: a focus on processing of the novel LAMP2 mutation and comments on the beneficial use of peripheral white blood cells in the diagnosis of LAMP2 deficiency. Gene 498, 183–195 (2012).
Bach, G. Mucolipidosis type IV. Mol. Genet. Metab. 73, 197–203 (2001).
Wakabayashi, K., Gustafson, A. M., Sidransky, E. & Goldin, E. Mucolipidosis type IV: an update. Mol. Genet. Metab. 104, 206–213 (2011).
Naureckiene, S. et al. Identification of HE1 as the second gene of Niemann-Pick C disease. Science 290, 2298–2301 (2000).
Isosomppi, J., Vesa, J., Jalanko, A. & Peltonen, L. Lysosomal localization of the neuronal ceroid lipofuscinosis CLN5 protein. Hum. Mol. Genet. 11, 885–891 (2002).
Savukoski, M. et al. CLN5, a novel gene encoding a putative transmembrane protein mutated in Finnish variant late infantile neuronal ceroid lipofuscinosis. Nat. Genet. 19, 286–288 (1998).
Sharifi, A. et al. Expression and lysosomal targeting of CLN7, a major facilitator superfamily transporter associated with variant late-infantile neuronal ceroid lipofuscinosis. Hum. Mol. Genet. 19, 4497–4514 (2010).
Wheeler, R. B. et al. A new locus for variant late infantile neuronal ceroid lipofuscinosis-CLN7. Mol. Genet. Metab. 66, 337–338 (1999).
Mole, S. E. & Cotman, S. L. Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochim. Biophys. Acta 1852, 2237–2241 (2015).
Cachon-Gonzalez, M. B. et al. Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proc. Natl Acad. Sci. USA 103, 10373–10378 (2006).
Chiricozzi, E. et al. Chaperone therapy for GM2 gangliosidosis: effects of pyrimethamine on β-hexosaminidase activity in Sandhoff fibroblasts. Mol. Neurobiol. 50, 159–167 (2014).
Cox, T. et al. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet 355, 1481–1485 (2000).
Wraith, J. E. et al. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol. Genet. Metab. 99, 351–357 (2010).
Marshall, J. et al. CNS-accessible inhibitor of glucosylceramide synthase for substrate reduction therapy of neuronopathic Gaucher disease. Mol. Ther. 24, 1019–1029 (2016).
Ashe, K. M. et al. Efficacy of enzyme and substrate reduction therapy with a novel antagonist of glucosylceramide synthase for Fabry disease. Mol. Med. 21, 389–399 (2015).
Lloyd-Evans, E. et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14, 1247–1255 (2008).
Acknowledgements
The author would like to thank B. Owen (Niemann–Pick UK) for helpful discussions on prevention, M. Hughes and A. Rahim for the current status of gene therapy trials, B. Winchester for comparing notes on the current status of enzyme replacement therapy trials and N. Platt and D. Priestman for comments on the manuscript. F.P. is a Wellcome Investigator in Science and a Royal Society Wolfson Merit Award Holder.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
F.P. is a consultant to Actelion, E3Bio and Orphazyme and a co-founder and consultant to IntraBio.
Glossary
- Lysosomal storage disorders
-
(LSDs). Disorders in which macromolecules build up in lysosomes, leading to so-called 'storage' as a result of an inherited mutation in a gene involved in lysosomal function.
- Multimorbidity
-
Individuals with multiple, typically chronic, clinical conditions.
- Biologic therapies
-
Treatments derived from living organisms to treat a disease. In the field of lysosomal storage disorders, enzyme replacement therapy, cell transplantation and gene therapy are all biologic therapies.
- Lysosome
-
An acidic organelle involved in macromolecule catabolism and recycling but also plays a role in nutrient sensing and calcium signalling.
- Proteostasis
-
The integrated process of protein synthesis, folding, trafficking and catabolism. Modulating this process is a therapeutic strategy for treating protein-misfolding diseases, including lysosomal storage disorders.
- Proteostasis modifiers
-
Drugs that increase the activity of the protein-folding machinery of the cell to aid folding of misfolded mutant proteins.
- Chemical chaperones
-
Drugs that either bind the active site of a mutant enzyme and stabilize it or are allosteric binders, binding away from the active site but still stabilizing the protein.
- Substrate reduction therapy
-
(SRT). A small-molecule drug that inhibits the biosynthesis of substrates that are stored in a lysosomal storage disorder.
- Polypharmacology
-
Treating a disease using a combination of therapies to maximize clinical benefit.
Rights and permissions
About this article
Cite this article
Platt, F. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat Rev Drug Discov 17, 133–150 (2018). https://doi.org/10.1038/nrd.2017.214
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2017.214
This article is cited by
-
Disease pathology signatures in a mouse model of Mucopolysaccharidosis type IIIB
Scientific Reports (2023)
-
The role of lysosomes in metabolic and autoimmune diseases
Nature Reviews Nephrology (2023)
-
A glycomic workflow for LC–MS/MS analysis of urine glycosaminoglycan biomarkers in mucopolysaccharidoses
Glycoconjugate Journal (2023)
-
Evolving therapies in neuronopathic LSDs: opportunities and challenges
Metabolic Brain Disease (2022)
-
Left Ventricular Hypertrophy: Etiology-Based Therapeutic Options
Cardiology and Therapy (2022)