Key Points
-
Nicotinamide phosphoribosyltransferase (NAMPT) is a regulator of the intracellular nicotinamide adenine dinucleotide (NAD) pool and, thus, regulates the activity of NAD-dependent enzymes
-
NAMPT is able to modulate processes involved in the pathogenesis of obesity and related disorders by influencing the oxidative stress response, apoptosis, lipid and glucose metabolism, inflammation and insulin resistance
-
Levels of extracellular NAMPT are associated with various metabolic disorders
-
NAMPT, which has a crucial role in cancer cell metabolism, is often overexpressed in tumour tissues and is an experimental target for antitumour therapies
Abstract
Nicotinamide phosphoribosyltransferase (NAMPT) is a regulator of the intracellular nicotinamide adenine dinucleotide (NAD) pool. NAD is an essential coenzyme involved in cellular redox reactions and is a substrate for NAD-dependent enzymes. In various metabolic disorders and during ageing, levels of NAD are decreased. Through its NAD-biosynthetic activity, NAMPT influences the activity of NAD-dependent enzymes, thereby regulating cellular metabolism. In addition to its enzymatic function, extracellular NAMPT (eNAMPT) has cytokine-like activity. Abnormal levels of eNAMPT are associated with various metabolic disorders. NAMPT is able to modulate processes involved in the pathogenesis of obesity and related disorders such as nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) by influencing the oxidative stress response, apoptosis, lipid and glucose metabolism, inflammation and insulin resistance. NAMPT also has a crucial role in cancer cell metabolism, is often overexpressed in tumour tissues and is an experimental target for antitumour therapies. In this Review, we discuss current understanding of the functions of NAMPT and highlight progress made in identifying the physiological role of NAMPT and its relevance in various human diseases and conditions, such as obesity, NAFLD, T2DM, cancer and ageing.
Similar content being viewed by others
Introduction
Nicotinamide phosphoribosyltransferase (NAMPT) was originally thought to be a cytokine that was a co-factor for B-cell maturation and was named pre-B-cell colony-enhancing factor (PBEF).1 Bacterial and murine homologues of NAMPT were later identified as enzymes involved in nicotinamide adenine dinucleotide (NAD) biosynthesis.2,3 However, NAMPT activity had been reported as early as 1957.4 NAMPT is highly expressed in visceral adipose tissue compared with subcutaneous adipose tissue,5 and plasma levels of NAMPT correlate with outcomes in patients with obesity.6 NAMPT was, thus, recognized as an adipokine and renamed visfatin.5 Although all three names (NAMPT, PBEF and visfatin) have been used in the literature, NAMPT is the official name of the protein and the gene (NAMPT), as approved by both the HUGO Gene Nomenclature Committee and the Mouse Genomic Nomenclature Committee.7 The term NAMPT is, therefore, used throughout this Review, whereas eNAMPT is used to designate extracellular NAMPT. In this Review, we discuss current understanding of the functions of NAMPT and place an emphasis on progress made in identifying the physiological role of NAMPT and its relevance in various human diseases and conditions, such as obesity, nonalcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM), cancer and ageing.
Physiological role of NAMPT
NAD metabolism and NAMPT enzyme activity
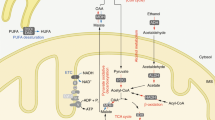
NAD is an essential coenzyme involved in cellular redox reactions and a substrate for NAD-dependent enzymes. In mammals, NAD can be synthesized de novo from tryptophan or NAD precursors such as nicotinamide (NAM), nicotinic acid, nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR, Figure 1). NAM, rather than nicotinic acid, is thought to be the predominant NAD precursor in mammals.8,9,10 NAM is also a product of deacetylation and ADP-ribosylation reactions, which are catalysed by NAD-dependent enzymes.11,12,13 NAMPT activity generates NMN from NAM and 5′-phosphoribosyl-1-pyrophosphate (PRPP), thereby catalysing the rate-limiting step in the mammalian NAD salvage pathway from NAM.3,14 NMN, together with ATP, is then converted into NAD by nicotinamide/nicotinic acid mononucleotide adenylyltransferases 1-3 (NMNAT1-3). NR is converted into NMN by nicotinamide riboside kinases (NRKs),15 which enters the NAD salvage pathway. Evidence of extracellular conversion of NAD and NMN to NR by the ectocellular enzymes CD38 and CD73 also exists (Figure 2).16,17,18
The NAD de novo kynurenine pathway is comprised of several steps. The first rate-limiting step is catalysed by TDOs (mainly in the liver) or IDOs. By multiple reactions, L-tryptophan is converted to NAMN. At this stage, NA enters the pathway and is phosphoribosylated by NAPRT. NAMN is then converted to NAAD by NMNAT1-3. Finally, NAAD is amidated by NADS to yield NAD. NAM is another NAD precursor and a product of deacetylation and ADP-ribosylation reactions, which are catalysed by NAD-dependent enzymes such as SIRTs, PARPs, MARTs and ADP-ribosyl cyclases such as CD38. NAMPT catalyses the formation of NMN from NAM and 5′-phosphoribosyl-1-pyrophosphate (PRPP), which is the rate-limiting step in the NAD salvage pathway from NAM. NMN is then converted into NAD by NMNAT1-3. NR enters the NAD biosynthesis pathway by phosphorylation to NMN, which is catalysed by NRKs. Abbreviations: IDOs, indoleamine 2,3-dioxygenases; MARTs, mono(ADP-ribosyl) transferases; NA, nicotinic acid; NAAD, nicotinic acid adenine dinucleotide; NAD, nicotinamide adenine dinucleotide; NADS, nicotinamide adenine dinucleotide synthase; NAM, nicotinamide; NAMN, nicotinic acid mononucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NAPRT, nicotinate phosphoribosyltransferase; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide/nicotinic acid mononucleotide adenylyltransferase; NR, nicotinamide riboside; NRKs, nicotinamide riboside kinases; PARPs, poly(ADP-ribose) polymerases; SIRTs, sirtuins; TDOs, tryptophan 2,3-dioxygenases.
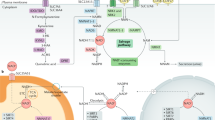
iNAMPT is found in the cytoplasm, nucleus and mitochondria. NAMPT exerts its effects by maintaining intracellular levels of NAD and recycling NAM, which is produced by the action of NAD-dependent enzymes such as SIRT1, SIRT6 and SIRT7, and PARP-1 in the nucleus, SIRT1 and SIRT2 in the cytoplasm and SIRT3, SIRT4 and SIRT5 in mitochondria. The ectoenzyme CD38 produces cADPR and regulates intracellular Ca2+ signalling. NAMPT expression is induced by the circadian regulators CLOCK and BMAL1 in complex with SIRT1. Other stimulators of NAMPT expression include mechanical stress and pro-inflammatory cytokines (such as TNF-α, IL-1β and IFN-γ). NAMPT is secreted from different cell types and is probably released from dying cells together with ATP and PRPP. Extracellular production of NMN, therefore, potentially, occurs and NMN might subsequently enter the cell, possibly after conversion to NR by the ectocellular enzymes CD38 and CD73. Apart from enzymatic activity, eNAMPT acts as a pro-inflammatory or anti-inflammatory cytokine in multiple signalling pathways, such as ERK1/2, IL-6–STAT3, PI3K–AKT, p38 MAPK and NF-κB, which influence the expression of several cytokines (such as TNF-α, IL-1β and TGF-β). A receptor for eNAMPT has not yet been identified. Abbreviations: AKT, protein kinase B; BMAL1, aryl hydrocarbon receptor translocator-like protein 1; cADPR, cyclic ADP-ribose; CLOCK, circadian locomoter output cycles protein kaput; eNAMPT, extracellular nicotinamide phosphoribosyltransferase; ERK, extracellular signal-regulated kinase; iNAMPT, intracellular nicotinamide phosphoribosyltransferase; NAD, nicotinamide adenine dinucleotide; NAM, nicotinamide; NF-κB, nuclear factor κB; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide/nicotinic acid mononucleotide adenylyltransferase; NR, nicotinamide riboside; NRKs, nicotinamide riboside kinases; p38 MAPK, mitogen-activated protein kinase p38; PARP, poly(ADP-ribose) polymerase; PI3K, phosphatidylinositol 3-kinase; PRPP, 5′-phosphoribosyl-1-pyrophosphate; SIRT, sirtuin; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor β; TNF-α, tumour necrosis factor α.
By analysing tissue sources of 719 cDNA clones, NAMPT was found to be expressed in nearly all organs, tissues and cells examined.1,19 This ubiquitous expression of NAMPT suggests pleiotropic functions of the protein in human physiology. NAMPT occurs intracellularly (mainly in the cytoplasm and nucleus)20 and extracellularly;21 NAMPT has also been reported to reside in mitochondria,22 although this finding has been disputed.23 Substantial sequence homology exists in NAMPT among prokaryotic organisms, primitive metazoans such as marine sponges and humans,2 which suggests a crucial role for NAMPT in cellular metabolism and survival. Several research groups have characterized the structural and enzymological features of mammalian NAMPT.24,25,26,27,28,29,30,31 Structural and mutagenesis studies have shown that mutations in NAMPT that impair dimerization attenuate enzymatic activity;26 furthermore, Asp219 is important in defining the substrate specificity of NAMPT,29 which does not include nicotinic acid.14,32 Autophosphorylation of NAMPT (by use of ATP) at His24724,26 creates a reaction intermediate that is hydrolysed during each catalytic cycle, which increases the affinity of NAMPT for NAM and its enzymatic activity up to 1,000-fold.24
NAMPT is one of the regulators of the intracellular NAD pool.3,14 Through its NAD-biosynthetic activity, NAMPT influences the activity of NAD-dependent enzymes, such as sirtuins14,22,33,34,35,36 and poly(ADP-ribose) polymerases,37 and thereby regulates cellular metabolism, mitochondrial biogenesis38,39,40 and adaptive responses to inflammatory, oxidative, proteotoxic and genotoxic stress.41 Further information on the roles of NAD-dependent enzymes in cellular metabolism can be found elsewhere.42,43,44,45,46 Mono-ADP-ribosyl transferases47 and ADP-ribosyl cyclases48 are involved in several physiological processes, which include calcium signalling and DNA repair. NAMPT activity probably influences the functions of these enzymes as they also use NAD as a substrate and are inhibited by the end-product NAM. Interestingly, the gene encoding NAMPT (NAMPT) is regulated by the circadian locomoter output cycles protein kaput (CLOCK)–aryl hydrocarbon receptor translocator-like protein 1 (BMAL1, also known as ARNTL) core clock machinery, which is responsible for circadian rhythmicity and leads to a circadian oscillation of NAD levels in vivo.49,50 Sirtuin (SIRT) 1 is recruited to the NAMPT promoter and contributes to the circadian synthesis of NAMPT, which completes a transcriptional–enzymatic feedback loop that connects cellular metabolism with circadian rhythms.49,50
Extracellular NAMPT
eNAMPT has been found in human circulation51 and mouse circulation21 and in human cerebrospinal fluid52 and seminal plasma,53 as well as in the supernatant of numerous cell types including differentiated adipocytes,21,54,55 hepatocytes,56,57 leucocytes,19 cardiomyocytes,58 neurons,59,60 amniotic epithelial cells,61 pancreatic β cells62 and lipopolysaccharide-activated monocytes,63,64 as well as spermatozoa (S. Thomas, personal communication). Several studies have indicated that eNAMPT might function as a growth factor;1,65,66,67,68,69 however, the mechanism of NAMPT secretion and its physiological function in the extracellular space is still uncertain. Another area of ambiguity is whether NAMPT occurs in the same form and configuration inside the cell as it does in the extracellular space. Some investigations of the action of eNAMPT have used recombinant NAMPT expressed from bacterial sources, which might not accurately represent the endogenous form of mammalian eNAMPT. A 2015 study reported that SIRT1 deacetylates intracellular NAMPT (iNAMPT), thereby predisposing NAMPT to secretion from adipocytes.55 Several studies have measured enzymatic activity of eNAMPT19,21,56,62,70 or detected the enzyme product (NMN) in the extracellular space;17,21,71 however, it has been reported that eNAMPT is not enzymatically active in mouse plasma owing to the low concentrations of PRPP and ATP.72 This observation raises the question as to whether enzymatic activity of eNAMPT is linked to pathophysiological conditions in which plasma PRPP and ATP levels increase due to cell death. Interestingly, the presence of a significant nicotinate phosphoribosyltransferase activity has been reported in human plasma, which suggests that nicotinic acid mononucleotide (NAMN) exists in the human circulation.70 As a receptor for eNAMPT has not been discovered yet, the mechanism of eNAMPT signal transduction continues to be the subject of research (Figure 2), owing to its importance in targeting eNAMPT in various pathological conditions. Improved understanding of the mechanism of action of eNAMPT is a prerequisite for exploitation of eNAMPT-related pathways as a therapeutic approach to treat relevant diseases.
Pathophysiological role of NAMPT
NAMPT and obesity
White adipose tissue (WAT) operates as a functional endocrine unit.73 Ever since eNAMPT was first described as an adipokine (also known as adipocytokine) with insulin-mimetic effects,5 the role of NAMPT in obesity and obesity-related disorders has been the subject of debate. Different adipocyte models including preadipocyte cell lines 3T3-L1 and SGBS as well as primary human adipocytes have been shown to secrete NAMPT into the supernatant via a non-classic pathway,21,54,74 thereby identifying adipose tissue as one of the major sources of eNAMPT. A number of SNPs in NAMPT are associated with obesity75 and obesity-related comorbidities such as coronary artery disease76 as well as glucose and lipid parameters.77 However, several studies have reported that genetic variation in NAMPT does not have a major role in the development of obesity or T2DM.78,79,80 The relevance of genetic variations in NAMPT in disease development might depend on whether the active site, assembly of the active NAMPT dimer or expression of NAMPT is affected.
A meta-analysis, which included human studies investigating the association between eNAMPT and obesity parameters, reported that levels of eNAMPT were generally increased in individuals with obesity;6 several human studies investigating the association between NAMPT and parameters of obesity have been published during the past 5 years (Table 1). Discrepancies between these studies, which have reported either a positive association or no association between circulating levels of eNAMPT and obesity-related parameters, might be due to differences in study populations, sample handling or systems to detect NAMPT.51
Several metabolic factors present at increased levels in individuals with obesity have been shown to modulate NAMPT expression and production and/or release of NAMPT (Table 2). Both glucose and oxidized LDL stimulate NAMPT expression and protein expression and release of NAMPT in human adipocytes via the PI3-kinase–AKT pathway.81,82,83 In addition, glucose administration in humans results in increased levels of eNAMPT.19 In vitro studies have shown that expression of NAMPT mRNA increases during adipogenesis84 and is stimulated by insulin-resistance-inducing factors such as IL-6, dexamethasone, growth hormone, tumour necrosis factor α (TNF-α) and isoproterenol.85,86,87 NAMPT is also upregulated in adipocytes under hypoxic conditions.88 The macrophage population in visceral WAT in individuals with obesity is another source of eNAMPT.89 Whether eNAMPT exhibits pro-inflammatory or anti-inflammatory activity is still the subject of debate.21,58,59,60,90,91 However, several studies have reported pro-inflammatory effects of eNAMPT on different cell types, which include induction of inducible nitric oxide synthase,92 activation of extracellular signal-regulated protein kinase 1/2 (ERK1/2),66 nuclear factor NF-κB activation92,93 and production of cytokines such as TNF-α, IL-6, IL-1β,93,94 transforming growth factor β95 and monocyte chemoattractant protein 1.96 Production of inflammatory cytokines and NAMPT expression in adipocytes, thus, seem to be regulated by a positive feedback activation loop. Furthermore, incubation with eNAMPT increases expression of lipoprotein lipase and peroxisome proliferator-activated receptor γ in preadipocytes and fatty acid synthase in differentiated adipocytes, which suggests that eNAMPT is a regulator of lipid metabolism.97
Evidence for an association between altered physical activity patterns (for example, those induced by shift work) and metabolic derangements in experimental rodent models98,99 and in humans100 exists. Hepatic NAMPT mRNA expression and NAD levels were downregulated in a rodent shift-work model and their synchrony with CLOCK and BMAL1 expression was lost.98 One human study showed that the diurnal rhythm of eNAMPT was disturbed by sleep loss and positively associated with impairment of postprandial glucose metabolism.101 A role for NAMPT in the regulation of food intake and behaviour was demonstrated in two different animal models.102,103 Intracerebroventricular injection of eNAMPT caused increased feed intake and pecking efficiency in chicks,102 whereas injection of eNAMPT into the arcuate nucleus of the hypothalamus of rats increased food intake and reduced hypothalamic expression of anorexigenic peptides.103 A 2015 study indicated that eNAMPT secreted by adipose tissue influences hypothalamic function.55 In mice with an adipose-tissue-specific knockout of NAMPT (designated ANKO), levels of eNAMPT were notably reduced. Remarkably, levels of NAD were markedly reduced specifically in the hypothalamus of female ANKO mice compared with wild-type littermates. These female ANKO mice also displayed reduced physical activity, which was normalized by injection of NMN.55 Another NAD intermediate, NR, was also found to be beneficial by ameliorating NAD levels.104 In mice, gain of body weight induced by a high-fat diet (HFD) was attenuated by NR, which was due to enhanced energy expenditure.104 In contrast to NMN, NR has been, or is currently, under investigation in three clinical trials that measured serum levels of NR and metabolites of NR,105 analysed NR and its metabolites in urine and blood,106 and investigated insulin sensitivity and substrate metabolism in men with obesity after 3 months of treatment with NR.107
Taken together, the findings of the clinical trials indicate that NAMPT gene and protein expression and eNAMPT levels are altered in obesity and obesity-related disorders. Functional studies suggest that both systemic NAMPT and adipocyte-specific NAMPT can regulate processes that contribute to the development of obesity.
NAMPT and nonalcoholic fatty liver disease
NAFLD—the most common liver disorder in the West—encompasses a wide range of pathophysiological conditions, ranging from simple steatosis (hepatic lipid accumulation) to inflammation (nonalcoholic steatohepatitis), which frequently leads to fibrosis and cirrhosis accompanied by an increased risk of hepatocellular carcinoma and eventually the need for liver transplantation.108 Hepatocytes have been identified as a source of eNAMPT.56 Furthermore, plasma levels of eNAMPT correlate with portal inflammation in individuals with NAFLD.109 Several, partly conflicting, studies investigating the association between NAMPT and the severity of NAFLD in humans have been published during the past few years (Table 1), with upregulation and downregulation of NAMPT observed in both animal models and patients with NAFLD.110,111,112,113,114,115 In mouse models of diet-induced obesity, upregulation of NAMPT by different strategies has proven beneficial in protecting against steatosis, inflammation and glucose intolerance.116,117,118 Supplementation with troxerutin (a trihydroxyethylated derivative of the natural bioflavonoid rutin)116 or leucine117 enhanced levels of NAMPT and NAD and, consequently, SIRT1 activity. In another mouse model of hepatic triglyceride accumulation induced by combined liver specific knockout of Foxo1, Foxo3 and Foxo4, levels of NAMPT were downregulated, which indicates that NAMPT expression is regulated by Foxo transcription factors in the mouse liver.118 In the same study, overexpression of NAMPT markedly reduced hepatic triglycerides in vivo.118
Another regulator of NAMPT is miRNA-34a, which is increased in individuals with obesity and substantially reduces NAD levels and SIRT1 activity in the liver by directly targeting NAMPT mRNA expression.119 In diet-induced obese mice, inhibition of miR-34 restored levels of NAMPT and NAD and improved steatosis, inflammation and glucose intolerance.120 In patients with NAFLD, hepatic expression of NAMPT was downregulated, possibly via activation of peroxisome proliferator-activated receptor α.113 Conversely, overexpression of NAMPT improved apoptosis in rat hepatocytes exposed to stress, which suggests an anti-apoptotic effect of NAMPT in NAFLD.113 In contrast to the beneficial effects of NAMPT overexpression in the liver, NAMPT overexpression in mouse skeletal muscle did not improve mitochondrial biogenesis or function.121 Furthermore, no change in hepatic NAD levels and NAMPT expression was found in mice fed a HFD for 4 weeks, even though SIRT1 activity was attenuated.122 These findings implicate factors other than levels of NAD in the regulation of sirtuin activity.
A pro-inflammatory action of NAMPT was reported in HepG2 cells.123 When treated with palmitate, a time-dependent and dose-dependent increase in NAMPT gene and protein expression as well as in levels of IL-6 and TNF-α was observed, whereas downregulation of NAMPT counteracted the inflammatory response.123 By inhibition of NF-κB, iNAMPT protein levels were normalized after stimulation with palmitate, which indicates that NAMPT might have a role in palmitate-induced inflammation in hepatocytes through the NF-κB pathway.123 Another in vitro study using HepG2 cells showed that incubation with eNAMPT activated gluconeogenesis via activation of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, independent of SIRT1 activation.65 Taken together, the findings demonstrate that NAMPT modulates processes involved in the pathogenesis of NAFLD by regulating oxidative stress (or mitochondrial biogenesis), apoptosis, lipid and glucose metabolism, inflammation and insulin resistance.
NAMPT and type 2 diabetes mellitus
Increased abdominal adiposity is associated with low-grade inflammation, abnormal hormone secretion and various metabolic disturbances that contribute to insulin resistance.124,125 Of late, interest has grown about the role of different adipokines in the pathogenesis of metabolic complications related to obesity.126,127 In particular, in the large spectrum of adipokines, eNAMPT is one of the most promising and interesting molecules that seems to be directly implicated in the regulation of glucose-stimulated insulin secretion (GSIS) in pancreatic β cells.21 Accumulating evidence shows a possible association between levels of eNAMPT and T2DM in individuals with and without obesity.128,129,130 Several studies have reported that patients with T2DM present with considerably higher levels of eNAMPT than healthy controls, independent of BMI.131,132,133 In addition, all these studies confirmed, by use of multiple logistic regression analysis, that eNAMPT can be considered as an independent risk factor for T2DM, even after adjustment for other risk factors.129,131,132
Subsequent studies showed that eNAMPT is not only associated with T2DM but also with complications of diabetes mellitus,126 such as endothelial dysfunction,134 diabetic nephropathy and impairment of lipid metabolism.130 A possible association between different NAMPT polymorphisms and T2DM or T2DM-related complications has been reported.76,135 Many human studies have reported an association between eNAMPT and T2DM (Table 3). Although some animal and in vitro studies have attributed the effects of NAMPT on glucose metabolism to the, still controversial, insulin-mimetic actions of NAMPT,5,136 other studies attribute the effects to NAMPT's role as a NAD biosynthetic enzyme. In particular, a mouse model with β-cell-specific overexpression of Sirt1 (BESTO mice) had improved glucose tolerance that worsened with age.137 Circulating levels of the NAMPT product NMN and, consequently, NAD levels in pancreatic β cells and islets were considerably lower in old BESTO mice than in young BESTO mice.137 Furthermore, administration of NMN restored the positive effect of Sirt1 on glucose tolerance and GSIS in aged BESTO female mice.137 In heterozygous Nampt knockout mice (Nampt−/+), a defect in NAD biosynthesis and GSIS was found in pancreatic islets.21 Female Nampt−/+ mice had impaired glucose tolerance, which was ameliorated by administration of the NAMPT enzyme product NMN.21 Moreover, administration of FK866, a potent NAMPT inhibitor, notably reduced NAD biosynthesis and GSIS in wild-type mice.21 Overall, these findings strongly suggest that NAMPT is able to control the regulation of insulin secretion by increasing levels of NAD in pancreatic β cells.21
In confirmation of this hypothesis, NAMPT and NMN were shown to induce increased insulin secretion compared with glucose alone in human islets after 1 h incubation with high glucose concentrations.138 In HFD-induced obese mice, a direct effect of NAMPT not only on glucose metabolism but also on the pathogenesis of T2DM was found.139 NAD biosynthesis mediated by NAMPT was impaired in HFD-fed mice with T2DM compared with that in regular chow-fed controls, whereas administration of NMN ameliorated glucose intolerance by restoring levels of NAD.139 In addition, NMN augmented hepatic insulin sensitivity and other biological pathways associated with oxidative stress, inflammation and lipid metabolism.139 Mice fed a fructose-rich diet had increased levels of NAMPT in brown adipose tissue and WAT, yet notably decreased levels of eNAMPT.140 Administration of NMN abolished the suppressive effects of the fructose-rich diet on insulin secretion.140 NMN also demonstrated protective effects against pro-inflammatory cytokine-mediated islet dysfunction.140 Furthermore, insulin secretion in islets cultured with pro-inflammatory cytokines was restored by NMN; the anti-inflammatory effects of NMN were partially blocked by inhibition of SIRT1.140
Considering all the evidence from in vitro and in vivo studies, NAMPT seems to be involved in the pathogenesis of T2DM and in the development of complications of diabetes mellitus. More interestingly, in vitro studies indicate a possible role for the NAD intermediates, NMN and NR, in ameliorating β-cell function and cellular homeostasis, glucose metabolism and stress responses.
NAMPT and ageing
Sirtuins have been comprehensively investigated as mediators of longevity.141 NAMPT, which regulates sirtuin function, can delay cellular senescence by increasing resistance to oxidative stress in human vascular smooth muscle cells.142 By contrast, eNAMPT induces telomere damage and premature senescence in human endothelial cells by activating NADPH oxidase.143 Reduced levels of NAMPT and NAD have been detected in peripheral tissue of old mice, such as the pancreas, WAT and skeletal muscle.139 In this study, administration of the NAMPT enzyme product, NMN, was an effective intervention for treating the pathophysiology of age-induced T2DM.139 In line with these results, rats treated with the NAMPT enzyme inhibitor FK866 failed to exhibit increased SIRT1 and SIRT3 activity in response to caloric restriction—a widely-used strategy to ameliorate age-associated diseases in animal models. Consequently, inhibition of NAMPT abolished caloric-restriction-induced mitochondrial biogenesis and enhanced insulin sensitivity.144
Interestingly, NAMPT is involved in the molecular mechanisms that lead to declining neuronal stem and/or progenitor cell (NSPC) numbers during ageing.145 NSPCs are able to proliferate and differentiate into the major cells of the brain, such as neurons, oligodendrocytes and astrocytes. Ageing is one of the strongest negative regulators of adult NSPC proliferation.145 One study raised the possibility that long-term administration of NMN might counteract age-related declines in NSPC functionality.146 A study on young and old Wistar rats demonstrated that NAMPT levels were lower in the aged group than in the young group.35 Interestingly, the age-associated decreases in NAMPT and NAD levels were reversed with regular exercise, which increased the specific activity of SIRT1.35 A clinical study on a large population of elderly people investigated the relationships between levels of eNAMPT, nutritional status and insulin resistance. Levels of eNAMPT declined with age and were associated with nutritional status, visceral obesity and inflammation.147
Several theories have been postulated to explain the declining levels of NAMPT and NAD with ageing. As discussed earlier, NAMPT is a major product of the action of the circadian transcription factors BMAL1 and CLOCK.49 One hypothesis is that a decline in central and peripheral circadian function during ageing results in a deficit in the production of NAMPT and NAD. Furthermore, ageing is accompanied by a state of chronic, low-grade inflammation,148 which is a major contributor to the development of many age-related chronic disorders. In this context, TNF-α, one of the major inflammatory cytokines, and oxidative stress markedly reduced levels of NAMPT and NAD in primary hepatocytes.139 TNF-α also suppresses CLOCK–BMAL1-mediated functions in mice,149 which might also contribute to a reduction in NAMPT-mediated NAD biosynthesis during ageing. As mentioned earlier, chronic inflammation, oxidative stress and DNA damage are crucial factors associated with ageing. Activation of NAD-dependent PARPs is induced immediately after DNA damage to facilitate repair and maintenance of genomic integrity. Thus, acute DNA damage can induce a sudden depletion of NAD levels owing to PARP activation. During ageing, damaged DNA accumulates in the nucleus, which causes activation of PARP and might be another possible explanation for age-induced reduction in NAD levels.
NAMPT in cancer
Cancer cells have a high capacity for glucose uptake and an increased rate of glycolysis even in the presence of oxygen—the so-called Warburg effect.150 The metabolic alterations leading to this phenotype require increased amounts of the redox cofactor NAD, which functions in many critical cellular processes necessary for cancer cell growth, including transcriptional regulation, cell-cycle progression, anti-apoptosis, DNA repair, regulation of chromatin dynamics and telomerase activity.42,151 NAMPT is essential for the replenishment of the intracellular NAD pool, as NAD is rapidly consumed by NAD-dependent enzymes in cancer cells and converted to NAM.32,152,153,154 The development of many cancers including colorectal, ovarian, breast, gastric, prostate, well-differentiated thyroid, endometrial carcinomas, myeloma, melanoma and astrocytomas is, thus, associated with increased NAMPT expression.155 NAMPT is differentially expressed in hepatocarcinoma cell lines compared with non-cancerous primary human hepatocytes, and can be regulated by resveratrol.57 In a meta-analysis of genome-wide expression data, which identified genes influenced by NAMPT, reduced NAMPT expression was strongly associated with dysregulation of cancer signalling pathways.156
Cancer cells are more susceptible to NAMPT inhibition than normal cells.157,158 Clinical studies have demonstrated that serum or blood levels of eNAMPT are increased in patients with cancer and a positive correlation exists between either tissue or eNAMPT levels and the stage of cancer progression.159,160,161,162,163 However, the molecular pathways of eNAMPT signalling in carcinogenesis are far from clear. eNAMPT affects redox adaptive responses and promotes tumour proliferation in human malignant melanoma cells,164 and influences resting monocytes, polarizing them towards a tumour-supporting M2 macrophage phenotype.163 By this latter mechanism, eNAMPT induces an immunosuppressive and tumour-promoting microenvironment in chronic lymphocytic leukaemia.163 Furthermore, eNAMPT stimulates vascular endothelial growth factor by activating the mitogen-activated protein kinase–ERK pathway and promoting angiogenesis, which is crucial during tumour growth and expansion.165
Cell lines overexpressing NAMPT are considerably more resistant to chemotherapeutic agents than control cells.166 By contrast, stable knock-down of NAMPT renders cells more sensitive to such treatments than control cells.22 Targeting NAMPT activity, thus, represents a novel therapeutic strategy for treating human cancers. For example, the specific NAMPT inhibitor FK866 has been evaluated in a broad variety of tumours, including solid tumours and leukemias,153,167,168 both in vitro and in nude-mouse xenografts,31,167,169,170,171 in which FK866 was able to reduce or attenuate tumour growth. NAMPT inhibition also attenuates glycolysis in conjunction with reduced NAD levels, which leads to blockade of the pentose phosphate pathway, serine biosynthesis and the tricarboxylic acid cycle.171 FK866 can induce delayed energy stress in hepatocarcinoma cells, which triggers activation of AMPKα and downregulation of mTOR signalling (which are associated with increased cancer cell death); noncancerous human hepatocytes are less sensitive to FK866 than are cancerous cells.128 In contrast to the strategy of NAD depletion by inhibition of NAD-producing enzymes, restoration of the pool of NAD with NR prevented DNA damage and tumour formation in a mouse model of hepatocarcinoma.172
Clinical trials using NAMPT inhibitors as monotherapies for the treatment of solid tumours (for example, melanoma), lymphomas and leukaemias have so far not been promising.173,174,175,176,177,178,179,180 One possible explanation could be that CD38 or CD73 reverse cell death induced by NAMPT inhibition through the supply of ectocellular NAD precursors.18 However, combining FK866 or other NAMPT inhibitors with antineoplastic agents, chemotherapy or radiotherapy might enhance their therapeutic efficacy.181
Conclusion
Levels of NAD are decreased in various metabolic disorders and during ageing. By recycling NAM to NAD, NAMPT is involved in regulating cellular energy metabolism by providing substrates for NAD-dependent enzymes. The role of NAMPT in various metabolic disorders is not completely known. One reason for this lack of clarity might be the difficulty in differentiating between the intracellular NMN-producing action of NAMPT and the extracellular NMN-producing and/or cytokine-like action of eNAMPT. Several in vitro and in vivo studies have shown that NAD precursors are successful in augmenting NAD levels and ameliorating the negative effects of pathophysiological conditions and ageing.
In the circulation, abnormal levels of eNAMPT are associated with metabolic disorders and cancer progression. However, association studies on eNAMPT levels in NAFLD are still controversial. This controversy might, in part, be explained by the wide range of disease phenotypes in NAFLD and perhaps a dependency of liver disease progression on levels of eNAMPT. In addition, NAMPT has a crucial role in cancer cell metabolism and is often overexpressed in tumour tissues. NAMPT inhibition and NAD depletion have been applied in in vitro studies and in animal studies to reduce tumour growth. However, clinical trials on NAMPT inhibitors as monotherapies have so far failed to show notable antitumour action.
Several questions, therefore, remain unanswered. Does NAMPT expression and function depend on disease progression and severity in metabolic disorders? Is NAMPT a pathogenetic factor in the development of NAFLD? How is NAMPT secretion regulated in different cell types and what is the role of eNAMPT? Does the secreting cell type influence eNAMPT function? Under which conditions is eNAMPT enzymatically active in vivo? What are the molecular differences between eNAMPT and iNAMPT besides differential acetylation? Are there any adverse effects of long-term NAD precursor treatment? Why does NAMPT inhibition lead to tumour cell death in vitro and tumour remission in animal models, but not in clinical trials? Finally, is a combination therapy with chemotherapeutics useful for patients? Answers to these questions are eagerly awaited.
References
Samal, B. et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 14, 1431–1437 (1994).
Martin, P. R., Shea, R. J. & Mulks, M. H. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J. Bacteriol. 183, 1168–1174 (2001).
Rongvaux, A. et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 32, 3225–3234 (2002).
Preiss, J. & Handler, P. Enzymatic synthesis of nicotinamide mononucleotide. J. Biol. Chem. 225, 759–770 (1957).
Fukuhara, A. et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307, 426–430 (2005).
Chang, Y.-H., Chang, D.-M., Lin, K.-C., Shin, S.-J. & Lee, Y.-J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab. Res. Rev. 27, 515–527 (2011).
Garten, A., Petzold, S., Körner, A., Imai, S. & Kiess, W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol. Metab. 20, 130–138 (2009).
Mori, V. et al. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS ONE 9, e113939 (2014).
Yang, S. J. et al. Nicotinamide improves glucose metabolism and affects the hepatic NAD–sirtuin pathway in a rodent model of obesity and type 2 diabetes. J. Nutr. Biochem. 25, 66–72 (2014).
Collins, P. B. & Chaykin, S. The management of nicotinamide and nicotinic acid in the mouse. J. Biol. Chem. 247, 778–783 (1972).
Ushiro, H., Yokoyama, Y. & Shizuta, Y. Purification and characterization of poly(ADP-ribose) synthetase from human placenta. J. Biol. Chem. 262, 2352–2357 (1987).
Guan, X., Lin, P., Knoll, E. & Chakrabarti, R. Mechanism of inhibition of the human sirtuin enzyme SIRT3 by nicotinamide: computational and experimental studies. PLoS ONE 9, e107729 (2014).
Bitterman, K. J., Anderson, R. M., Cohen, H. Y., Latorre-Esteves, M. & Sinclair, D. A. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 277, 45099–45107 (2002).
Revollo, J. R., Grimm, A. A. & Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 (2004).
Bieganowski, P. & Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss–Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502 (2004).
Nikiforov, A., Dölle, C., Niere, M. & Ziegler, M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 286, 21767–21778 (2011).
Garavaglia, S. et al. The high-resolution crystal structure of periplasmic Haemophilus influenzae NAD nucleotidase reveals a novel enzymatic function of human CD73 related to NAD metabolism. Biochem. J. 441, 131–141 (2012).
Grozio, A. et al. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J. Biol. Chem. 288, 25938–25949 (2013).
Friebe, D. et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia 54, 1200–1211 (2011).
Kitani, T., Okuno, S. & Fujisawa, H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 544, 74–78 (2003).
Revollo, J. R. et al. Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 6, 363–375 (2007).
Yang, H. et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 (2007).
Pittelli, M. et al. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem. 285, 34106–34114 (2010).
Burgos, E. S. & Schramm, V. L. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry 47, 11086–11096 (2008).
Kim, M.-K. et al. Crystal structure of visfatin/pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866. J. Mol. Biol. 362, 66–77 (2006).
Wang, T. et al. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat. Struct. Mol. Biol. 13, 661–662 (2006).
Kang, G. B. et al. Crystal structure of Rattus norvegicus visfatin/PBEF/Nampt in complex with an FK866-based inhibitor. Mol. Cells 27, 667–671 (2009).
Burgos, E. S., Vetticatt, M. J. & Schramm, V. L. Recycling nicotinamide. The transition-state structure of human nicotinamide phosphoribosyltransferase. J. Am. Chem. Soc. 135, 3485–3493 (2013).
Khan, J. A., Tao, X. & Tong, L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat. Struct. Mol. Biol. 13, 582–588 (2006).
Takahashi, R., Nakamura, S., Yoshida, T., Kobayashi, Y. & Ohkubo, T. Crystallization of human nicotinamide phosphoribosyltransferase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63, 375–377 (2007).
Christensen, M. K. et al. Nicotinamide phosphoribosyltransferase inhibitors, design, preparation, and structure–activity relationship. J. Med. Chem. 56, 9071–9088 (2013).
Hasmann, M. & Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 63, 7436–7442 (2003).
Bowlby, S. C., Thomas, M. J., D'Agostino, R. B. & Kridel, S. J. Nicotinamide phosphoribosyl transferase (Nampt) is required for de novo lipogenesis in tumor cells. PLoS ONE 7, e40195 (2012).
Bruzzone, S. et al. Catastrophic NAD+ depletion in activated T lymphocytes through Nampt inhibition reduces demyelination and disability in EAE. PLoS ONE 4, e7897 (2009).
Koltai, E. et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech. Ageing Dev. 131, 21–28 (2010).
Van Gool, F. et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat. Med. 15, 206–210 (2009).
Pillai, J. B., Isbatan, A., Imai, S. & Gupta, M. P. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J. Biol. Chem. 280, 43121–43130 (2005).
Rodgers, J. T., Lerin, C., Gerhart-Hines, Z. & Puigserver, P. Metabolic adaptations through the PGC-1 α and SIRT1 pathways. FEBS Lett. 582, 46–53 (2008).
Van der Horst, A. et al. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1). J. Biol. Chem. 279, 28873–28879 (2004).
Bordone, L. et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol. 4, e31 (2006).
Luo, X. & Kraus, W. L. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 26, 417–432 (2012).
Mouchiroud, L., Houtkooper, R. H. & Auwerx, J. NAD+ metabolism: a therapeutic target for age-related metabolic disease. Crit. Rev. Biochem. Mol. Biol. 48, 397–408 (2013).
Dölle, C., Skoge, R. H., Vanlinden, M. R. & Ziegler, M. NAD biosynthesis in humans—enzymes, metabolites and therapeutic aspects. Curr. Top. Med. Chem. 13, 2907–2917 (2013).
Chang, H.-C. & Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 25, 138–145 (2014).
Imai, S. & Kiess, W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front. Biosci. (Landmark Ed.) 14, 2983–2995 (2009).
Bürkle, A. & Virág, L. Poly(ADP-ribose): PARadigms and PARadoxes. Mol. Aspects Med. 34, 1046–1065 (2013).
Scarpa, E. S., Fabrizio, G. & Di Girolamo, M. A role of intracellular mono-ADP-ribosylation in cancer biology. FEBS J. 280, 3551–3562 (2013).
Lee, H. C. & Aarhus, R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 2, 203–209 (1991).
Ramsey, K. M. et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 (2009).
Nakahata, Y., Sahar, S., Astarita, G., Kaluzova, M. & Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK–SIRT1. Science 324, 654–657 (2009).
Korner, A. et al. Molecular characteristics of serum visfatin and differential detection by immunoassays. J. Clin. Endocrinol. Metab. 92, 4783–4791 (2007).
Hallschmid, M., Randeva, H., Tan, B. K., Kern, W. & Lehnert, H. Relationship between cerebrospinal fluid visfatin (PBEF/Nampt) levels and adiposity in humans. Diabetes 58, 637–640 (2009).
Thomas, S. et al. Seminal plasma adipokine levels are correlated with functional characteristics of spermatozoa. Fertil. Steril. 99, 1256–1263 (2013).
Tanaka, M. et al. Visfatin is released from 3T3-L1 adipocytes via a non-classical pathway. Biochem. Biophys. Res. Commun. 359, 194–201 (2007).
Yoon, M. J. et al. SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell. Metab. 21, 706–717 (2015).
Garten, A. et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem. Biophys. Res. Commun. 391, 376–381 (2010).
Schuster, S. et al. Resveratrol differentially regulates NAMPT and SIRT1 in hepatocarcinoma cells and primary human hepatocytes. PLoS ONE 9, e91045 (2014).
Pillai, V. B. et al. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 304, H415–H426 (2013).
Zhao, B. et al. Cerebral ischemia is exacerbated by extracellular nicotinamide phosphoribosyltransferase via a non-enzymatic mechanism. PLoS ONE 8, e85403 (2013).
Jing, Z. et al. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J. Cereb. Blood Flow Metab. 34, 1613–1621 (2014).
Ognjanovic, S. & Bryant-Greenwood, G. D. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am. J. Obstet. Gynecol. 187, 1051–1058 (2002).
Kover, K. et al. Expression and regulation of nampt in human islets. PLoS ONE 8, e58767 (2013).
Schilling, E. & Hauschildt, S. Extracellular ATP induces P2X7-dependent nicotinamide phosphoribosyltransferase release in LPS-activated human monocytes. Innate Immun. 18, 738–744 (2012).
Van den Bergh, R. et al. Monocytes contribute to differential immune pressure on R5 versus X4 HIV through the adipocytokine visfatin/NAMPT. PLoS ONE 7, e35074 (2012).
Choi, Y. J. et al. Extracellular visfatin activates gluconeogenesis in HepG2 cells through the classical PKA/CREB-dependent pathway. Horm. Metab. Res. 46, 233–239 (2014).
Kim, S.-R. et al. Visfatin promotes angiogenesis by activation of extracellular signal-regulated kinase 1/2. Biochem. Biophys. Res. Commun. 357, 150–156 (2007).
Bae, Y.-H. et al. Upregulation of fibroblast growth factor-2 by visfatin that promotes endothelial angiogenesis. Biochem. Biophys. Res. Commun. 379, 206–211 (2009).
Kang, Y.-S. et al. Visfatin induces neurite outgrowth in PC12 cells via ERK1/2 signaling pathway. Neurosci. Lett. 504, 121–126 (2011).
Park, H.-J. et al. Visfatin promotes cell and tumor growth by upregulating Notch1 in breast cancer. Oncotarget 5, 5087–5099 (2014).
Zamporlini, F. et al. Novel assay for simultaneous measurement of pyridine mononucleotides synthesizing activities allows dissection of NAD+ biosynthetic machinery in mammalian cells. FEBS J. 281, 5104–5119 (2014).
Formentini, L., Moroni, F. & Chiarugi, A. Detection and pharmacological modulation of nicotinamide mononucleotide (NMN) in vitro and in vivo. Biochem. Pharmacol. 77, 1612–1620 (2009).
Hara, N., Yamada, K., Shibata, T., Osago, H. & Tsuchiya, M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS ONE 6, e22781 (2011).
Khan, M. & Joseph, F. Adipose tissue and adipokines: the association with and application of adipokines in obesity. Scientifica (Cairo) 2014, 328592 (2014).
Olarescu, N. C. et al. Adipocytes as a source of increased circulating levels of nicotinamide phosphoribosyltransferase/visfatin in active acromegaly. J. Clin. Endocrinol. Metab. 97, 1355–1362 (2012).
Blakemore, A. I. et al. A rare variant in the visfatin gene (NAMPT/PBEF1) is associated with protection from obesity. Obesity (Silver Spring) 17, 1549–1553 (2009).
Saddi-Rosa, P. et al. Association of circulating levels of nicotinamide phosphoribosyltransferase (NAMPT/Visfatin) and of a frequent polymorphism in the promoter of the NAMPT gene with coronary artery disease in diabetic and non-diabetic subjects. Cardiovasc. Diabetol. 12, 119 (2013).
Jian, W.-X. et al. The visfatin gene is associated with glucose and lipid metabolism in a Chinese population. Diabet. Med. 23, 967–973 (2006).
Paschou, P. et al. Genetic variation in the visfatin (PBEF1/NAMPT) gene and type 2 diabetes in the Greek population. Cytokine 51, 25–27 (2010).
Körner, A. et al. Effects of genetic variation in the visfatin gene (PBEF1) on obesity, glucose metabolism, and blood pressure in children. Metabolism. 56, 772–777 (2007).
Böttcher, Y. et al. Genetic variation in the visfatin gene (PBEF1) and its relation to glucose metabolism and fat-depot-specific messenger ribonucleic acid expression in humans. J. Clin. Endocrinol. Metab. 91, 2725–2731 (2006).
Haider, D. G. et al. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia 49, 1909–1914 (2006).
Haider, D. G. et al. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J. Clin. Endocrinol. Metab. 91, 1578–1581 (2006).
Chen, Y., Chen, M., Wu, Z. & Zhao, S. Ox-LDL induces ER stress and promotes the adipokines secretion in 3T3-L1 adipocytes. PLoS ONE 8, e81379 (2013).
Kralisch, S. et al. Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J. Endocrinol. 185, R1–R8 (2005).
Kralisch, S. et al. Interleukin-6 is a negative regulator of visfatin gene expression in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 289, E586–E590 (2005).
Friebe, D. et al. Impact of metabolic regulators on the expression of the obesity associated genes FTO and NAMPT in human preadipocytes and adipocytes. PLoS ONE 6, e19526 (2011).
Kim, H.-S. et al. Blockade of visfatin induction by oleanolic acid via disturbing IL-6-TRAF6-NF-κB signaling of adipocytes. Exp. Biol. Med. (Maywood) 239, 284–292 (2014).
Segawa, K. et al. Visfatin in adipocytes is upregulated by hypoxia through HIF1α-dependent mechanism. Biochem. Biophys. Res. Commun. 349, 875–882 (2006).
Curat, C. A. et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49, 744–747 (2006).
Romacho, T., Sánchez-Ferrer, C. F. & Peiró, C. Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm. 2013, 946427 (2013).
Li, Y. et al. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J. Biol. Chem. 283, 34833–34843 (2008).
Romacho, T. et al. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia 52, 2455–2463 (2009).
Moschen, A. R., Gerner, R. R. & Tilg, H. Pre-B cell colony enhancing factor/NAMPT/visfatin in inflammation and obesity-related disorders. Curr. Pharm. Des. 16, 1913–1920 (2010).
Hector, J. et al. TNF-α alters visfatin and adiponectin levels in human fat. Horm. Metab. Res. 39, 250–255 (2007).
Song, H. K. et al. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am. J. Physiol. Renal Physiol. 295, F1485–F1494 (2008).
Sommer, G. et al. Visfatin is a positive regulator of MCP-1 in human adipocytes in vitro and in mice in vivo. Obesity (Silver Spring) 18, 1486–1492 (2010).
Yang, C. C. et al. Visfatin regulates genes related to lipid metabolism in porcine adipocytes. J. Anim. Sci. 88, 3233–3241 (2010).
Salgado-Delgado, R. C. et al. Shift work or food intake during the rest phase promotes metabolic disruption and desynchrony of liver genes in male rats. PLoS ONE 8, e60052 (2013).
Barclay, J. L. et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE 7, e37150 (2012).
Karlsson, B., Knutsson, A. & Lindahl, B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup. Environ. Med. 58, 747–752 (2001).
Benedict, C. et al. Diurnal rhythm of circulating nicotinamide phosphoribosyltransferase (Nampt/visfatin/PBEF): impact of sleep loss and relation to glucose metabolism. J. Clin. Endocrinol. Metab. 97, E218–E222 (2012).
Cline, M. A., Nandar, W., Prall, B. C., Bowden, C. N. & Denbow, D. M. Central visfatin causes orexigenic effects in chicks. Behav. Brain Res. 186, 293–297 (2008).
Brunetti, L. et al. Effects of visfatin/PBEF/NAMPT on feeding behaviour and hypothalamic neuromodulators in the rat. J. Biol. Regul. Homeost. Agents 26, 295–302 (2012).
Cantó, C. et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
Tarantino, G. & Finelli, C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J. Gastroenterol. 19, 3375–3384 (2013).
Aller, R. et al. Influence of visfatin on histopathological changes of non-alcoholic fatty liver disease. Dig. Dis. Sci. 54, 1772–1777 (2009).
Auguet, T. et al. Plasma visfatin levels and gene expression in morbidly obese women with associated fatty liver disease. Clin. Biochem. 46, 202–208 (2013).
Genc, H. et al. Association of plasma visfatin with hepatic and systemic inflammation in nonalcoholic fatty liver disease. Ann. Hepatol. 12, 548–555 (2013).
Akbal, E., Kocak, E., Tas, A., Yuksel, E. & Koklu, S. Visfatin levels in nonalcoholic fatty liver disease. J. Clin. Lab. Anal. 26, 115–119 (2012).
Dahl, T. B. et al. Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down-regulated in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 95, 3039–3047 (2010).
Gaddipati, R. et al. Visceral adipose tissue visfatin in nonalcoholic fatty liver disease. Ann. Hepatol. 9, 266–270 (2010).
Kukla, M. M. et al. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Polish J. Pathol. 61, 147–153 (2010).
Zhang, Z.-F. et al. Troxerutin improves hepatic lipid homeostasis by restoring NAD+-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem. Pharmacol. 91, 74–86 (2014).
Li, H., Xu, M., Lee, J., He, C. & Xie, Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 303, E1234–E1244 (2012).
Tao, R. et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J. Biol. Chem. 286, 14681–14690 (2011).
Castro, R. E. et al. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J. Hepatol. 58, 119–125 (2013).
Choi, S.-E. et al. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 12, 1062–1072 (2013).
Frederick, D. W. et al. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J. Biol. Chem. 290, 1546–1558 (2015).
Escande, C. et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J. Clin. Invest. 120, 545–558 (2010).
Choi, Y. J. et al. Involvement of visfatin in palmitate-induced upregulation of inflammatory cytokines in hepatocytes. Metabolism 60, 1781–1789 (2011).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Al-Goblan, A. S., Al-Alfi, M. A. & Khan, M. Z. Mechanism linking diabetes mellitus and obesity. Diabetes. Metab. Syndr. Obes. 7, 587–591 (2014).
Gulcelik, N. E., Usman, A. & Gürlek, A. Role of adipocytokines in predicting the development of diabetes and its late complications. Endocrine 36, 397–403 (2009).
Blüher, M. Adipokines—removing road blocks to obesity and diabetes therapy. Mol. Metab. 3, 230–240 (2014).
Hug, C. & Lodish, H. F. Medicine. Visfatin: a new adipokine. Science 307, 366–367 (2005).
Varma, V. et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J. Clin. Endocrinol. Metab. 92, 666–672 (2007).
Hajianfar, H., Bahonar, A., Entezari, M. H., Askari, G. & Yazdani, M. Lipid profiles and serum visfatin concentrations in patients with type II diabetes in comparison with healthy controls. Int. J. Prev. Med. 3, 326–331 (2012).
El-Mesallamy, H. O., Kassem, D. H., El-Demerdash, E. & Amin, A. I. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism 60, 63–70 (2011).
Chen, M.-P. et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 91, 295–299 (2006).
López-Bermejo, A. et al. Serum visfatin increases with progressive β-cell deterioration. Diabetes 55, 2871–2875 (2006).
Yilmaz, M. I. et al. Endothelial dysfunction in type-2 diabetics with early diabetic nephropathy is associated with low circulating adiponectin. Nephrol. Dial. Transplant. 23, 1621–1627 (2008).
Motawi, T. M., Shaker, O. G., El-Sawalhi, M. M. & Abdel-Nasser, Z. M. Visfatin −948G/T and resistin −420C/G. polymorphisms in Egyptian type 2 diabetic patients with and without cardiovascular diseases. Genome 57, 259–266 (2014).
Xie, H. et al. Insulin-like effects of visfatin on human osteoblasts. Calcif. Tissue Int. 80, 201–210 (2007).
Ramsey, K. M., Mills, K. F., Satoh, A. & Imai, S.-I. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in β cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7, 78–88 (2008).
Spinnler, R. et al. The adipocytokine Nampt and its product NMN have no effect on β-cell survival but potentiate glucose stimulated insulin secretion. PLoS ONE 8, e54106 (2013).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
Caton, P. W., Kieswich, J., Yaqoob, M. M., Holness, M. J. & Sugden, M. C. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia 54, 3083–3092 (2011).
Imai, S. & Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471 (2014).
Van der Veer, E. et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J. Biol. Chem. 282, 10841–10845 (2007).
Villalobos, L. A. et al. Visfatin/Nampt induces telomere damage and senescence in human endothelial cells. Int. J. Cardiol. 175, 573–575 (2014).
Song, J. et al. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J. Gerontol. A Biol. Sci. Med. Sci. 69, 44–57 (2014).
Artegiani, B. & Calegari, F. Age-related cognitive decline: can neural stem cells help us? Aging (Albany NY) 4, 176–186 (2012).
Stein, L. R. & Imai, S. I. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 33, 1321–1340 (2014).
Olszanecka-Glinianowicz, M. et al. Relationship between circulating visfatin/NAMPT, nutritional status and insulin resistance in an elderly population—results from the PolSenior substudy. Metabolism 63, 1409–1418 (2014).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Cavadini, G. et al. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc. Natl Acad. Sci. USA 104, 12843–12848 (2007).
Warburg, O. On respiratory impairment in cancer cells. Science 124, 269–270 (1956).
Chiarugi, A., Dölle, C., Felici, R. & Ziegler, M. The NAD metabolome—a key determinant of cancer cell biology. Nat. Rev. Cancer 12, 741–752 (2012).
Schuster, S. et al. FK866-induced NAMPT inhibition activates AMPK and downregulates mTOR signalling in hepatocarcinoma cells. Biochem. Biophys. Res. Commun. 458, 334–340 (2015).
Nahimana, A. et al. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood 113, 3276–3286 (2009).
Olesen, U. H. et al. Anticancer agent CHS-828 inhibits cellular synthesis of NAD. Biochem. Biophys. Res. Commun. 367, 799–804 (2008).
Galli, U. et al. Medicinal chemistry of nicotinamide phosphoribosyltransferase (NAMPT) inhibitors. J. Med. Chem. 56, 6279–6296 (2013).
Zhou, T., Wang, T. & Garcia, J. G. Expression of nicotinamide phosphoribosyltransferase-influenced genes predicts recurrence-free survival in lung and breast cancers. Sci. Rep. 4, 6107 (2014).
Cea, M. et al. Targeting NAD+ salvage pathway induces autophagy in multiple myeloma cells via mTORC1 and extracellular signal-regulated kinase (ERK1/2) inhibition. Blood 120, 3519–3529 (2012).
Thakur, B. K. et al. Involvement of p53 in the cytotoxic activity of the NAMPT inhibitor FK866 in myeloid leukemic cells. Int. J. Cancer 132, 766–774 (2013).
Zhang, K. et al. Genetic variants in NAMPT predict bladder cancer risk and prognosis in individuals from southwest Chinese Han group. Tumour Biol. 35, 4031–4040 (2014).
Tian, W. et al. Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol. Oncol. 129, 505–512 (2013).
Reddy, P. S. et al. PBEF1/NAmPRTase/Visfatin: a potential malignant astrocytoma/glioblastoma serum marker with prognostic value. Cancer Biol. Ther. 7, 663–668 (2008).
Ninomiya, S. et al. Possible role of visfatin in hepatoma progression and the effects of branched-chain amino acids on visfatin-induced proliferation in human hepatoma cells. Cancer Prev. Res. (Phila.) 4, 2092–2100 (2011).
Audrito, V. et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood 125, 111–123 (2015).
Bułdak, R. J. et al. Visfatin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: an in vitro study. Oncol. Rep. 29, 771–778 (2013).
Adya, R., Tan, B. K., Punn, A., Chen, J. & Randeva, H. S. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 78, 356–365 (2008).
Olesen, U. H. et al. Target enzyme mutations are the molecular basis for resistance towards pharmacological inhibition of nicotinamide phosphoribosyltransferase. BMC Cancer 10, 677 (2010).
Bi, T.-Q. et al. Overexpression of Nampt in gastric cancer and chemopotentiating effects of the Nampt inhibitor FK866 in combination with fluorouracil. Oncol. Rep. 26, 1251–1257 (2011).
Gehrke, I. et al. On-target effect of FK866, a nicotinamide phosphoribosyl transferase inhibitor, by apoptosis-mediated death in chronic lymphocytic leukemia cells. Clin. Cancer Res. 20, 4861–4872 (2014).
Cea, M. et al. APO866 activity in hematologic malignancies: a preclinical in vitro study. Blood 113, 6035–6037 (2009).
Soncini, D. et al. Nicotinamide phosphoribosyltransferase promotes epithelial-to-mesenchymal transition as a soluble factor independent of its enzymatic activity. J. Biol. Chem. 289, 34189–34204 (2014).
Tan, B. et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. J. Biol. Chem. 288, 3500–3511 (2013).
Tummala, K. S. et al. Inhibition of de novo NAD+ synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 26, 826–839 (2014).
Von Heideman, A., Berglund, A., Larsson, R. & Nygren, P. Safety and efficacy of NAD depleting cancer drugs: results of a phase I clinical trial of CHS 828 and overview of published data. Cancer Chemother. Pharmacol. 65, 1165–1172 (2010).
Holen, K., Saltz, L. B., Hollywood, E., Burk, K. & Hanauske, A.-R. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Invest. New Drugs 26, 45–51 (2008).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
US National Library of Medicine. ClinicalTrials.gov[online], (2015).
Chan, M. et al. Synergy between the NAMPT inhibitor GMX1777(8) and pemetrexed in non-small cell lung cancer cells is mediated by PARP activation and enhanced NAD consumption. Cancer Res. 74, 5948–5954 (2014).
Hosseinzadeh-Attar, M. J., Golpaie, A., Janani, L. & Derakhshanian, H. Effect of weight reduction following bariatric surgery on serum visfatin and adiponectin levels in morbidly obese subjects. Obes. Facts 6, 193–202 (2013).
Saboori, S. et al. The comparison of serum vaspin and visfatin concentrations in obese and normal weight women. Diabetes Metab. Syndr. http://dx.doi.org/10.1016/j.dsx.2013.10.009.
Li, R.-Z. et al. Elevated visfatin levels in obese children are related to proinflammatory factors. J. Pediatr. Endocrinol. Metab. 26, 111–118 (2013).
Jaleel, A. et al. Association of adipokines with obesity in children and adolescents. Biomark. Med. 7, 731–735 (2013).
Terra, X. et al. Increased levels and adipose tissue expression of visfatin in morbidly obese women: the relationship with pro-inflammatory cytokines. Clin. Endocrinol. (Oxf.) 77, 691–698 (2012).
Taskesen, D., Kirel, B. & Us, T. Serum visfatin levels, adiposity and glucose metabolism in obese adolescents. J. Clin. Res. Pediatr. Endocrinol. 4, 76–81 (2012).
Catalan, V. et al. Association of increased visfatin/PBEF/NAMPT circulating concentrations and gene expression levels in peripheral blood cells with lipid metabolism and fatty liver in human morbid obesity. Nutr. Metab. Cardiovasc. Dis. 21, 245–253 (2011).
Reda, R., Shehab, A., Soliman, D., Gabr, A. & Abbass, A. Serum visfatin levels in a group of Egyptian obese individuals. Egypt. J. Immunol. 18, 25–32 (2011).
Krzystek-Korpacka, M., Patryn, E., Bednarz-Misa, I., Hotowy, K. & Noczynska, A. Visfatin in juvenile obesity—the effect of obesity intervention and sex. Eur. J. Clin. Invest. 41, 1284–1291 (2011).
Martos-Moreno, G. Á. et al. Serum visfatin and vaspin levels in prepubertal children: effect of obesity and weight loss after behavior modifications on their secretion and relationship with glucose metabolism. Int. J. Obes. (Lond.) 35, 1355–1362 (2011).
Ersoy, C. et al. Body fat distribution has no effect on serum visfatin levels in healthy female subjects. Cytokine 49, 275–278 (2010).
Kaminska, A. et al. An evaluation of visfatin levels in obese subjects. Endokrynol. Pol. 61, 169–173 (2010).
Barth, S. et al. Expression of neuropeptide Y, omentin and visfatin in visceral and subcutaneous adipose tissues in humans: relation to endocrine and clinical parameters. Obes. Facts 3, 245–251 (2010).
Costford, S. R. et al. Skeletal muscle NAMPT is induced by exercise in humans. Am. J. Physiol. Endocrinol. Metab. 298, E117–E126 (2010).
Nowell, M. A. et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 54, 2084–2095 (2006).
Bae, S.-K. et al. Hypoxic induction of human visfatin gene is directly mediated by hypoxia-inducible factor-1. FEBS Lett. 580, 4105–4113 (2006).
Acknowledgements
The authors thank colleagues from the Kiess laboratory for valuable discussions and acknowledge support from the German Diabetes Society, the German Competence Network Obesity, the Federal Ministry of Education and Research (grant 01GI1330 to A.G.), the Mitteldeutsche Kinderkrebsstiftung, the University of Chieti, Italy (for a scholarship to T.d.G.) and LIFE (Leipzig Research Centre for Civilization Diseases, University of Leipzig, Germany). LIFE is funded by the European Union, the European Regional Development Fund (ERDF; grant number: 4-7,531.70/5/4) and the Free State of Saxony within the framework of the excellence initiative.
Author information
Authors and Affiliations
Contributions
S.S., M.P., T.G. and T.d.G. researched data for the article. A.G., S.S., M.P., T.G., T.d.G. and W.K. provided substantial contributions to discussions of the content. A.G., S.S., M.P., T.G. and T.d.G. wrote the article. A.G., S.S. and W.K. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Garten, A., Schuster, S., Penke, M. et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11, 535–546 (2015). https://doi.org/10.1038/nrendo.2015.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.117
This article is cited by
-
Hypothalamic astrocyte NAD+ salvage pathway mediates the coupling of dietary fat overconsumption in a mouse model of obesity
Nature Communications (2024)
-
Discovering therapeutic possibilities for polycystic ovary syndrome by targeting XIST and its associated ceRNA network through the analysis of transcriptome data
Scientific Reports (2024)
-
The evolution and heterogeneity of neutrophils in cancers: origins, subsets, functions, orchestrations and clinical applications
Molecular Cancer (2023)
-
Identification and immunological characterization of lipid metabolism-related molecular clusters in nonalcoholic fatty liver disease
Lipids in Health and Disease (2023)
-
NAD+ metabolism-based immunoregulation and therapeutic potential
Cell & Bioscience (2023)