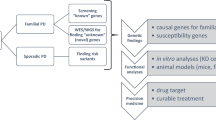
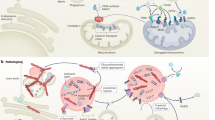
Key Points
-
Parkinson disease (PD) is the second most prevalent, age-associated, neurodegenerative disorder after Alzheimer disease.
-
PD is the major cause of parkinsonism, the clinical features of which include resting tremor, slowness and rigidity. Disease onset is insidious and progressive, and clinical symptoms are highly variable.
-
Epidemiology and twin studies once refuted a genetic aetiology, but pathogenic mutations were recently described in 7 genes. In the last decade, monogenic parkinsonism has become the most frequent definitive cause of sporadic and familial PD.
-
Symptomatic therapy is currently based on neurotransmitter (dopamine) replacement, but temporal improvement is limited and typically incurs troubling side effects as the disease progresses.
-
Molecular findings continue to nominate targets for rational drug design: primarily pharmacogenomic and neuroprotective therapies.
-
The pathology of PD consists of neuronal loss in the substantia nigra with Lewy body inclusions, whereas many forms of parkinsonism are associated with tauopathy. However, both lesions can represent alternative end points of the same genetic cause.
Abstract
Parkinson disease is a complex, multifactorial neurodegenerative disease. Although a heritable basis was originally thought unlikely, recent studies have implicated several genes in its pathogenesis, and molecular findings now allow accurate diagnosis and challenge past criteria for defining Parkinson disease. Most importantly, genetic insights provide the rationale for new strategies for prevention or therapy, and have led to animal models of disease in which these strategies can be tested. Neuroprotective therapies can now be designed to slow or halt disease progression in affected subjects and asymptomatic carriers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tanner, C. M. Is the cause of Parkinson's disease environmental or hereditary? Evidence from twin studies. Adv. Neurol. 91, 133–142 (2003).
Wirdefeldt, K., Gatz, M., Schalling, M. & Pedersen, N. L. No evidence for heritability of Parkinson disease in Swedish twins. Neurology 63, 305–311 (2004).
Zimprich, A. et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 (2004). Describes the identification of the PARK8 gene, LRRK2 , as a cause of late-onset Parkinson disease that might be associated with a pleomorphic pathology.
Valente, E. M. et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 (2004).
Singleton, A. B. et al. α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 (2003). Describes the discovery of α-synuclein multiplication mutations, which showed that simple overexpression of the wild-type protein is sufficient to cause disease. This work also suggests that Parkinson disease and dementia with Lewy bodies share the same aetiology.
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Paisan-Ruiz, C. et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595–600 (2004).
Leroy, E. et al. The ubiquitin pathway in Parkinson's disease. Nature 395, 451–452 (1998).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Bonifati, V. et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 (2003).
Maraganore, D. et al. High-resolution whole genome association study of Parkinson's disease. Am. J. Hum. Genet. 77, 685–693 (2005).
Fahn, S. Description of Parkinson's disease as a clinical syndrome. Ann. NY Acad. Sci. 991, 1–14 (2003). An excellent review of the clinical symptomatology of Parkinson disease.
Van Den Eeden, S. K. et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 157, 1015–1022 (2003).
Hughes, A. J., Daniel, S. E., Ben-Shlomo, Y. & Lees, A. J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125, 861–870 (2002).
Chen, L. & Feany, M. B. α-Synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nature Neurosci. 8, 657–663 (2005). Provides functional insights to suggest that α-synuclein inclusions and Lewy bodies might be protective rather than pathogenic.
Firestone, J. A. et al. Pesticides and risk of Parkinson disease: a population-based case-control study. Arch. Neurol. 62, 91–95 (2005).
Jankovic, J. Searching for a relationship between manganese and welding and Parkinson's disease. Neurology 64, 2021–2028 (2005).
Priyadarshi, A., Khuder, S. A., Schaub, E. A. & Priyadarshi, S. S. Environmental risk factors and Parkinson's disease: a metaanalysis. Environ. Res. 86, 122–127 (2001).
Allam, M. F., Campbell, M. J., Hofman, A., Del Castillo, A. S. & Fernandez-Crehuet Navajas, R. Smoking and Parkinson's disease: systematic review of prospective studies. Mov. Disord. 19, 614–621 (2004).
Leroux, P.-D. Contribution à l'Étude des Causes de la Paralysie Agitante. Thèse de Paris, Imprimeur de la Faculté de Médecine (1880) (in French).
Allen, W. Inheritance of the shaking palsy. Arch. Int. Med. 60, 424–436 (1937).
Mjones, H. Paralysis Agitans. A clinical and genetic study. Acta Psychiatr. Neurol. Scand. Supplement 54, 1–195 (Ejnar Munksgaard, Copenhagen, 1949).
Piccini, P., Burn, D. J., Ceravolo, R., Maraganore, D. & Brooks, D. J. The role of inheritance in sporadic Parkinson's disease: evidence from a longitudinal study of dopaminergic function in twins. Ann. Neurol. 45, 577–582 (1999).
Tanner, C. M. et al. Parkinson disease in twins: an etiologic study. JAMA 281, 341–346 (1999).
Sveinbjornsdottir, S. et al. Familial aggregation of Parkinson's disease in Iceland. N. Engl. J. Med. 343, 1765–1770 (2000).
Rocca, W. A. et al. Familial aggregation of Parkinson's disease: The Mayo Clinic family study. Ann. Neurol. 56, 495–502 (2004).
Simon, D. K., Lin, M. T. & Pascual-Leone, A. 'Nature versus nurture' and incompletely penetrant mutations. J. Neurol. Neurosurg. Psychiatry 72, 686–689 (2002).
Valente, E. M. et al. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann. Neurol. 56, 336–341 (2004).
Maraganore, D. M. et al. UCHL1 is a Parkinson's disease susceptibility gene. Ann. Neurol. 55, 512–521 (2004).
Lotharius, J. & Brundin, P. Pathogenesis of Parkinson's disease: dopamine, vesicles and α-synuclein. Nature Rev. Neurosci. 3, 932–942 (2002).
Sidhu, A., Wersinger, C., Moussa, C. E. & Vernier, P. The role of α-synuclein in both neuroprotection and neurodegeneration. Ann. NY Acad. Sci. 1035, 250–270 (2004).
Bostantjopoulou, S. et al. Clinical features of parkinsonian patients with the α-synuclein (G209A) mutation. Mov. Disord. 16, 1007–1013 (2001).
Kruger, R. et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nature Genet. 18, 106–108 (1998).
Zarranz, J. J. et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 (2004).
Hope, A. & Farrer, M. in Molecular Mechanisms in Parkinson's Disease (eds Philipp, K. & Haass, C.) (Landes Bioscience, Georgetown, Texas, 2004).
Lashuel, H. A. et al. α-Synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J. Mol. Biol. 322, 1089–1102 (2002).
Farrer, M. et al. Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann. Neurol. 55, 174–179 (2004).
Chartier-Harlin, M. C. et al. α-Synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 (2004).
Ibanez, P. et al. Causal relation between α-synuclein gene duplication and familial Parkinson's disease. Lancet 364, 1169–1171 (2004).
Nishioka, K. et al. Clinical heterogeneity of α-synuclein gene duplication in autosomal dominant familial Parkinson's disease. Ann. Neurol. 59, 298–309 (2006).
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc. Natl Acad. Sci. USA 95, 6469–6473 (1998).
McKeith, I. G. et al. Dementia with Lewy bodies. Semin. Clin. Neuropsychiatry 8, 46–57 (2003).
Poewe, W. Treatment of dementia with Lewy bodies and Parkinson's disease dementia. Mov. Disord. 20, S77–S82 (2005).
Pals, P. et al. α-Synuclein promoter confers susceptibility to Parkinson's disease. Ann. Neurol. 56, 591–595 (2004).
Mueller, J. C. et al. Multiple regions of α-synuclein are associated with Parkinson's disease. Ann. Neurol. 57, 535–541 (2005).
Chiba-Falek, O., Kowalak, J. A., Smulson, M. E. & Nussbaum, R. L. Regulation of α-synuclein expression by poly (ADP ribose) polymerase-1 (PARP-1) binding to the NACP-Rep1 polymorphic site upstream of the SNCA gene. Am. J. Hum. Genet. 76, 478–492 (2005).
Mellick, G. D., Maraganore, D. M. & Silburn, P. A. Australian data and meta-analysis lend support for α-synuclein (NACP-Rep1) as a risk factor for Parkinson's disease. Neurosci. Lett. 375, 112–116 (2005).
Outeiro, T. F. & Lindquist, S. Yeast cells provide insight into α-synuclein biology and pathobiology. Science 302, 1772–1775 (2003).
Lakso, M. et al. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human α-synuclein. J. Neurochem. 86, 165–172 (2003).
Fernagut, P. O. & Chesselet, M. F. α-Synuclein and transgenic mouse models. Neurobiol. Dis. 17, 123–130 (2004).
Yamada, M., Iwatsubo, T., Mizuno, Y. & Mochizuki, H. Overexpression of α-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of α-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J. Neurochem. 91, 451–461 (2004).
Perez, R. G. & Hastings, T. G. Could a loss of α-synuclein function put dopaminergic neurons at risk? J. Neurochem. 89, 1318–1324 (2004).
Feany, M. B. & Bender, W. W. A Drosophila model of Parkinson's disease. Nature 404, 394–398 (2000).
Takahashi, M. et al. Phosphorylation of α-synuclein characteristic of synucleinopathy lesions is recapitulated in αsynuclein transgenic Drosophila. Neurosci. Lett. 336, 155–158 (2003).
Auluck, P. K., Meulener, M. C. & Bonini, N. M. Mechanisms of suppression of α-synuclein neurotoxicity by geldanamycin in Drosophila. J. Biol. Chem. 280, 2873–2878 (2005).
Fujiwara, H. et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biol. 4, 160–164 (2002).
Nonaka, T., Iwatsubo, T. & Hasegawa, M. Ubiquitination of α-synuclein. Biochemistry 44, 361–368 (2005).
Funayama, M. et al. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2–q13.1. Ann. Neurol. 51, 296–301 (2002).
Kachergus, J. et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet. 76, 672–680 (2005). This work shows that a common founder was responsible for the most frequent mutation to cause Parkinson disease that has been identified so far: LRRK2 Gly2019Ser. It also provides an age-associated penetrance estimate.
Paisan-Ruiz, C. et al. Familial Parkinson's disease: clinical and genetic analysis of four Basque families. Ann. Neurol. 57, 365–372 (2005).
Funayama, M. et al. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann. Neurol. 57, 918–921 (2005).
Mata, I. F. et al. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics 6, 171–177 (2005).
Gilks, W. P. et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet 365, 415–416 (2005).
Di Fonzo, A. et al. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet 365, 412–415 (2005).
Nichols, W. C. et al. Genetic screening for a single common LRRK2 mutation in familial Parkinson's disease. Lancet 365, 410–412 (2005).
Farrer, M. et al. LRRK2 mutations in Parkinson disease. Neurology 65, 738–740 (2005).
Tomiyama, H. et al. Clinicogenetic study of mutations in LRRK2 exon 41 in Parkinson's disease patients from 18 different countries. Mov. Disord. 2006 (doi: 10.1002/mds.20886).
Ozelius, L. J. et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 354, 424–425 (2006).
Lesage, S. et al. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N. Engl. J. Med. 354, 422–423 (2006).
Lesage, S. et al. G2019S LRRK2 mutation in French and North African families with Parkinson's disease. Ann. Neurol. 58, 784–787 (2005).
Aasly, J. O. et al. Clinical features of LRRK2-associated Parkinson's disease in central Norway. Ann. Neurol. 57, 762–765 (2005).
West, A. B. et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl Acad. Sci. USA 102, 16842–16847 (2005).
Gloeckner, C. J. et al. The Parkinson disease causing LRRK2 mutation I20202T is associated with increased kinase activity. Hum. Mol. Genet. 15, 223–232 (2006). References 72 and 73 show that LRRK2 substitutions might enhance kinase activity, which indicates the therapeutic possibility of kinase inhibition as a neuroprotective therapy in Parkinson disease.
Ross, O. A. et al. Lrrk2 and Lewy body disease. Ann. Neurol. 59, 388–393 (2006).
Wszolek, Z. K. et al. Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology 62, 1619–1622 (2004).
Hasegawa, K. & Kowa, H. Autosomal dominant familial Parkinson disease: older onset of age, and good response to levodopa therapy. Eur. Neurol. 38, 39–43 (1997).
Giasson, B. I. et al. Biochemical and pathological characterization of Lrrk2. Ann. Neurol. 59, 315–322 (2005).
Bosgraaf, L. et al. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 21, 4560–4570 (2002).
Katzenschlager, R. & Lees, A. J. Olfaction and Parkinson's syndromes: its role in differential diagnosis. Curr. Opin. Neurol. 17, 417–423 (2004).
Meylan, E. & Tschopp, J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem. Sci. 30, 151–159 (2005).
Mata, I. F., Lockhart, P. J. & Farrer, M. J. Parkin genetics: one model for Parkinson's disease. Hum. Mol. Genet. 13, R127–R133 (2004).
Abou-Sleiman, P. M., Healy, D. G. & Wood, N. W. Causes of Parkinson's disease: genetics of DJ-1. Cell Tissue Res 318, 185–188 (2004).
Shimura, H. et al. Familial Parkinson disease gene product, parkin, is a ubiquitin–protein ligase. Nature Genet. 25, 302–305 (2000). Functional insights from the first gene to be implicated in early-onset parkinsonism, Parkin, highlighted the role of the ubiquitin–proteasome pathway in pure nigral neuronal degeneration.
Pramstaller, P. P. et al. Lewy body Parkinson's disease in a large pedigree with 77 Parkin mutation carriers. Ann. Neurol. 58, 411–422 (2005).
Sriram, S. R. et al. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum. Mol. Genet. 14, 2571–2586 (2005).
Betarbet, R., Sherer, T. B. & Greenamyre, J. T. Ubiquitin–proteasome system and Parkinson's diseases. Exp. Neurol. 191, S17–S27 (2005).
Kahle, P. J. & Haass, C. How does parkin ligate ubiquitin to Parkinson's disease? EMBO Rep. 5, 681–685 (2004).
Ko, H. S. et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J. Neurosci. 25, 7968–7978 (2005).
Lo Bianco, C. et al. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an α-synuclein rat model of Parkinson's disease. Proc. Natl Acad. Sci. USA 101, 17510–17515 (2004).
Greene, J. C., Whitworth, A. J., Andrews, L. A., Parker, T. J. & Pallanck, L. J. Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Hum. Mol. Genet. 14, 799–811 (2005).
Valente, E. M. et al. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35–p36. Am. J. Hum. Genet. 68, 895–900 (2001).
Hatano, Y. et al. Novel PINK1 mutations in early-onset parkinsonism. Ann. Neurol. 56, 424–427 (2004).
Petit, A. et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson's disease-related mutations. J. Biol. Chem. 280, 34025–34032 (2005).
Beilina, A. et al. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc. Natl Acad. Sci. USA 102, 5703–5708 (2005).
Deng, H., Jankovic, J., Guo, Y., Xie, W. & Le, W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 337, 1133–1138 (2005).
Kessler, K. R. et al. Dopaminergic function in a family with the PARK6 form of autosomal recessive Parkinson's syndrome. J. Neural Transm. 112, 1345–1353 (2005).
Khan, N. L. et al. Clinical and subclinical dopaminergic dysfunction in PARK6-linked parkinsonism: an 18F-dopa PET study. Ann. Neurol. 52, 849–853 (2002).
Lockhart, P. J. et al. DJ-1 mutations are a rare cause of recessively inherited early onset parkinsonism mediated by loss of protein function. J. Med. Genet. 41, e22 (2004).
Tao, X. & Tong, L. Crystal structure of human DJ-1, a protein associated with early onset Parkinson's disease. J. Biol. Chem. 278, 31372–31379 (2003).
Zhang, L. et al. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum. Mol. Genet. 14, 2063–2073 (2005).
Goldberg, M. S. et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron 45, 489–496 (2005).
Kim, R. H. et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl Acad. Sci. USA 102, 5215–5220 (2005).
Yang, Y. et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl Acad. Sci. USA 102, 13670–13675 (2005).
Meulener, M. et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr. Biol. 15, 1572–1577 (2005).
Dekker, M. C. et al. PET neuroimaging and mutations in the DJ-1 gene. J. Neural Transm. 111, 1575–1581 (2004).
Rizzu, P. et al. DJ-1 colocalizes with tau inclusions:a link between parkinsonism and dementia. Ann. Neurol. 55, 113–118 (2004).
Scott, W. K. et al. Complete genomic screen in Parkinson disease: evidence for multiple genes. JAMA 286, 2239–2244 (2001).
Hicks, A. A. et al. A susceptibility gene for late-onset idiopathic Parkinson's disease. Ann. Neurol. 52, 549–555 (2002).
DeStefano, A. L. et al. PARK3 influences age at onset in Parkinson disease: a genome scan in the GenePD study. Am. J. Hum. Genet. 70, 1089–1095 (2002).
Li, Y. J. et al. Age at onset in two common neurodegenerative diseases is genetically controlled. Am. J. Hum. Genet. 70, 985–993 (2002).
Pankratz, N. et al. Genome-wide linkage analysis and evidence of gene-by-gene interactions in a sample of 362 multiplex Parkinson disease families. Hum. Mol. Genet. 12, 2599–2608 (2003).
Martinez, M. et al. Genome-wide scan linkage analysis for Parkinson's disease: the European genetic study of Parkinson's disease. J. Med. Genet. 41, 900–907 (2004).
Hutton, M. Molecular genetics of chromosome 17 tauopathies. Ann. NY Acad. Sci. 920, 63–73 (2000).
Skipper, L. et al. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am. J. Hum. Genet. 75, 669–677 (2004).
Rademakers, R. et al. High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum. Mol. Genet. 14, 3281–3292 (2005).
Liu, Y., Fallon, L., Lashuel, H. A., Liu, Z. & Lansbury, P. T. Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect α-synuclein degradation and Parkinson's disease susceptibility. Cell 111, 209–218 (2002).
Shen, J. & Cookson, M. R. Mitochondria and dopamine: new insights into recessive parkinsonism. Neuron 43, 301–304 (2004).
Uitti, R. J., Calne, D. B., Dickson, D. W. & Wszolek, Z. K. Is the neuropathological 'gold standard' diagnosis dead? Implications of clinicopathological findings in an autosomal dominant neurodegenerative disorder. Parkinsonism Relat. Disord. 10, 461–463 (2004).
Forman, M. S., Lee, V. M. & Trojanowski, J. Q. Nosology of Parkinson's disease: looking for the way out of a quackmire. Neuron 47, 479–482 (2005).
Trojanowski, J. Q. & Lee, V. M. Transgenic models of tauopathies and synucleinopathies. Brain Pathol. 9, 733–739 (1999).
Parkkinen, L., Kauppinen, T., Pirttila, T., Autere, J. M. & Alafuzoff, I. α-Synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann. Neurol. 57, 82–91 (2005).
Wittmann, C. W. et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293, 711–714 (2001).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Olanow, C. W. et al. Levodopa in the treatment of Parkinson's disease: current controversies. Mov. Disord. 19, 997–1005 (2004).
Fahn, S. The spectrum of levodopa-induced dyskinesias. Ann. Neurol. 47, S2–S9; discussion S9–S11 (2000).
Walter, B. L. & Vitek, J. L. Surgical treatment for Parkinson's disease. Lancet Neurol. 3, 719–728 (2004).
Masliah, E. et al. Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron 46, 857–868 (2005). A demonstration of the power of genetic insights in nominating targets for translational advances and creating the models in which to test them.
Ross, O. A. & Farrer, M. J. Pathophysiology, pleiotrophy and paradigm shifts: genetic lessons from Parkinson's disease. Biochem. Soc. Trans. 33, 586–590 (2005).
Silva, R. M., Kuan, C. Y., Rakic, P. & Burke, R. E. Mixed lineage kinase-c-jun N-terminal kinase signaling pathway: a new therapeutic target in Parkinson's disease. Mov. Disord. 20, 653–664 (2005).
Langston, J. W., Ballard, P., Tetrud, J. W. & Irwin, I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983).
Hirsch, E. C. et al. Animal models of Parkinson's disease in rodents induced by toxins: an update. J. Neural Transm. Suppl. 65, 89–100 (2003).
Hirano, A., Kurland, L. T., Krooth, R. S. & Lessell, S. Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain 84, 642–661 (1961).
Ince, P. G. & Codd, G. A. Return of the cycad hypothesis — does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol. Appl. Neurobiol. 31, 345–353 (2005).
Hof, P. R. et al. Amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: quantitative neuropathology, immunohistochemical analysis of neuronal vulnerability, and comparison with related neurodegenerative disorders. Acta Neuropathol. (Berl.) 88, 397–404 (1994).
Sebeo, J., Hof, P. R. & Perl, D. P. Occurrence of α-synuclein pathology in the cerebellum of Guamanian patients with parkinsonism-dementia complex. Acta Neuropathol. (Berl.) 107, 497–503 (2004).
Caparros-Lefebvre, D. et al. Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy. Brain 125, 801–811 (2002).
Economo Von, C. Encephalytis Lethargica: its Sequelae and Treatment (Oxford University Press, London, 1931).
Dale, R. C. et al. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain 127, 21–33 (2004).
Josephs, K. A., Parisi, J. E. & Dickson, D. W. α-Synuclein studies are negative in postencephalic parkinsonism of von Economo. Neurology 59, 645–646 (2002).
Henry, J. M. & Jellinger, K. in Neurodegeneration: The molecular pathology of dementia and movement disorders (ed. Dickson, D.) (ISN Neuropath Press, Basel, 2003).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Linkage mapping
-
A method for localizing genes that is based on the coinheritance of genetic markers and phenotypes in families over several generations.
- Association study
-
A gene-discovery strategy that compares cases with controls to assess the contribution of genetic variants to phenotypes in specific populations. Such studies can test for association with variants in a candidate gene, or with large sets of variants that are located throughout the genome.
- Bradykinesia
-
Slowing of and difficulty in initiating movement that is characteristic of Parkinson disease.
- Substantia nigra
-
The region of the brain that includes the pars compacta and harbours the neurons that produce the neurotransmitter dopamine, which is required for controlled movement. Substantia nigra neurons degenerate in Parkinson disease.
- Proteasome
-
Part of the ubiquitin–proteasome system, in which ubiquitin molecules are attached to a target protein that is subsequently degraded by the proteasome complex.
- Relative risk
-
The ratio of the risk of developing a disease in individuals who have been exposed to a risk factor to that in individuals who have not been exposed to the risk factor.
- Longitudinal twin studies
-
The concordance rate of disease in monozygotic versus dizygotic pairs of twins, which is assessed longitudinally over time.
- Cross-sectional twin studies
-
An assessment of the concordance rate of disease in monozygotic versus dizygotic pairs of twins, carried out at a specific time point.
- Penetrance
-
The frequency with which individuals that carry a given gene will show the manifestations that are associated with the gene. If a disease allele is 100% penetrant then all individuals carrying that allele will express the associated disorder.
- Diffuse Lewy body disease
-
Brain-stem and cortical Lewy body pathology typically associated with clinical dementia and parkinsonism, and with parkinsonism with dementia.
- Astrocytosis
-
The process whereby supporting glial cells in the CNS become activated in response to insult.
- Dopaminergic
-
Refers to neurons that predominantly use dopamine as a neurotransmitter.
- Argyrophilic grains disease
-
A late-onset dementia, with tau-positive 'grains' in neuronal processes and coiled bodies in oligodendrocytes post mortem.
- Compound heterozygous
-
Refers to individuals that carry a diploid genotype in which the two copies of a gene carry different mutations.
- Positron emission tomography
-
Imaging of the emission of positrons from the brain after a small amount of radioactive isotope has been injected into the blood stream. This method is used to quantitatively measure metabolic, biochemical and functional activity in living tissue.
- Dyskinesia
-
Uncontrolled or excessive involuntary movement, which is typically induced as a side-effect of long-term L-DOPA administration.
Rights and permissions
About this article
Cite this article
Farrer, M. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet 7, 306–318 (2006). https://doi.org/10.1038/nrg1831
Issue Date:
DOI: https://doi.org/10.1038/nrg1831
This article is cited by
-
Toxic interactions between dopamine, α-synuclein, monoamine oxidase, and genes in mitochondria of Parkinson’s disease
Journal of Neural Transmission (2024)
-
Apolipoprotein D concentration in Parkinson’s disease patients
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2023)
-
Different pieces of the same puzzle: a multifaceted perspective on the complex biological basis of Parkinson’s disease
npj Parkinson's Disease (2023)
-
Lysosomal Pathogenesis of Parkinson's Disease: Insights From LRRK2 and GBA1 Rodent Models
Neurotherapeutics (2023)
-
Circular RNAs in Parkinson’s Disease: Reliable Biological Markers and Targets for Rehabilitation
Molecular Neurobiology (2023)