Key Points
-
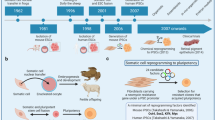
This Review describes recent progress in directing human pluripotent stem cells (hPSCs) into specific progeny that could have therapeutic purposes for a range of diseases. It also addresses major hurdles in the transition of hPSC-based cell therapies from the bench to the bedside.
-
Neural induction of hPSCs can be achieved in several ways. Recent protocols use defined neural inducers — such as inhibitors of transforming growth factor-β (TGFβ) and bone morphogenetic protein (BMP) (that is, dual SMAD inhibition) — to greatly enhance the efficiency and the speed of neural induction.
-
The derivation of dopamine neurons from hPSCs has been achieved a decade ago, but the cells did not show good engraftment. Recent data shows that those neurons lacked expression of forkhead box protein A2 (FOXA2), which is a DNA-binding transcription factor that is fundamental for authentic midbrain identity.
-
A novel protocol derives dopamine neurons through a floor plate intermediate, which show genetic, biochemical and physiological features of authentic midbrain neurons. They also survive and ameliorate Parkinson's disease-like behaviour in vivo.
-
Improved protocols for the derivation of medium spiny striatal neurons from hPSCs has been reported, and evidence shows survival and behavioural improvement in a lesion model of Huntington's disease.
-
The derivation of glial cells from hPSCs is faced with the challenge of protracted developmental timing in vitro, which is similar to the in vivo situation. The derivation of oligodendrocytes has been achieved using long-term in vitro cultures; these cells have been grafted in neonatal Shiverer-expressing mice with good cell survival, remyelination and extended lifespan in these mice.
-
The current derivation of non-neural cell types — such as cardiomyocytes, pancreatic islet cells and engraftable haematopoietic stem cells — faces substantial challenges owing to the immature nature of the differentiated cells (for cardiomyocytes), the need for in vivo differentiation (for pancreatic islet cells) and poor in vivo homing (for haematopoietic stem cells).
-
New developments in cell differentiation include the use of potent small molecules that allow the direct manipulation of multiple signalling pathways and, in some cases, the acceleration of differentiation timelines. Other approaches include cell purification and three-dimensional cultures that harness the self-organizing potential of hPSC-derived lineages.
-
Defining cell identity in vitro is a fundamental element in designing directed differentiation strategies and includes expression of cell type-specific markers, transcriptional profiles and assessments of the epigenetic or enhancer landscapes. Assessment of in vivo function includes electrophysiology, the use of genetically encoded calcium sensors, microdialysis and optogenetic techniques, as well as behavioural studies.
-
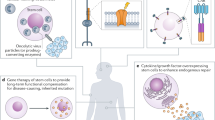
Autologous cell sources, such as patient-derived induced pluripotent stem cells, are of great interest but currently face substantial hurdles for clinical implementation that are related to safety and regulatory requirements. The translation of direct reprogramming and nuclear transfer strategies are in early stages of development.
-
A spinal cord trial using human embryonic stem cell (hESC)-derived oligodendrocytes has not reported any major adverse effects, although the trial has been abandoned. Ongoing clinical trials using hESC-derived retinal pigment epithelial in eye repair are promising.
Abstract
After years of incremental progress, several recent studies have succeeded in deriving disease-relevant cell types from human pluripotent stem cell (hPSC) sources. The prospect of an unlimited cell source, combined with promising preclinical data, indicates that hPSC technology may be on the verge of clinical translation. In this Review, we discuss recent progress in directed differentiation, some of the new technologies that have facilitated the success of hPSC therapies and the remaining hurdles on the road towards developing hPSC-based cell therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551 (2011).
Ma, L. et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 10, 455–464 (2012).
Wang, S. et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252–264 (2013).
Shiba, Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012).
Bellin, M., Marchetto, M. C., Gage, F. H. & Mummery, C. L. Induced pluripotent stem cells: the new patient? Nature Rev. Mol. Cell Biol. 13, 713–726 (2012).
Zhang, S. C., Wernig, M., Duncan, I. D., Brustle, O. & Thomson, J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotech. 19, 1129–1133 (2001).
Reubinoff, B. E. et al. Neural progenitors from human embryonic stem cells. Nature Biotech. 19, 1134–1140 (2001).
Ladewig, J., Koch, P. & Brustle, O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nature Rev. Mol. Cell Biol. 14, 225–236 (2013).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotech. 25, 681–686 (2007).
Perrier, A. L. et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl Acad. Sci. USA 101, 12543–12548 (2004).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotech. 27, 275–280 (2009).
Lindvall, O. Dopaminergic neurons for Parkinson's therapy. Nature Biotech. 30, 56–58 (2012).
Olanow, C. W. et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 54, 403–414 (2003).
Freed, C. R. et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 344, 710–719 (2001).
Barker, R. A., Barrett, J., Mason, S. L. & Bjorklund, A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 12, 84–91 (2013).
Yan, Y. et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781–790 (2005).
Sonntag, K. C. et al. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells 25, 411–418 (2007).
Kirkeby, A. et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714 (2012).
Xi, J. et al. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells 30, 1655–1663 (2012).
MacDonald, M. E. et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Perrier, A. & Peschanski, M. How can human pluripotent stem cells help decipher and cure Huntington's disease? Cell Stem Cell 11, 153–161 (2012).
Aubry, L. et al. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc. Natl Acad. Sci. USA 105, 16707–16712 (2008).
Carri, A. D. et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development 140, 301–312 (2013).
Nistor, G. I., Totoiu, M. O., Haque, N., Carpenter, M. K. & Keirstead, H. S. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49, 385–396 (2005).
Alper, J. Geron gets green light for human trial of ES cell-derived product. Nature Biotech. 27, 213–214 (2009).
Hu, B. Y., Du, Z. W., Li, X. J., Ayala, M. & Zhang, S. C. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development 136, 1443–1452 (2009).
Goldman, S. A., Nedergaard, M. & Windrem, M. S. Glial progenitor cell-based treatment and modeling of neurological disease. Science 338, 491–495 (2012).
Touboul, T. et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51, 1754–1765 (2010).
James, D. et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFβ inhibition is Id1 dependent. Nature Biotech. 28, 161–166 (2010).
Kelly, O. G. et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nature Biotech. 29, 750–756 (2011).
Ledran, M. H. et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell 3, 85–98 (2008).
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotech. 25, 1015–1024 (2007).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008).
Nunes, S. S. et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nature Methods 10, 781–787 (2013).
Chen, S. et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nature Chem. Biol. 5, 258–265 (2009).
D'Amour, K. A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotech. 24, 1392–1401 (2006).
Nostro, M. C. et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138, 861–871 (2011).
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotech. 26, 443–452 (2008).
Barberi, T. et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nature Med. 13, 642–648 (2007).
Darabi, R. et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619 (2012).
Li, W., Li, K., Wei, W. & Ding, S. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell 13, 270–283 (2013).
Li, W. & Ding, S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol. Sci. 31, 36–45 (2010).
Longmire, T. A. et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10, 398–411 (2012).
Chambers, S. M. et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nature Biotech. 30, 715–720 (2012).
Morizane, A., Doi, D., Kikuchi, T., Nishimura, K. & Takahashi, J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J. Neurosci. Res. 89, 117–126 (2011).
Boulting, G. L. et al. A functionally characterized test set of human induced pluripotent stem cells. Nature Biotech. 29, 279–286 (2011).
Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 493, 318–326 (2013).
Kusuma, S. et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl Acad. Sci. USA 110, 12601–12606 (2013).
Marolt, D. et al. Engineering bone tissue from human embryonic stem cells. Proc. Natl Acad. Sci. USA 109, 8705–8709 (2012).
Diekman, B. O. et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 109, 19172–19177 (2012).
Kraehenbuehl, T. P., Langer, R. & Ferreira, L. S. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nature Methods 8, 731–736 (2011).
Redmond, D. E. Jr., Vinuela, A., Kordower, J. H. & Isacson, O. Influence of cell preparation and target location on the behavioral recovery after striatal transplantation of fetal dopaminergic neurons in a primate model of Parkinson's disease. Neurobiol. Dis. 29, 103–116 (2008).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Giudice, A. & Trounson, A. Genetic modification of human embryonic stem cells for derivation of target cells. Cell Stem Cell 2, 422–433 (2008).
Gaj, T., Gersbach, C. A. & Barbas, C. F. 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
Ganat, Y. M. et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J. Clin. Invest. 122, 2928–2939 (2012).
Nicholas, C. R. et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 12, 573–586 (2013).
Maroof, A. M. et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12, 559–572 (2013).
Yuan, S. H. et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 6, e17540 (2011).
Dubois, N. C. et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nature Biotech. 29, 1011–1018 (2011).
Paige, S. L. et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell 151, 221–232 (2012).
Rada-Iglesias, A. et al. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell 11, 633–648 (2012).
Arbab, A. S., Liu, W. & Frank, J. A. Cellular magnetic resonance imaging: current status and future prospects. Expert Rev. Med. Devices 3, 427–439 (2006).
Budde, M. D. & Frank, J. A. Magnetic tagging of therapeutic cells for MRI. J. Nucl. Med. 50, 171–174 (2009).
Kretzschmar, K. & Watt, F. M. Lineage tracing. Cell 148, 33–45 (2012).
Kiuru, M., Boyer, J. L., O'Connor, T. P. & Crystal, R. G. Genetic control of wayward pluripotent stem cells and their progeny after transplantation. Cell Stem Cell 4, 289–300 (2009).
Björklund, A. et al. Mechanisms of action of intracerebral neural implants: studies on nigral and striatal grafts to the lesioned striatum. Trends Neurosciences 10, 509–516 (1987).
Weick, J. P., Liu, Y. & Zhang, S. C. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proc. Natl Acad. Sci. USA 108, 20189–20194 (2011).
Cummings, B. J. et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl Acad. Sci. USA 102, 14069–14074 (2005).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. New Engl. J. Med. 365, 1673–1683 (2011).
Suzuki, M. & Svendsen, C. N. Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci. 31, 192–198 (2008).
Papapetrou, E. P. et al. Genomic safe harbors permit high β-globin transgene expression in thalassemia induced pluripotent stem cells. Nature Biotech. 29, 73–78 (2011).
Battista, D., Ganat, Y., El Maarouf, A., Studer, L. & Rutishauser, U. Enhancement of polysialic acid expression improves function of embryonic stem-derived dopamine neuron grafts in Parkinsonian mice. Stem Cells Transl Med. http://dx.doi.org/10.5966/sctm.2013-0084 (2013).
Araki, R. et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 494, 100–104 (2013).
Zhao, T., Zhang, Z. N., Rong, Z. & Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 474, 212–215 (2011).
Guha, P., Morgan, J. W., Mostoslavsky, G., Rodrigues, N. P. & Boyd, A. S. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell 12, 407–412 (2013).
Gore, A. et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 471, 63–67 (2011).
Lister, R. et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471, 68–73 (2011).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Fusaki, N., Ban, H., Nishiyama, A., Saeki, K. & Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 85, 348–362 (2009).
Polo, J. M. et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature Biotech. 28, 848–855 (2010).
Kim, K. et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature Biotech. 29, 1117–1119 (2011).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010).
Bar-Nur, O., Russ, H. A., Efrat, S. & Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet β cells. Cell Stem Cell 9, 17–23 (2011).
Cyranoski, D. Stem cells cruise to clinic. Nature 494, 413 (2013).
Tachibana, M. et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 153, 1228–1238 (2013).
Vierbuchen, T. & Wernig, M. Molecular roadblocks for cellular reprogramming. Mol. Cell 47, 827–838 (2012).
Chambers, S. M. & Studer, L. Cell fate plug and play: direct reprogramming and induced pluripotency. Cell 145, 827–830 (2011).
Rouaux, C. & Arlotta, P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nature Cell Biol. 15, 214–221 (2013).
De la Rossa, A. et al. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nature Neurosci. 16, 193–200 (2013).
Torper, O. et al. Generation of induced neurons via direct conversion in vivo. Proc. Natl Acad. Sci. USA 110, 7038–7043 (2013).
Niu, W. et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature Cell Biol. 15, 1164–1175 (2013).
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D. A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 (2008).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Frantz, S. Embryonic stem cell pioneer Geron exits field, cuts losses. Nature Biotech. 30, 12–13 (2012).
Ausubel, L. J., Lopez, P. M. & Couture, L. A. GMP scale-up and banking of pluripotent stem cells for cellular therapy applications. Methods Mol. Biol. 767, 147–159 (2011).
Okun, M. S. Deep-brain stimulation for Parkinson's disease. New Engl. J. Med. 367, 1529–1538 (2012).
Larson, P. S. et al. An optimized system for interventional magnetic resonance imaging-guided stereotactic surgery: preliminary evaluation of targeting accuracy. Neurosurgery 70, 95–103 (2012).
Draft guidance for industry: considerations for the design of early-phase clinical trials of cellular and gene therapy products. US Department of Health and Human Services, The Food and Drug Administration [online], (2013).
Acknowledgements
The authors' own work described in this Review was supported by New York State Stem Cell Board (NYSTEM) (C028503) and the US National Institute of Neurological Disorders (5R01NS054009 to V.T.; NS052671 and NS084334 to L.S.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Directed differentiation
-
A method to control the differentiation of pluripotent stem cells into specific cell types, which is typically achieved by providing cells with extrinsic signals in a precise temporal sequence that mimicks development.
- Morphogen
-
A substance that is active in pattern formation and that varies in spatial concentration or activity; cells respond differently at different threshold concentrations of morphogens.
- Embryoid body
-
A clump of cells that arises when embryonic cells are cultured and differentiated in suspension and that can give rise to cell types from all three germ layers (that is, the endoderm, mesoderm and ectoderm).
- Stromal
-
Pertaining to connective tissue that is made up of both cells (such as fibroblasts) and matrix (such as collagen).
- Dopamine neuron
-
A nerve cell that uses the neurotransmitter dopamine; those in the midbrain are affected in Parkinson's disease.
- Dual SMAD inhibition
-
(dSMADi). The concomitant inhibition of bone morphogenetic protein and Nodal–activin–transforming growth factor-β signalling, which is used to obtain neural cells from human pluripotent stem cell sources.
- Floor plate
-
A transient developmental structure along the midline of the embryo that is important for brain development.
- Striatal neurons
-
Neurons that lie in the striatum, which is an area of the brain that is involved in fine movements, emotion and cognition.
- Globus pallidus
-
A subcortical structure of the brain that is a major element of the basal ganglia system.
- Oligodendrocytes
-
One of the three main cell types that make up the brain parenchyma, the other two being neurons and astrocytes. They produce myelin, which insulates axons to alter the conduction properties of neurons.
- Self-organizing
-
Pertaining to an intrinsic programme in pluripotent stem cell-derived lineages that enables cells in vitro to assemble into tissue-like and organoid structures.
- Transcription activator-like effector nucleases
-
(TALENs). Fusions of truncated TALEs to a nonspecific DNA-cleavage domain of the FokI endonuclease. Each TALE contains an amino terminus, a custom-designed DNA-binding domain and a carboxyl terminus with the activation domain being removed.
- Positron emission tomography
-
(PET). An imaging technique that detects the emission of positrons from the brain after a small amount of radioactive isotopes have been injected into the blood stream; it is used to quantitatively measure metabolic, biochemical and functional activity in living tissues.
- Good manufacturing practice
-
(GMP). A set of standardized production and testing conditions that are required for developing a clinical-grade cell product and for obtaining regulatory approval for trials in human subjects.
Rights and permissions
About this article
Cite this article
Tabar, V., Studer, L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 15, 82–92 (2014). https://doi.org/10.1038/nrg3563
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3563
This article is cited by
-
An in vitro culture platform for studying the effect of collective cell migration on spatial self-organization within induced pluripotent stem cell colonies
Journal of Biological Engineering (2023)
-
SUPT4H1-edited stem cell therapy rescues neuronal dysfunction in a mouse model for Huntington’s disease
npj Regenerative Medicine (2022)
-
Functional human cell-based vascularised cardiac tissue model for biomedical research and testing
Scientific Reports (2022)
-
Spatial profiling of early primate gastrulation in utero
Nature (2022)
-
Effects of Magnetite Nanoparticles and Static Magnetic Field on Neural Differentiation of Pluripotent Stem Cells
Stem Cell Reviews and Reports (2022)