Key Points
-
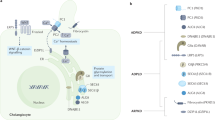
Proteins encoded by the genes that cause polycystic liver diseases are predominantly localized in the primary cilium, plasma membrane and/or the endoplasmic reticulum of cholangiocytes
-
Current treatments are based on surgical procedures and/or pharmacological management; however, their beneficial effects are modest, leaving liver transplantation as the only definitive remedy
-
Elucidating the molecular mechanisms involved in the pathogenesis of these disorders is crucial in order to identify new potential targets for therapy
-
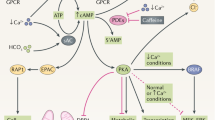
Hepatic cystogenesis is characterized by ductal plate malformation, abnormalities of the cholangiocyte primary cilium, centrosome amplification, hyperproliferation, hypersecretion, matrix-metalloprotease hyperactivity, angiogenesis, epigenetic alterations and atypical levels of key intracellular mediators
-
Preclinical studies have revealed new potential therapeutic targets that need to be validated in future clinical trials
Abstract
Polycystic liver diseases are genetic disorders characterized by progressive bile duct dilatation and/or cyst development. The large volume of hepatic cysts causes different symptoms and complications such as abdominal distension, local pressure with back pain, hypertension, gastro-oesophageal reflux and dyspnea as well as bleeding, infection and rupture of the cysts. Current therapeutic strategies are based on surgical procedures and pharmacological management, which partially prevent or ameliorate the disease. However, as these treatments only show short-term and/or modest beneficial effects, liver transplantation is the only definitive therapy. Therefore, interest in understanding the molecular mechanisms involved in disease pathogenesis is increasing so that new targets for therapy can be identified. In this Review, the genetic mechanisms underlying polycystic liver diseases and the most relevant molecular pathways of hepatic cystogenesis are discussed. Moreover, the main clinical and preclinical studies are highlighted and future directions in basic as well as clinical research are indicated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gevers, T. J. & Drenth, J. P. Diagnosis and management of polycystic liver disease. Nat. Rev. Gastroenterol. Hepatol. 10, 101–108 (2013).
Drenth, J. P., te Morsche, R. H., Smink, R., Bonifacino, J. S. & Jansen, J. B. Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat. Genet. 33, 345–347 (2003).
Li, A. et al. Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am. J. Hum. Genet. 72, 691–703 (2003).
Davila, S. et al. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat. Genet. 36, 575–577 (2004).
Cnossen, W. R. et al. Whole-exome sequencing reveals LRP5 mutations and canonical Wnt signaling associated with hepatic cystogenesis. Proc. Natl Acad. Sci. USA 111, 5343–5348 (2014).
[No authors listed] The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. The European Polycystic Kidney Disease Consortium. Cell 77, 881–894 (1994).
Mochizuki, T. et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 (1996).
Ward, C. J. et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat. Genet. 30, 259–269 (2002).
Strazzabosco, M. & Somlo, S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology 140, 1855–1859 (2011).
Rossetti, S. et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 18, 2143–2160 (2007).
Waanders, E., te Morsche, R. H., de Man, R. A., Jansen, J. B. & Drenth, J. P. Extensive mutational analysis of PRKCSH and SEC63 broadens the spectrum of polycystic liver disease. Hum. Mutat. 27, 830 (2006).
Waanders, E. et al. Secondary and tertiary structure modeling reveals effects of novel mutations in polycystic liver disease genes PRKCSH and SEC63. Clin. Genet. 78, 47–56 (2010).
Ong, A. C. & Harris, P. C. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 67, 1234–1247 (2005).
Geng, L. et al. Syntaxin 5 regulates the endoplasmic reticulum channel-release properties of polycystin-2. Proc. Natl Acad. Sci. USA 105, 15920–15925 (2008).
Al-Bhalal, L. & Akhtar, M. Molecular basis of autosomal recessive polycystic kidney disease (ARPKD). Adv. Anat. Pathol. 15, 54–58 (2008).
Janssen, M. J., Salomon, J., te Morsche, R. H. & Drenth, J. P. Loss of heterozygosity is present in SEC63 germline carriers with polycystic liver disease. PLoS ONE 7, e50324 (2012).
Janssen, M. J. et al. Secondary, somatic mutations might promote cyst formation in patients with autosomal dominant polycystic liver disease. Gastroenterology 141, 2056–2063 (2011).
Watnick, T. J. et al. Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease. Mol. Cell 2, 247–251 (1998).
Pei, Y. et al. Somatic PKD2 mutations in individual kidney and liver cysts support a “two-hit” model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 10, 1524–1529 (1999).
Watnick, T. et al. Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat. Genet. 25, 143–144 (2000).
Banales, J. M., Munoz-Garrido, P. & Bujanda, L. Somatic second-hit mutations leads to polycystic liver diseases. World J. Gastroenterol. 19, 141–143 (2013).
Chandok, N. Polycystic liver disease: a clinical review. Ann. Hepatol. 11, 819–826 (2012).
Raynaud, P., Carpentier, R., Antoniou, A. & Lemaigre, F. P. Biliary differentiation and bile duct morphogenesis in development and disease. Int. J. Biochem. Cell Biol. 43, 245–256 (2011).
Wills, E. S., Roepman, R. & Drenth, J. P. Polycystic liver disease: ductal plate malformation and the primary cilium. Trends Mol. Med. 20, 261–270 (2014).
Temmerman, F. et al. Systematic review: the pathophysiology and management of polycystic liver disease. Aliment. Pharmacol. Ther. 34, 702–713 (2011).
Gunay-Aygun, M. Liver and kidney disease in ciliopathies. Am. J. Med. Genet. C. Semin. Med. Genet. 151C, 296–306 (2009).
Godlewski, G., Gaubert-Cristol, R., Rouy, S. & Prudhomme, M. Liver development in the rat and in man during the embryonic period (Carnegie stages 11–23). Microsc. Res. Tech. 39, 314–327 (1997).
Carpentier, R. et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 141, 1432–1438 (2011).
Desmet, V. J. Ludwig symposium on biliary disorders—part I. Pathogenesis of ductal plate abnormalities. Mayo Clin. Proc. 73, 80–89 (1998).
Raynaud, P. et al. A classification of ductal plate malformations based on distinct pathogenic mechanisms of biliary dysmorphogenesis. Hepatology 53, 1959–1966 (2011).
Crawford, J. M. Development of the intrahepatic biliary tree. Semin. Liver Dis. 22, 213–226 (2002).
Ezratty, E. J. et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145, 1129–1141 (2011).
Lopes, S. S. et al. Notch signalling regulates left-right asymmetry through ciliary length control. Development 137, 3625–3632 (2010).
Shih, H. P. et al. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 139, 2488–2499 (2012).
Sato, Y. et al. Cholangiocytes with mesenchymal features contribute to progressive hepatic fibrosis of the polycystic kidney rat. Am. J. Pathol. 171, 1859–1871 (2007).
Hassane, S. et al. Elevated TGFβ-Smad signalling in experimental Pkd1 models and human patients with polycystic kidney disease. J. Pathol. 222, 21–31 (2010).
Decaens, T. et al. Stabilization of β-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology 47, 247–258 (2008).
May-Simera, H. L. & Kelley, M. W. Cilia, Wnt signaling, and the cytoskeleton. Cilia 1, 7 (2012).
Benzing, T., Simons, M. & Walz, G. Wnt signaling in polycystic kidney disease. J. Am. Soc. Nephrol. 18, 1389–1398 (2007).
Yanai, M. et al. FGF signaling segregates biliary cell-lineage from chick hepatoblasts cooperatively with BMP4 and ECM components in vitro. Dev. Dyn. 237, 1268–1283 (2008).
Neugebauer, J. M., Amack, J. D., Peterson, A. G., Bisgrove, B. W. & Yost, H. J. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458, 651–654 (2009).
Pavik, I. et al. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 79, 234–240 (2011).
Clotman, F. et al. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development 129, 1819–1828 (2002).
Hunter, M. P. et al. The homeobox gene Hhex is essential for proper hepatoblast differentiation and bile duct morphogenesis. Dev. Biol. 308, 355–367 (2007).
Pierreux, C. E. et al. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology 130, 532–541 (2006).
Guay-Woodford, L. M., Green, W. J., Lindsey, J. R. & Beier, D. R. Germline and somatic loss of function of the mouse cpk gene causes biliary ductal pathology that is genetically modulated. Hum. Mol. Genet. 9, 769–778 (2000).
Hou, X. et al. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J. Clin. Invest. 109, 533–540 (2002).
Hiesberger, T. et al. Mutation of hepatocyte nuclear factor-1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J. Clin. Invest. 113, 814–825 (2004).
Gresh, L. et al. A transcriptional network in polycystic kidney disease. EMBO J. 23, 1657–1668 (2004).
Yamasaki, H. et al. Suppression of C/EBPα expression in periportal hepatoblasts may stimulate biliary cell differentiation through increased Hnf6 and Hnf1b expression. Development 133, 4233–4243 (2006).
Hand, N. J. et al. The microRNA-30 family is required for vertebrate hepatobiliary development. Gastroenterology 136, 1081–1090 (2009).
Masyuk, A. I., Masyuk, T. V. & LaRusso, N. F. Cholangiocyte primary cilia in liver health and disease. Dev. Dyn. 237, 2007–2012 (2008).
Masyuk, A. I. et al. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131, 911–920 (2006).
Masyuk, T. V. et al. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology 125, 1303–1310 (2003).
Masyuk, T. V. et al. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am. J. Pathol. 165, 1719–1730 (2004).
Stroope, A. et al. Hepato-renal pathology in Pkd2ws25/− mice, an animal model of autosomal dominant polycystic kidney disease. Am. J. Pathol. 176, 1282–1291 (2010).
Alvaro, D. & Mancino, M. G. New insights on the molecular and cell biology of human cholangiopathies. Mol. Aspects Med. 29, 50–57 (2008).
Masyuk, T. V. et al. Centrosomal abnormalities characterize human and rodent cystic cholangiocytes and are associated with Cdc25A overexpression. Am. J. Pathol. 184, 110–121 (2014).
Battini, L. et al. Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum. Mol. Genet. 17, 2819–2833 (2008).
Woollard, J. R. et al. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int. 72, 328–336 (2007).
Torres, V. E. & Harris, P. C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J. Am. Soc. Nephrol. 25, 18–32 (2014).
Torrice, A. et al. Polycystins play a key role in the modulation of cholangiocyte proliferation. Dig. Liver Dis. 42, 377–385 (2010).
Masyuk, A. I. et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1013–G1024 (2013).
Gradilone, S. A. et al. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc. Natl Acad. Sci. USA 104, 19138–19143 (2007).
Gradilone, S. A. et al. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology 139, 304–314 (2010).
Onori, P. et al. Polycystic liver diseases. Dig. Liver Dis. 42, 261–271 (2010).
Muchatuta, M. N., Gattone, V. H. 2nd, Witzmann, F. A. & Blazer-Yost, B. L. Structural and functional analyses of liver cysts from the BALB/c-cpk mouse model of polycystic kidney disease. Exp. Biol. Med. (Maywood) 234, 17–27 (2009).
Banales, J. M. et al. Hepatic cystogenesis is associated with abnormal expression and location of ion transporters and water channels in an animal model of autosomal recessive polycystic kidney disease. Am. J. Pathol. 173, 1637–1646 (2008).
Fedeles, S. V. et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet. 43, 639–647 (2011).
Fedeles, S. V., Gallagher, A. R. & Somlo, S. Polycystin-1: a master regulator of intersecting cystic pathways. Trends Mol. Med. 20, 251–260 (2014).
Ma, M., Tian, X., Igarashi, P., Pazour, G. J. & Somlo, S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet. 45, 1004–1012 (2013).
Alvaro, D. et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am. J. Pathol. 172, 321–332 (2008).
Spirli, C. et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin 2 defective mice. Hepatology 51, 1778–1788 (2010).
Spirli, C. et al. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology 138, 360–371 (2010).
Masyuk, T. V. et al. Inhibition of Cdc25A suppresses hepato-renal cystogenesis in rodent models of polycystic kidney and liver disease. Gastroenterology 142, 622–633 (2012).
Waanders, E., Van Krieken, J. H., Lameris, A. L. & Drenth, J. P. Disrupted cell adhesion but not proliferation mediates cyst formation in polycystic liver disease. Mod. Pathol. 21, 1293–1302 (2008).
Nichols, M. T. et al. Secretion of cytokines and growth factors into autosomal dominant polycystic kidney disease liver cyst fluid. Hepatology 40, 836–846 (2004).
Fabris, L. et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 43, 1001–1012 (2006).
Amura, C. R. et al. CXCR2 agonists in ADPKD liver cyst fluids promote cell proliferation. Am. J. Physiol. Cell Physiol. 294, C786–C796 (2008).
Sato, Y. et al. Activation of the MEK5/ERK5 cascade is responsible for biliary dysgenesis in a rat model of Caroli's disease. Am. J. Pathol. 166, 49–60 (2005).
Torres, V. E. et al. Epidermal growth factor receptor tyrosine kinase inhibition is not protective in PCK rats. Kidney Int. 66, 1766–1773 (2004).
Amura, C. R. et al. VEGF receptor inhibition blocks liver cyst growth in pkd2WS25/− mice. Am. J. Physiol. Cell Physiol. 293, C419–C428 (2007).
Brodsky, K. S., McWilliams, R. R., Amura, C. R., Barry, N. P. & Doctor, R. B. Liver cyst cytokines promote endothelial cell proliferation and development. Exp. Biol. Med. (Maywood) 234, 1155–1165 (2009).
Renken, C., Fischer, D. C., Kundt, G., Gretz, N. & Haffner, D. Inhibition of mTOR with sirolimus does not attenuate progression of liver and kidney disease in PCK rats. Nephrol. Dial. Transplant 26, 92–100 (2011).
Qian, Q. et al. Sirolimus reduces polycystic liver volume in ADPKD patients. J. Am. Soc. Nephrol. 19, 631–638 (2008).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Chrispijn, M. et al. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. J. Hepatol. 59, 153–159 (2013).
Spirli, C. et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology 56, 2363–2374 (2012).
Yoshihara, D. et al. PPAR-γ agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am. J. Physiol. Renal Physiol. 300, F465–F474 (2011).
Yoshihara, D. et al. Global gene expression profiling in PPAR-γ agonist-treated kidneys in an orthologous rat model of human autosomal recessive polycystic kidney disease. PPAR Res. 2012, 695898 (2012).
Yoshihara, D. et al. Telmisartan ameliorates fibrocystic liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. PLoS ONE 8, e81480 (2013).
Chapman, A. B. Cystic disease in women: clinical characteristics and medical management. Adv. Ren. Replace. Ther. 10, 24–30 (2003).
Gabow, P. A. et al. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 11, 1033–1037 (1990).
Sherstha, R. et al. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 26, 1282–1286 (1997).
Alvaro, D. et al. Estrogens and the pathophysiology of the biliary tree. World J. Gastroenterol. 12, 3537–3545 (2006).
Masyuk, T. V., Masyuk, A. I., Torres, V. E., Harris, P. C. & Larusso, N. F. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′, 5′-cyclic monophosphate. Gastroenterology 132, 1104–1116 (2007).
Banales, J. M. et al. The cAMP effectors Epac and protein kinase A (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49, 160–174 (2009).
Masyuk, T. V. et al. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology 58, 409–421 (2013).
Caroli, A. et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin. J. Am. Soc. Nephrol. 5, 783–789 (2010).
Hogan, M. C. et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J. Am. Soc. Nephrol. 21, 1052–1061 (2010).
Hogan, M. C. et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol. Dial. Transplant 27, 3532–3539 (2012).
van Keimpema, L. et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 137, 1661–1668 (2009).
Chrispijn, M. et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment. Pharmacol. Ther. 35, 266–274 (2012).
Temmerman, F. et al. Safety and efficacy of different lanreotide doses in the treatment of polycystic liver disease: pooled analysis of individual patient data. Aliment. Pharmacol. Ther. 38, 397–406 (2013).
Gevers, T. J. et al. Young women with polycystic liver disease respond best to somatostatin analogues: a pooled analysis of individual patient data. Gastroenterology 145, 357–365 (2013).
US National Library of Medicine. ClincalTrials.gov [online], (2012).
Spirli, C. et al. Altered store operated calcium entry increases cyclic 3′, 5′-adenosine monophosphate production and extracellular signal-regulated kinases 1 and 2 phosphorylation in polycystin 2 defective cholangiocytes. Hepatology 55, 856–868 (2012).
Banales, J. M., Prieto, J. & Medina, J. F. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J. Gastroenterol. 12, 3496–3511 (2006).
Banales, J. M. et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology 43, 266–275 (2006).
Tietz, P. S. et al. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J. Biol. Chem. 278, 20413–20419 (2003).
Perrone, R. D. et al. Autosomal dominant polycystic kidney disease decreases anion exchanger activity. Am. J. Physiol. 272, C1748–C1756 (1997).
Wang, X. et al. Insignificant effect of secretin in rodent models of polycystic kidney and liver disease. Am. J. Physiol. Renal. Physiol. 303, F1089–F1098 (2012).
Urribarri, A. D. et al. Inhibition of metalloprotease hyperactivity in cystic cholangiocytes halts the development of polycystic liver diseases. Gut http://dx.doi.org/10.1136/gutjnl-2013-305281.
Yasoshima, M. et al. Matrix proteins of basement membrane of intrahepatic bile ducts are degraded in congenital hepatic fibrosis and Caroli's disease. J. Pathol. 217, 442–451 (2009).
Nemunaitis, J. et al. Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: selection of a biologically active and tolerable dose for longer-term studies. Clin. Cancer Res. 4, 1101–1109 (1998).
Rosenbaum, E. et al. Marimastat in the treatment of patients with biochemically relapsed prostate cancer: a prospective randomized, double-blind, phase I/II trial. Clin. Cancer Res. 11, 4437–4443 (2005).
Lee, S. O. et al. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J. Clin. Invest. 118, 3714–3724 (2008).
Gradilone, S. A. et al. HDAC6 is overexpressed in cystic cholangiocytes and its inhibition reduces cystogenesis. Am. J. Pathol. 184, 600–608 (2014).
Gevers, T. J. & Drenth, J. P. Somatostatin analogues for treatment of polycystic liver disease. Curr. Opin. Gastroenterol. 27, 294–300 (2011).
Takiar, V. & Caplan, M. J. Polycystic kidney disease: pathogenesis and potential therapies. Biochim. Biophys. Acta 1812, 1337–1343 (2011).
Abu-Wasel, B., Walsh, C., Keough, V. & Molinari, M. Pathophysiology, epidemiology, classification and treatment options for polycystic liver diseases. World J. Gastroenterol. 19, 5775–5786 (2013).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Perugorria, M., Masyuk, T., Marin, J. et al. Polycystic liver diseases: advanced insights into the molecular mechanisms. Nat Rev Gastroenterol Hepatol 11, 750–761 (2014). https://doi.org/10.1038/nrgastro.2014.155
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.155
This article is cited by
-
Protocol for a randomized controlled multicenter trial assessing the efficacy of leuprorelin for severe polycystic liver disease: the AGAINST-PLD study
BMC Gastroenterology (2022)
-
Genetics, pathobiology and therapeutic opportunities of polycystic liver disease
Nature Reviews Gastroenterology & Hepatology (2022)
-
Imaging of fibropolycystic liver disease
Abdominal Radiology (2022)
-
Polycystic liver disease with lethal abdominal wall rupture: a case report
Journal of Medical Case Reports (2021)
-
Novel GANAB variants associated with polycystic liver disease
Orphanet Journal of Rare Diseases (2020)