Key Points
-
Monocytes are progenitors of both tissue macrophage and dendritic-cell subsets.
-
Two classes of circulating monocytes have been identified; these have different migration properties and chemokine-receptor expression patterns.
-
The trafficking of monocytes into tissues requires the orchestrated activation of integrins by specific chemokines.
-
Signal transduction induced by chemokines targets cytosolic integrin-activating proteins, such as RHOA, RAP1, talin or PYK2, which results in cell adhesion to the blood-vessel wall.
-
Transendothelial migration of adherent monocytes requires polarization of the adherent monocytes and remodelling of the endothelial-cell junctions.
-
The trafficking of monocytes to their final tissue depends on chemotactic gradients and specific expression of adhesion molecules, which are regulated by inflammation.
Abstract
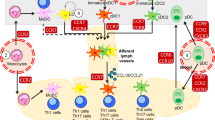
Because of their phagocytic activity and their ability to differentiate into antigen-presenting cells, monocytes participate in both innate and adaptive immune responses. They derive from bone-marrow progenitor cells, circulate in the blood as monocytes and differentiate into tissue macrophages or myeloid dendritic cells in the periphery. After activation by an antigenic challenge in the tissues, they can contribute to the local resolution of the injury or can migrate farther to secondary lymphoid organs. Recruitment of these cells from the blood to the tissue and from the tissue to the lymph nodes requires orchestrated adhesive interactions between leukocytes and the vascular or lymphatic endothelium. Here, we discuss the signals by which chemokines regulate the leukocyte-adhesion molecules that are essential for transendothelial migration, and we describe the routes taken by monocytes and myeloid dendritic cells to reach their final destination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nature Rev. Immunol. 3, 867–878 (2003).
van Furth, R. & Cohn, Z. A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Steinman, R. M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9, 271–296 (1991).
Warren, M. K. & Vogel, S. N. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J. Immunol. 134, 982–989 (1985).
Zhou, L. J. & Tedder, T. F. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl Acad. Sci. USA 93, 2588–2592 (1996).
del Hoyo, G. M. et al. Characterization of a common precursor population for dendritic cells. Nature 415, 1043–1047 (2002).
Randolph, G. J., Inaba, K., Robbiani, D. F., Steinman, R. M. & Muller, W. A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 (1999). The first demonstration that monocytes recruited into tissues by latex beads can migrate to draining lymph nodes and differentiate into DCs. This population differs from Langerhans cells, which are activated and recruited to the draining lymph nodes after the epicutaneous application of contact sensitizers.
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003). Reports the identification of two mouse monocyte subsets, which are based on the cell-surface expression of CX 3 CR1 and Gr1. These subsets are comparable to the two human monocyte subpopulations: CD14hiCD16low and CD14low CD16hi. Using adoptive transfer experiments, the CX 3 CR1lowGr1+ subset of monocytes were shown to migrate preferentially to inflamed tissues.
Ancuta, P. et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 197, 1701–1707 (2003).
Ingulli, E., Ulman, D. R., Lucido, M. M. & Jenkins, M. K. In situ analysis reveals physical interactions between CD11b+ dendritic cells and antigen-specific CD4 T cells after subcutaneous injection of antigen. J. Immunol. 169, 2247–2252 (2002).
Palframan, R. T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Janatpour, M. J., Hudak, S., Sathe, M., Sedgwick, J. D. & McEvoy, L. M. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J. Exp. Med. 194, 1375–1384 (2001).
Kim, M., Carman, C. V. & Springer, T. A. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720–1725 (2003).
Spillmann, D., Witt, D. & Lindahl, U. Defining the interleukin-8-binding domain of heparan sulfate. J. Biol. Chem. 273, 15487–15493 (1998).
Halden, Y. et al. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem. J. 377, 533–538 (2004).
Tanaka, Y. et al. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature 361, 79–82 (1993). Describes the concept that soluble chemokines have two sites of interaction: one that immobilizes them on vascular proteoglycans and another that reacts with chemokine receptors on rolling leukocytes.
Roscic-Mrkic, B. et al. RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood 102, 1169–1177 (2003).
Proudfoot, A. E., Power, C. A. & Wells, T. N. The strategy of blocking the chemokine system to combat disease. Immunol. Rev. 177, 246–256 (2000).
Thelen, M. Dancing to the tune of chemokines. Nature Immunol. 2, 129–134 (2001).
Katagiri, K. et al. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 20, 1956–1969 (2000).
Reedquist, K. A. et al. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell Biol. 148, 1151–1158 (2000).
Bos, J. L., de Rooij, J. & Reedquist, K. A. Rap1 signalling: adhering to new models. Nature Rev. Mol. Cell Biol. 2, 369–377 (2001).
Caron, E., Self, A. J. & Hall, A. The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Curr. Biol. 10, 974–978 (2000).
McLeod, S. J., Shum, A. J., Lee, R. L., Takei, F. & Gold, M. R. The Rap GTPases regulate integrin-mediated adhesion, cell spreading, actin polymerization, and Pyk2 tyrosine phosphorylation in B lymphocytes. J. Biol. Chem. 279, 12009–12019 (2004).
Bos, J. L. Epac: a new cAMP target and new avenues in cAMP research. Nature Rev. Mol. Cell Biol. 4, 733–738 (2003).
Shimonaka, M. et al. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J. Cell Biol. 161, 417–427 (2003). This key paper shows that chemokines induce shear-force-resistant integrin activation by signal transduction through RAP1. Most importantly, it shows that RAP1 activity polarizes the leukocyte-migration machinery and induces transendothelial migration of leukocytes.
Imhof, B. A. & Dunon, D. Leukocyte migration and adhesion. Adv. Immunol. 58, 345–416 (1995).
Tohyama, Y. et al. The critical cytoplasmic regions of the αLβ2 integrin in Rap1-induced adhesion and migration. Mol. Biol. Cell 14, 2570–2582 (2003).
Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nature Immunol. 4, 741–748 (2003). Demonstrates that the RAP1–RAPL interaction leads to polarized integrin activation in the lamellipodium, thereby enabling cell migration.
Tadokoro, S. et al. Talin binding to integrin-β tails: a final common step in integrin activation. Science 302, 103–106 (2003). Shows that the binding of talin to the β-chains of integrins activates them by enhancing their affinity. Talin binding is therefore a final step in the signalling cascades that control integrin activation.
Calderwood, D. A. Integrin activation. J. Cell Sci. 117, 657–666 (2004).
Di Paolo, G. et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature 420, 85–89 (2002).
Ling, K., Doughman, R. L., Firestone, A. J., Bunce, M. W. & Anderson, R. A. Type I γ-phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420, 89–93 (2002).
Martel, V. et al. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 276, 21217–21227 (2001).
Datta, A., Huber, F. & Boettiger, D. Phosphorylation of β3 integrin controls ligand binding strength. J. Biol. Chem. 277, 3943–3949 (2002).
Sakai, T., Jove, R., Fassler, R. & Mosher, D. F. Role of the cytoplasmic tyrosines of β1A integrins in transformation by v-src. Proc. Natl Acad. Sci. USA 98, 3808–3813 (2001).
Alblas, J., Ulfman, L., Hordijk, P. & Koenderman, L. Activation of RhoA and ROCK are essential for detachment of migrating leukocytes. Mol. Biol. Cell 12, 2137–2145 (2001).
Ridley, A. J. & Hall, A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 13, 2600–2610 (1994).
Laudanna, C., Kim, J. Y., Constantin, G. & Butcher, E. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 186, 37–46 (2002).
Worthylake, R. A. & Burridge, K. RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 278, 13578–13584 (2003).
Smith, A., Bracke, M., Leitinger, B., Porter, J. C. & Hogg, N. LFA-1-induced T-cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J. Cell Sci. 116, 3123–3133 (2003).
Giagulli, C. et al. RhoA and ζ-PKC control distinct modalities of LFA-1 activation by chemokines. Critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity 20, 25–35 (2004). Examines three different functional domains of the GTPase RHOA. It shows that RHOA increases the affinity of integrins for their ligands through a signal-transduction pathway involving PI3K. It also increases the mobility and polarization of activated integrins by signalling involving the atypical protein kinase, PKC-ζ.
Fujisawa, K. et al. Different regions of Rho determine Rho-selective binding of different classes of Rho target molecules. J. Biol. Chem. 273, 18943–18949 (1998).
Aspenstrom, P. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11, 95–102 (1999).
Reid, T. et al. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the Rho-binding domain. J. Biol. Chem. 271, 13556–13560 (1996).
Wang, H. R. et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 (2003).
Etienne-Manneville, S. & Hall, A. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421, 753–756 (2003).
Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J. V. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nature Immunol. 1, 31–36 (2000). An in vivo proof that chemokines induce Pyk2-mediated cell adhesion and migration, which is also shown to be crucial for the positioning of immune cells in lymphoid organs. Pyk2 deficiency was found to mainly affect the humoral immune responses of mice.
Avraham, H., Park, S. Y., Schinkmann, K. & Avraham, S. RAFTK/Pyk2-mediated cellular signalling. Cell. Signal. 12, 123–133 (2000).
Gismondi, A. et al. Proline-rich tyrosine kinase-2 activation by β1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J. Immunol. 159, 4729–4736 (1997).
Okigaki, M. et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl Acad. Sci. USA 100, 10740–10745 (2003).
Owen, J. D., Ruest, P. J., Fry, D. W. & Hanks, S. K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19, 4806–4818 (1999).
Wehrle-Haller, B. & Imhof, B. The inner lives of focal adhesions. Trends Cell Biol. 12, 382–389 (2002).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Sanchez-Madrid, F. & del Pozo, M. A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 18, 501–511 (1999).
Imhof, B. A. et al. Cross talk between αvβ3 and α4β1 integrins regulates lymphocyte migration on vascular cell adhesion molecule 1. Eur. J. Immunol. 27, 3242–3252 (1997).
Plant, P. J. et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nature Cell Biol. 5, 301–308 (2003). The downstream effectors of signal transduction cascades that lead to cell migration are called polarity complexes. This paper indicates that the protein complex PAR6, atypical PKC and LGL are involved in the delivery of new lipid membrane to zones of lamellipodium propulsion.
Hughes, P. E. et al. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Biol. Chem. 271, 6571–6574 (1996).
Johnson-Leger, C. & Imhof, B. A. Forging the endothelium during inflammation: pushing at a half-open door? Cell Tissue Res. 314, 93–105 (2003).
Feng, D., Nagy, J. A., Pyne, K., Dvorak, H. F. & Dvorak, A. M. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J. Exp. Med. 187, 903–915 (1998).
Johnson-Leger, C., Aurrand-Lions, M. & Imhof, B. A. The parting of the endothelium: miracle, or simply a junctional affair? J. Cell Sci. 113, 921–933 (2000).
Luscinskas, F. W., Ma, S., Nusrat, A., Parkos, C. A. & Shaw, S. K. Leukocyte transendothelial migration: a junctional affair. Semin. Immunol. 14, 105–113 (2002).
Tsukita, S., Furuse, M. & Itoh, M. Multifunctional strands in tight junctions. Nature Rev. Mol. Cell Biol. 2, 285–293 (2001).
Ebnet, K., Suzuki, A., Ohno, S. & Vestweber, D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 117, 19–29 (2004).
Bazzoni, G. The JAM family of junctional adhesion molecules. Curr. Opin. Cell Biol. 15, 525–530 (2003).
Martin-Padura, I. et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142, 117–127 (1998).
Malergue, F. et al. A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol. Immunol. 35, 1111–1119 (1998).
Ostermann, G., Weber, K. S., Zernecke, A., Schroder, A. & Weber, C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nature Immunol. 3, 151–158 (2002).
Bazzoni, G. et al. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 275, 20520–20526 (2000).
Martinez-Estrada, O. M. et al. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial Caco-2 cells. J. Biol. Chem. 276, 9291–9296 (2001).
Ebnet, K., Schulz, C. U., Meyer zu Brickwedde, M. -K., Pendl, G. G. & Vestweber, D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275, 27979–27988 (2000).
Ebnet, K. et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J. 20, 3738–3748 (2001).
Ozaki, H. et al. Cutting edge: Combined treatment of TNF-α and IFN-γ causes redistribution of junctional adhesion molecule in human endothelial cells. J. Immunol. 163, 553–557 (1999).
Shaw, S. K. et al. Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-α plus interferon-γ does not reduce leukocyte transmigration under flow. Am. J. Pathol. 159, 2281–2291 (2001).
Aurrand-Lions, M. A., Duncan, L., Ballestrem, C. & Imhof, B. A. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem. 276, 2733–2741 (2001).
Cunningham, S. A. et al. A novel protein with homology to the junctional adhesion molecule. Characterization of leukocyte interactions. J. Biol. Chem. 275, 34750–34756 (2000).
Arrate, M. P., Rodriguez, J. M., Tran, T. M., Brock, T. A. & Cunningham, S. A. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J. Biol. Chem. 276, 45826–45832 (2001).
Palmeri, D., van Zante, A., Huang, C. C., Hemmerich, S. & Rosen, S. D. Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J. Biol. Chem. 275, 19139–19145 (2000).
Santoso, S. et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 196, 679–691 (2002).
Cunningham, S. A., Rodriguez, J. M., Arrate, M. P., Tran, T. M. & Brock, T. A. JAM2 interacts with α4β1. Facilitation by JAM3. J. Biol. Chem. 277, 27589–27592 (2002).
Johnson-Leger, C. A., Aurrand-Lions, M., Beltraminelli, N., Fasel, N. & Imhof, B. A. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood 100, 2479–2486 (2002).
Yagi, T. & Takeichi, M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 14, 1169–1180 (2000).
Huelsken, J. & Behrens, J. The Wnt signalling pathway. J. Cell Sci. 115, 3977–3978 (2002).
Lampugnani, M. G. et al. A novel endothelial-specific membrane protein is a marker of cell–cell contacts. J. Cell Biol. 118, 1511–1522 (1992).
Carmeliet, P. et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98, 147–157 (1999).
Allport, J. R., Muller, W. A. & Luscinskas, F. W. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J. Cell Biol. 148, 203–216 (2000).
Shaw, S. K., Bamba, P. S., Perkins, B. N. & Luscinskas, F. W. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 167, 2323–2330 (2001).
Mamdouh, Z., Chen, X., Pierini, L. M., Maxfield, F. R. & Muller, W. A. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature 421, 748–753 (2003).
Wong, C. W. et al. PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin αvβ3 in cis. Mol. Biol. Cell 11, 3109–3121 (2000).
Muller, W. A. Leukocyte–endothelial cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24, 327–334 (2003).
Duncan, G. S. et al. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J. Immunol. 162, 3022–3030 (1999).
Dangerfield, J., Larbi, K. Y., Huang, M. T., Dewar, A. & Nourshargh, S. PECAM-1 (CD31) homophilic interaction up-regulates α6β1 on transmigrated neutrophils in vivo and plays a functional role in the ability of α6 integrins to mediate leukocyte migration through the perivascular basement membrane. J. Exp. Med. 196, 1201–1211 (2002).
Schenkel, A. R., Mamdouh, Z., Chen, X., Liebman, R. M. & Muller, W. A. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nature Immunol. 3, 143–150 (2002).
Aurrand-Lions, M., Johnson-Leger, C. & Imhof, B. A. The last molecular fortress in leukocyte trans-endothelial migration. Nature Immunol. 3, 116–118 (2002).
Beyer, E. C. Gap junctions. Int. Rev. Cytol. 137C, 1–37 (1993).
Van Rijen, H. et al. Gap junctions in human umbilical cord endothelial cells contain multiple connexins. Am. J. Physiol. 272, C117–C130 (1997).
van Rijen, H. V., van Kempen, M. J., Postma, S. & Jongsma, H. J. Tumour necrosis factor-α alters the expression of connexin43, connexin40, and connexin37 in human umbilical vein endothelial cells. Cytokine 10, 258–264 (1998).
Eugenin, E. A., Branes, M. C., Berman, J. W. & Saez, J. C. TNF-α plus IFN-γ induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J. Immunol. 170, 1320–1328 (2003).
Jara, P. I., Boric, M. P. & Saez, J. C. Leukocytes express connexin 43 after activation with lipopolysaccharide and appear to form gap junctions with endothelial cells after ischemia-reperfusion. Proc. Natl Acad. Sci. USA 92, 7011–7015 (1995).
Oviedo-Orta, E., Errington, R. J. & Evans, W. H. Gap junction intercellular communication during lymphocyte transendothelial migration. Cell Biol. Int. 26, 253–263 (2002).
Zahler, S., Hoffmann, A., Gloe, T. & Pohl, U. Gap-junctional coupling between neutrophils and endothelial cells: a novel modulator of transendothelial migration. J. Leukoc. Biol. 73, 118–126 (2003).
Kurth, I. et al. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J. Exp. Med. 194, 855–861 (2001).
Yang, D., Biragyn, A., Kwak, L. W. & Oppenheim, J. J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23, 291–296 (2002).
Yang, D. et al. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J. Leukoc. Biol. 74, 448–455 (2003).
Hume, D. A., Robinson, A. P., MacPherson, G. G. & Gordon, S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J. Exp. Med. 158, 1522–1536 (1983).
Witmer-Pack, M. D. et al. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J. Cell Sci. 104, 1021–1029 (1993).
Takahashi, K., Naito, M., Shultz, L. D., Hayashi, S. & Nishikawa, S. Differentiation of dendritic cell populations in macrophage colony-stimulating factor-deficient mice homozygous for the osteopetrosis (op) mutation. J. Leukoc. Biol. 53, 19–28 (1993).
Kennedy, D. W. & Abkowitz, J. L. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood 90, 986–993 (1997).
Hochrein, H. et al. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J. Immunol. 166, 5448–5455 (2001).
Holt, P. G., Haining, S., Nelson, D. J. & Sedgwick, J. D. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 153, 256–261 (1994).
Vermaelen, K. Y., Carro-Muino, I., Lambrecht, B. N. & Pauwels, R. A. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193, 51–60 (2001).
Schneeberger, E. E., Vu, Q., LeBlanc, B. W. & Doerschuk, C. M. The accumulation of dendritic cells in the lung is impaired in CD18−/− but not in ICAM-1−/− mutant mice. J. Immunol. 164, 2472–2478 (2000).
De Smedt, T. et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184, 1413–1424 (1996).
Huang, F. P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Scheinecker, C., McHugh, R., Shevach, E. M. & Germain, R. N. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196, 1079–1090 (2002). Provides evidence that constitutive presentation of one self-antigen is mediated by DCs under non-inflammatory conditions and that this does not necessarily result in the depletion of autoreactive T cells. Tissue destruction is also shown to increase the number of lymph-node DCs containing self-antigen, possibly contributing to the amplification of disease.
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Figdor, C. G. et al. Isolation of functionally different human monocytes by counterflow centrifugation elutriation. Blood 60, 46–53 (1982).
Grage-Griebenow, E., Flad, H. D. & Ernst, M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 69, 11–20 (2001).
Weber, C. et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J. Leukoc. Biol. 67, 699–704 (2000).
Gerszten, R. E. et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398, 718–723. (1999).
Boring, L. et al. Impaired monocyte migration and reduced type 1 (TH1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100, 2552–2561 (1997).
Kuziel, W. A. et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl Acad. Sci. USA 94, 12053–12058 (1997).
Kurihara, T., Warr, G., Loy, J. & Bravo, R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186, 1757–1762 (1997).
Lu, B. et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187, 601–608 (1998).
Tedder, T. F., Steeber, D. A. & Pizcueta, P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J. Exp. Med. 181, 2259–2264 (1995).
Henderson, R. B., Hobbs, J. A., Mathies, M. & Hogg, N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood 102, 328–335 (2003).
Rosen, H. Role of CR3 in induced myelomonocytic recruitment: insights from in vivo monoclonal antibody studies in the mouse. J. Leukoc. Biol. 48, 465–469 (1990).
Rosen, H. & Gordon, S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J. Exp. Med. 166, 1685–1701 (1987).
Issekutz, T. B. In vivo blood monocyte migration to acute inflammatory reactions, IL-1α, TNF-α, IFN-γ, and C5a utilizes LFA-1, Mac-1, and VLA-4. The relative importance of each integrin. J. Immunol. 154, 6533–6540 (1995).
Meerschaert, J. & Furie, M. B. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J. Immunol. 154, 4099–4112 (1995).
Aurrand-Lions, M., Johnson-Leger, C., Wong, C., Du Pasquier, L. & Imhof, B. A. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood 98, 3699–3707 (2001).
Muller, W. A., Weigl, S. A., Deng, X. & Phillips, D. M. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 178, 449–460 (1993).
Liao, F. et al. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 182, 1337–1343 (1995).
Liao, F., Ali, J., Greene, T. & Muller, W. A. Soluble domain 1 of platelet/endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J. Exp. Med. 185, 1349–1357 (1997).
Zhao, T. & Newman, P. J. Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J. Cell Biol. 152, 65–73 (2001).
Savill, J., Dransfield, I., Gregory, C. & Haslett, C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nature Rev. Immunol. 2, 965–975 (2002).
Bellingan, G. J. et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J. Exp. Med. 196, 1515–1521 (2002).
Randolph, G. J., Beaulieu, S., Lebecque, S., Steinman, R. M. & Muller, W. A. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 (1998).
Randolph, G. J., Sanchez-Schmitz, G., Liebman, R. M. & Schakel, K. The CD16+ (FcγRIII+) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J. Exp. Med. 196, 517–527 (2002).
Sallusto, F. & Lanzavecchia, A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J. Exp. Med. 189, 611–614 (1999).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Martin-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003). A careful examination of the mechanisms involved in DC migration from tissues to lymph nodes and their functional consequences on lymph-node cellularity and T-cell response. The migration of bone-marrow-derived DCs is dependent on CCR7 and correlates with the level of CCL21 expressed by lymphatic endothelial cells after exposure to inflammatory stimuli.
Rotta, G. et al. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J. Exp. Med. 198, 1253–1263 (2003). Illustrates the complexity of the mechanisms regulating the ability of monocytes to differentiate into DCs that subsequently migrate to lymph nodes. The migration of monocyte-derived DCs to draining lymph nodes is inhibited by LPS, whereas the migration of Langerhans cells is not, indicating that local inflammatory signals are a negative regulator of adaptive immune responses to particulate antigens.
Gretz, J. E., Norbury, C. C., Anderson, A. O., Proudfoot, A. E. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
McEvoy, L. M., Jutila, M. A., Tsao, P. S., Cooke, J. P. & Butcher, E. C. Anti-CD43 inhibits monocyte–endothelial adhesion in inflammation and atherogenesis. Blood 90, 3587–3594 (1997).
Hart, D. N. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90, 3245–3287 (1997).
Robert, C. et al. Interaction of dendritic cells with skin endothelium: a new perspective on immunosurveillance. J. Exp. Med. 189, 627–636 (1999).
Geijtenbeek, T. B. et al. DC-SIGN–ICAM-2 interaction mediates dendritic cell trafficking. Nature Immunol. 1, 353–357. (2000).
Pendl, G. G. et al. Immature mouse dendritic cells enter inflamed tissue, a process that requires E- and P-selectin, but not P-selectin glycoprotein ligand 1. Blood 99, 946–956 (2002).
Arbones, M. L. et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1, 247–260. (1994).
Johnston, B. & Kubes, P. The α4-integrin: an alternative pathway for neutrophil recruitment? Immunol. Today 20, 545–550 (1999).
Vaporciyan, A. A. et al. Involvement of platelet/endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science 262, 1580–1582 (1993).
Christofidou-Solomidou, M., Nakada, M. T., Williams, J., Muller, W. A. & DeLisser, H. M. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravasation. J. Immunol. 158, 4872–4878 (1997).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Lagasse, E. & Weissman, I. L. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197, 139–150 (1996).
Ajuebor, M. N. et al. Endogenous monocyte chemoattractant protein–1 recruits monocytes in the zymosan peritonitis model. J. Leukoc. Biol. 63, 108–116 (1998).
Acknowledgements
This work was supported by grants from the Swiss National Science Foundation and the Ligne National Centre Le Cancer. We thank B. Wehrle-Haller for his valuable advice and suggestions concerning integrin activation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
Glossary
- GLY-PHE-PHE-LYS-ARG MOTIF
-
A conserved amino-acid motif present in the cytoplasmic domain of the α-chains of integrins. It enables the α- and β-chain of an integrin to be held in close contact, which then keeps the extracellular ligand-binding domain in a folded inactive form. Lysines (amino acids proximal to the Gly-Phe-Phe-Lys-Arg motif) are essential for the interaction of the integrin α-chain with RAPL. The binding of RAPL contributes to the spatial separation of the cytoplasmic domains of the integrin chains, allowing the unfolding of the extracellular portion into a functional ligand-binding integrin.
- ASN-PRO-XAA-TYR/PHE MOTIF
-
A conserved amino-acid motif present in the cytoplasmic domain of the β-chains of integrins. It is a docking sequence for talin. Talin–integrin interactions mediate integrin activation. It is probable (but not yet experimentally proven) that this occurs through facilitating an optimal spatial separation of the cytoplasmic domains of the integrin chains, thereby allowing the unfolding of the extracellular portion into a functional ligand-binding integrin.
- MEMBRANE RUFFLING
-
A cellular zone undergoing rapid reorganization of the plasma membrane and the actin cytoskeleton. Membrane ruffling often precedes the formation of a lamellipodium.
- LAMELLIPODIUM
-
A flattened projection protruding from the anterior region of a cell. It is an actin-rich zone that is formed in response to chemokine signals, and it propels a migrating cell forward.
- POLARITY COMPLEX
-
Polarity complex molecules were first discovered in Drosophila melanogaster and Caenorhabditis elegans. They are required for the development of anterior–posterior polarity in leukocytes and apical–basal polarity in epithelial and endothelial cells. In migrating cells, some of these complexes are enriched in the lamellipodium.
- FOCAL ADHESION COMPLEXES
-
The closest contact sites of a cell with its environment, which are formed by integrin clustering. The integrins link the extracellular environment to the actin cytoskeleton by a complex assembly of adaptor proteins.
- UROPOD
-
A structure formed at the posterior of a migrating leukocyte. Adhesion molecules, such as intercellular adhesion molecule 1 (ICAM1) or CD44, are enriched in this zone.
- DIAPEDESIS
-
The last step in the leukocyte–endothelial adhesion cascade. The cascade includes tethering, triggering, tight adhesion and transmigration. Diapedesis is the migration of leukocytes across the endothelium, which occurs by squeezing through the junctions between adjacent endothelial cells.
- DEFENSINS
-
Defensins are small (2–6 kDa) cationic microbicidal peptides that participate in innate immunity. Their tertiary structures are stabilized by intradisulphide bridges and clusters of positively charged amino acids, which resemble those found in chemokines. Several defensins have chemotactic activity for leukocytes.
- OP/OP MICE
-
A inbred strain of mice that suffer from osteopetrosis. The defect has been localized to the gene encoding macrophage colony-stimulating factor.
- THIOGLYCOLLATE-INDUCED PERITONITIS
-
Inflammation of the peritoneum induced by sterile injection of thioglycollate. This results in the sequential recruitment of granulocytes (within hours), monocytes (within 2 days) and lymphocytes (after 2–3 days). It is widely used to study acute inflammation.
Rights and permissions
About this article
Cite this article
Imhof, B., Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 4, 432–444 (2004). https://doi.org/10.1038/nri1375
Issue Date:
DOI: https://doi.org/10.1038/nri1375
This article is cited by
-
Amyloid-β (Aβ) immunotherapy induced microhemorrhages are associated with activated perivascular macrophages and peripheral monocyte recruitment in Alzheimer’s disease mice
Molecular Neurodegeneration (2023)
-
Comparative study of the effects of cigarette smoke versus next-generation tobacco and nicotine product extracts on inflammatory biomarkers of human monocytes
Pflügers Archiv - European Journal of Physiology (2023)
-
Non-infectious granulomatous disorders of the upper lip: clinicopathological analysis of 11 patients
BMC Oral Health (2022)
-
A Novel Role of Nogo Proteins: Regulating Macrophages in Inflammatory Disease
Cellular and Molecular Neurobiology (2022)
-
Opioid Effects on the Central Nervous System and the Peripheral Immune System: Implications for Opioid Tolerance
Current Pharmacology Reports (2021)