Key Points
-
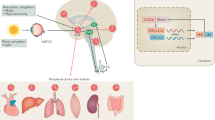
The effects of neurodegenerative syndromes extend beyond cognitive function to involve key physiological systems
-
Key changes in eating and metabolism, autonomic function, sleep and motor function have been demonstrated across several neurodegenerative conditions
-
Key neural structures that mediate physiological change across these conditions include neuroendocrine and hypothalamic pathways, reward pathways, motor systems and the autonomic nervous system
-
Evidence from human and animal studies suggests that these changes can arise presymptomatically and worsen with disease progression, offering a potential avenue for developing biomarkers to track disease progression
-
Further research is required to determine whether physiological changes simply result from the neurodegeneration process or also modify the neurodegenerative process, thereby providing a novel therapeutic target
Abstract
The effects of neurodegenerative syndromes extend beyond cognitive function to involve key physiological processes, including eating and metabolism, autonomic nervous system function, sleep, and motor function. Changes in these physiological processes are present in several conditions, including frontotemporal dementia, amyotrophic lateral sclerosis, Alzheimer disease and the parkinsonian plus conditions. Key neural structures that mediate physiological changes across these conditions include neuroendocrine and hypothalamic pathways, reward pathways, motor systems and the autonomic nervous system. In this Review, we highlight the key changes in physiological processing in neurodegenerative syndromes and the similarities in these changes between different progressive neurodegenerative brain conditions. The changes and similarities between disorders might provide novel insights into the human neural correlates of physiological functioning. Given the evidence that physiological changes can arise early in the neurodegenerative process, these changes could provide biomarkers to aid in the early diagnosis of neurodegenerative diseases and in treatment trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Boeve, B. F. The multiple phenotypes of corticobasal syndrome and corticobasal degeneration: implications for further study. J. Mol. Neurosci. 45, 350–353 (2011).
Perry, D. C. et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 140, 3329–3345 (2017).
Ahmed, R. M. et al. Neuronal network disintegration: common pathways linking neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 87, 1234–1241 (2016). This paper provides a good overview of network disintegration in neurodegeneration.
Ott, A. et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ 310, 970–973 (1995).
Rohrer, J. D. et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134, 2565–2581 (2011). This article is a good overview of the pathologies of FTD.
Josephs, K. A. et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 122, 137–153 (2011).
Mitsuyama, Y. & Inoue, T. Clinical entity of frontotemporal dementia with motor neuron disease. Neuropathology 29, 649–654 (2009).
Burrell, J. R., Kiernan, M. C., Vucic, S. & Hodges, J. R. Motor neuron dysfunction in frontotemporal dementia. Brain 134, 2582–2594 (2011).
Lomen-Hoerth, C., Anderson, T. & Miller, B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 59, 1077–1079 (2002).
Ahmed, R. M. et al. Amyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol. 15, 332–342 (2016). This provides a good overview of changes in eating and metabolism that occur in FTD and ALS.
Ahmed, R. M. et al. Assessment of eating behavior disturbance and associated neural networks in frontotemporal dementia. JAMA Neurol. 73, 282–290 (2016).
Ahmed, R. M. et al. Cognition and eating behavior in amyotrophic lateral sclerosis: effect on survival. J. Neurol. 263, 1593–1603 (2016).
Aziz, N. A. et al. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology 71, 1506–1513 (2008).
Aziz, N. A. et al. Weight loss in neurodegenerative disorders. J. Neurol. 255, 1872–1880 (2008).
Westfall, S. et al. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. Life Sci. 74, 3769–3787 (2017).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Piguet, O., Hornberger, M., Shelley, B. P., Kipps, C. M. & Hodges, J. R. Sensitivity of current criteria for the diagnosis of behavioral variant frontotemporal dementia. Neurology 72, 732–737 (2009).
Woolley, J. D. et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology 69, 1424–1433 (2007).
Miller, B. L., Darby, A. L., Swartz, J. R., Yener, G. G. & Mena, I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia 6, 195–199 (1995).
Snowden, J. S. et al. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg. Psychiatry 70, 323–332 (2001).
Ikeda, M., Brown, J., Holland, A. J., Fukuhara, R. & Hodges, J. R. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 73, 371–376 (2002).
Ahmed, R. M. et al. Quantifying the eating abnormalities in frontotemporal dementia. JAMA Neurol. 71, 1540–1546 (2014).
Van Langenhove, T., Leyton, C. E., Piguet, O. & Hodges, J. R. Comparing longitudinal behavior changes in the primary progressive aphasias. J. Alzheimers Dis. 53, 1033–1042 (2016).
Langmore, S. E., Olney, R. K., Lomen-Hoerth, C. & Miller, B. L. Dysphagia in patients with frontotemporal lobar dementia. Arch. Neurol. 64, 58–62 (2007).
Liu, W. et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 62, 742–748 (2004).
Kuhnlein, P. et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat. Clin. Pract. Neurol. 4, 366–374 (2008).
Holm, T. et al. Severe loss of appetite in amyotrophic lateral sclerosis patients: online self-assessment study. Interact. J. Med. Res. 2, e8 (2013).
Huisman, M. H. et al. Effect of presymptomatic body mass index and consumption of fat and alcohol on amyotrophic lateral sclerosis. JAMA Neurol. 72, 1155–1162 (2015).
Palamiuc, L. et al. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol. Med. 7, 526–546 (2015).
Dupuis, L., Oudart, H., Rene, F., Gonzalez de Aguilar, J. L. & Loeffler, J. P. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc. Natl Acad. Sci. USA 101, 11159–11164 (2004).
Kai, K. et al. Relationship between eating disturbance and dementia severity in patients with Alzheimer's disease. PLOS ONE 10, e0133666 (2015).
Frisardi, V. et al. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer's disease. Ageing Res. Rev. 9, 399–417 (2010).
Panza, F. et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J. Alzheimers Dis. 21, 691–724 (2010).
Cramer, C., Haan, M. N., Galea, S., Langa, K. M. & Kalbfleisch, J. D. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 71, 344–350 (2008).
Kronmal, R. A., Cain, K. C., Ye, Z. & Omenn, G. S. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch. Intern. Med. 153, 1065–1073 (1993).
Piguet, O. et al. Vascular risk factors, cognition and dementia incidence over 6 years in the Sydney Older Persons Study. Neuroepidemiology 22, 165–171 (2003).
Rizvi, B. et al. The effect of white matter hyperintensities on cognition is mediated by cortical atrophy. Neurobiol. Aging 64, 25–32 (2017).
Bos, I. et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res. Ther. 9, 101 (2017).
Reich-Slotky, R. et al. Body mass index (BMI) as predictor of ALSFRS-R score decline in ALS patients. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 212–216 (2013).
Bouteloup, C. et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. J. Neurol. 256, 1236–1242 (2009).
Vaisman, N. et al. Do patients with amyotrophic lateral sclerosis (ALS) have increased energy needs? J. Neurol. Sci. 279, 26–29 (2009).
Dupuis, L., Pradat, P. F., Ludolph, A. C. & Loeffler, J. P. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 10, 75–82 (2011).
Kasarskis, E. J., Berryman, S., Vanderleest, J. G., Schneider, A. R. & McClain, C. J. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am. J. Clin. Nutr. 63, 130–137 (1996).
Menzies, F. M., Ince, P. G. & Shaw, P. J. Mitochondrial involvement in amyotrophic lateral sclerosis. Neurochem. Int. 40, 543–551 (2002).
Chiang, P. M. et al. Deletion of TDP-43 down-regulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc. Natl Acad. Sci. USA 107, 16320–16324 (2010).
Xu, Y. F. et al. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J. Neurosci. 30, 10851–10859 (2010).
Shan, X., Chiang, P. M., Price, D. L. & Wong, P. C. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc. Natl Acad. Sci. USA 107, 16325–16330 (2010).
Ahmed, R. M. et al. Energy expenditure in frontotemporal dementia: a behavioural and imaging study. Brain 140, 171–183 (2017).
Dupuis, L. et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70, 1004–1009 (2008).
Dorst, J. et al. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J. Neurol. 258, 613–617 (2011).
Sutedja, N. A. et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 82, 638–642 (2011).
Ikeda, K., Hirayama, T., Takazawa, T., Kawabe, K. & Iwasaki, Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern. Med. 51, 1501–1508 (2012).
Yang, J. W. et al. Hypolipidemia in patients with amyotrophic lateral sclerosis: a possible gender difference? J. Clin. Neurol. 9, 125–129 (2013).
Chio, A. et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology 73, 1681–1685 (2009).
Jawaid, A. et al. ALS disease onset may occur later in patients with pre-morbid diabetes mellitus. Eur. J. Neurol. 17, 733–739 (2010).
Reyes, E. T., Perurena, O. H., Festoff, B. W., Jorgensen, R. & Moore, W. V. Insulin resistance in amyotrophic lateral sclerosis. J. Neurol. Sci. 63, 317–324 (1984).
Dupuis, L. et al. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLOS ONE 7, e37885 (2012).
Jawaid, A., Paganoni, S., Hauser, C. & Schulz, P. E. Trials of antidiabetic drugs in amyotrophic lateral sclerosis: proceed with caution? Neurodegener Dis. 13, 205–208 (2014).
Gao, S. et al. Accelerated weight loss and incident dementia in an elderly African-American cohort. J. Am. Geriatr. Soc. 59, 18–25 (2011).
Chakrabarti, S. et al. Metabolic risk factors of sporadic Alzheimer's disease: implications in the pathology, pathogenesis and treatment. Aging Dis. 6, 282–299 (2015).
Meng, X. F. et al. Midlife vascular risk factors and the risk of Alzheimer's disease: a systematic review and meta-analysis. J. Alzheimers Dis. 42, 1295–1310 (2014).
Noyce, A. J. et al. Estimating the causal influence of body mass index on risk of Parkinson disease: a Mendelian randomisation study. PLOS Med. 14, e1002314 (2017).
Whitwell, J. L. et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. NeuroImage 35, 207–213 (2007).
Perry, D. C. et al. Anatomical correlates of reward-seeking behaviours in behavioural variant frontotemporal dementia. Brain 137, 1621–1626 (2014).
Irish, M., Piguet, O. & Hodges, J. R. Self-projection and the default network in frontotemporal dementia. Nat. Rev. Neurol. 8, 152–161 (2011).
Woolley, J. D. et al. Satiety-related hormonal dysregulation in behavioral variant frontotemporal dementia. Neurology 82, 512–520 (2014).
Ahmed, R. M. et al. Eating behavior in frontotemporal dementia: peripheral hormones versus hypothalamic pathology. Neurology 85, 1310–1317 (2015).
Piguet, O. et al. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann. Neurol. 69, 312–319 (2011).
Coll, A. P., Farooqi, I. S., Challis, B. G., Yeo, G. S. & O'Rahilly, S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J. Clin. Endocrinol. Metabol. 89, 2557–2562 (2004).
Coll, A. P., Farooqi, I. S. & O'Rahilly, S. The hormonal control of food intake. Cell 129, 251–262 (2007).
Swaab, D. F. et al. Functional neuroanatomy and neuropathology of the human hypothalamus. Anat. Embryol. 187, 317–330 (1993).
Ahmed, R. M. et al. Autonomic dysregulation in frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 86, 1048–1049 (2015).
Cong, N. D. et al. Reduced 24 hour ambulatory blood pressure and abnormal heart rate variability in patients with dysorexia nervosa. Heart 90, 563–564 (2004).
Vercruysse, P. et al. Alterations in the hypothalamic melanocortin pathway in amyotrophic lateral sclerosis. Brain 139, 1106–1122 (2016).
Cykowski, M. D., Takei, H., Schulz, P. E., Appel, S. H. & Powell, S. Z. TDP-43 pathology in the basal forebrain and hypothalamus of patients with amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2, 171 (2014).
Ahmed, R. & Farooqi, I. S. Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 1006–1007 (2017).
Gorges, M. et al. Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 1033–1041 (2017).
Alberici, A. et al. Serum leptin levels are higher in females affected by frontotemporal lobar degeneration than Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 79, 712–715 (2008).
Warren, M. W., Hynan, L. S. & Weiner, M. F. Lipids and adipokines as risk factors for Alzheimer's disease. J. Alzheimers Dis. 29, 151–157 (2012).
Walker, K. A., Power, M. C. & Gottesman, R. F. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr. Hypertens. Rep. 19, 24 (2017).
Bocti, C. et al. Orthostatic hypotension associated with executive dysfunction in mild cognitive impairment. J. Neurol. Sci. 382, 79–83 (2017).
McLeod, K. J. & Jain, T. Postural hypotension and cognitive function in older adults. Gerontol. Geriatr. Med. 3, 2333721417733216 (2017).
Diehl-Schmid, J. et al. Extrapyramidal signs, primitive reflexes and incontinence in fronto-temporal dementia. Eur. J. Neurol. 14, 860–864 (2007).
Hoefer, M. et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain 131, 1646–1657 (2008).
Fletcher, P. D. et al. Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain 138, 3360–3372 (2015).
Neary, D. et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554 (1998).
Guo, C. C. et al. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc. Natl Acad. Sci. USA 113, E2430–E2439 (2016).
Fletcher, P. D. et al. A physiological signature of sound meaning in dementia. Cortex 77, 13–23 (2016).
Merico, A. & Cavinato, M. Autonomic dysfunction in the early stage of ALS with bulbar involvement. Amyotroph. Lateral Scler. 12, 363–367 (2011).
Baltadzhieva, R., Gurevich, T. & Korczyn, A. D. Autonomic impairment in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 18, 487–493 (2005).
Shimizu, T. et al. Circulatory collapse and sudden death in respirator-dependent amyotrophic lateral sclerosis. J. Neurol. Sci. 124, 45–55 (1994).
Shimizu, T., Kato, S., Hayashi, M., Hayashi, H. & Tanabe, H. Amyotrophic lateral sclerosis with hypertensive attacks: blood pressure changes in response to drug administration. Clin. Auton. Res. 6, 241–244 (1996).
Vucic, S. Sensory and autonomic nervous system dysfunction in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 43, 99–101 (2017).
Beck, M. et al. Progressive sudomotor dysfunction in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 73, 68–70 (2002).
Toepfer, M. et al. Gastrointestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 15–19 (1999).
Nolano, M. et al. Non-motor involvement in amyotrophic lateral sclerosis: new insight from nerve and vessel analysis in skin biopsy. Neuropathol. Appl. Neurobiol. 43, 119–132 (2017).
Giubilei, F. et al. Cardiac autonomic dysfunction in patients with Alzheimer disease: possible pathogenetic mechanisms. Alzheimer Dis. Assoc. Disord. 12, 356–361 (1998).
Pascualy, M. et al. Hypothalamic pituitary adrenocortical and sympathetic nervous system responses to the cold pressor test in Alzheimer's disease. Biol. Psychiatry 48, 247–254 (2000).
Perry, E. K., Smith, C. J., Court, J. A. & Perry, R. H. Cholinergic nicotinic and muscarinic receptors in dementia of Alzheimer, Parkinson and Lewy body types. J. Neural Transm. Park Dis. Dement. Sect. 2, 149–158 (1990).
Royall, D. R., Gao, J. H. & Kellogg, D. L. Jr. Insular Alzheimer's disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med. Hypotheses 67, 747–758 (2006).
Santos, C. Y., Machan, J. T., Wu, W. C. & Snyder, P. J. Autonomic cardiac function in preclinical Alzheimer's disease. J. Alzheimers Dis. 59, 1057–1065 (2017).
Lattanzi, S., Luzzi, S., Provinciali, L. & Silvestrini, M. Blood pressure variability in Alzheimer's disease and frontotemporal dementia: the effect on the rate of cognitive decline. J. Alzheimers Dis. 45, 387–394 (2015).
Femminella, G. D. et al. Autonomic dysfunction in Alzheimer's disease: tools for assessment and review of the literature. J. Alzheimers Dis. 42, 369–377 (2014).
Boxer, A. L. et al. Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 16, 552–563 (2017). This study provides a good overview of the new diagnostic criteria for PSP syndrome.
Schmidt, C. et al. Autonomic dysfunction in patients with progressive supranuclear palsy. Mov. Disord. 23, 2083–2089 (2008).
Reimann, M. et al. Comprehensive autonomic assessment does not differentiate between Parkinson's disease, multiple system atrophy and progressive supranuclear palsy. J. Neural Transm. 117, 69–76 (2010).
Jones, S. E. Imaging for autonomic dysfunction. Cleve. Clin. J. Med. 78, S69–S74 (2011).
Critchley, H. D., Nagai, Y., Gray, M. A. & Mathias, C. J. Dissecting axes of autonomic control in humans: insights from neuroimaging. Auton. Neurosci. 161, 34–42 (2011). This is a good overview of the neural control of autonomic function.
Seeley, W. W. et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol. 65, 249–255 (2008).
Kumfor, F., Irish, M., Hodges, J. R. & Piguet, O. Discrete neural correlates for the recognition of negative emotions: insights from frontotemporal dementia. PLOS ONE 8, e67457 (2013).
Galton, C. J. et al. Temporal lobe rating scale: application to Alzheimer's disease and frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 70, 165–173 (2001).
Irish, M., Piguet, O., Hodges, J. R. & Hornberger, M. Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer's disease. Hum. Brain Mapp. 35, 1422–1435 (2014).
Kipps, C. M., Nestor, P. J., Acosta-Cabronero, J., Arnold, R. & Hodges, J. R. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain 132, 592–603 (2009).
Perneczky, R. et al. Urinary incontinence and its functional anatomy in frontotemporal lobar degenerations. Eur. J. Nucl. Med. Mol. Imaging 35, 605–610 (2008).
Rosen, H. J. & Levenson, R. W. The emotional brain: combining insights from patients and basic science. Neurocase 15, 173–181 (2009).
Craig, A. D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Critchley, H. D. et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126, 2139–2152 (2003).
Luu, P. & Posner, M. I. Anterior cingulate cortex regulation of sympathetic activity. Brain 126, 2119–2120 (2003).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
Thayer, J. F. et al. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756 (2012).
Mieda, M. The roles of orexins in sleep/wake regulation. Neurosci. Res. 118, 56–65 (2017).
Bonakis, A. et al. Sleep in frontotemporal dementia is equally or possibly more disrupted, and at an earlier stage, when compared to sleep in Alzheimer's disease. J. Alzheimers Dis. 38, 85–91 (2014).
Anderson, K. N., Hatfield, C., Kipps, C., Hastings, M. & Hodges, J. R. Disrupted sleep and circadian patterns in frontotemporal dementia. Eur. J. Neurol. 16, 317–323 (2009).
Pawlak, C., Blois, R., Gaillard, J. M. & Richard, J. Sleep in Pick disease. Encephale 12, 327–334 (1986).
McCarter, S. J., St Louis, E. K. & Boeve, B. F. Sleep disturbances in frontotemporal dementia. Curr. Neurol. Neurosci. Rep. 16, 85 (2016). This paper is a good overview of sleep changes that occur in FTD.
Ahmed, R. M. et al. Sleep disorders and respiratory function in amyotrophic lateral sclerosis. Sleep Med. Rev. 26, 33–42 (2016). This article provides a good overview of sleep changes in ALS.
Atalaia, A., De Carvalho, M., Evangelista, T. & Pinto, A. Sleep characteristics of amyotrophic lateral sclerosis in patients with preserved diaphragmatic function. Amyotroph. Lateral Scler. 8, 101–105 (2007).
Yamauchi, R. et al. Respiratory insufficiency with preserved diaphragmatic function in amyotrophic lateral sclerosis. Intern. Med. 53, 1325–1331 (2014).
Santos, C. et al. Sleep-related breathing disorders in amyotrophic lateral sclerosis. Monaldi Arch. Chest Dis. 59, 160–165 (2003).
David, W. S., Bundlie, S. R. & Mahdavi, Z. Polysomnographic studies in amyotrophic lateral sclerosis. J. Neurol. Sci. 152, S29–S35 (1997).
Park, S. Y. et al. Nocturnal hypoxia in ALS is related to cognitive dysfunction and can occur as clusters of desaturations. PLOS ONE 8, e75324 (2013).
Turek, F. W., Dugovic, C. & Zee, P. C. Current understanding of the circadian clock and the clinical implications for neurological disorders. Arch. Neurol. 58, 1781–1787 (2001).
Bede, P. et al. Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 81, 361–369 (2013).
Newsom-Davis, I. C., Lyall, R. A., Leigh, P. N., Moxham, J. & Goldstein, L. H. The effect of non-invasive positive pressure ventilation (NIPPV) on cognitive function in amyotrophic lateral sclerosis (ALS): a prospective study. J. Neurol. Neurosurg. Psychiatry 71, 482–487 (2001).
Raggi, A. & Ferri, R. Sleep disorders in neurodegenerative diseases. Eur. J. Neurol. 17, 1326–1338 (2010).
Vitiello, M. V. & Borson, S. Sleep disturbances in patients with Alzheimer's disease: epidemiology, pathophysiology and treatment. CNS Drugs 15, 777–796 (2001).
Prinz, P. N. et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol. Aging 3, 361–370 (1982).
Vitiello, M. V. & Prinz, P. N. Alzheimer's disease. Sleep and sleep/wake patterns. Clin. Geriatr. Med. 5, 289–299 (1989).
Hita-Yanez, E., Atienza, M., Gil-Neciga, E. & Cantero, J. L. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE epsilon4 genotype. Curr. Alzheimer Res. 9, 290–297 (2012).
Mander, B. A., Winer, J. R., Jagust, W. J. & Walker, M. P. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci. 39, 552–566 (2016).
Aldrich, M. S., Foster, N. L., White, R. F., Bluemlein, L. & Prokopowicz, G. Sleep abnormalities in progressive supranuclear palsy. Ann. Neurol. 25, 577–581 (1989).
Arnulf, I. et al. REM sleep behavior disorder and REM sleep without atonia in patients with progressive supranuclear palsy. Sleep 28, 349–354 (2005).
Kimura, K., Tachibana, N., Aso, T., Kimura, J. & Shibasaki, H. Subclinical REM sleep behavior disorder in a patient with corticobasal degeneration. Sleep 20, 891–894 (1997).
Wetter, T. C., Brunner, H., Collado-Seidel, V., Trenkwalder, C. & Winkelmann, J. Sleep and periodic limb movements in corticobasal degeneration. Sleep Med. 3, 33–36 (2002).
Moore, R. Y. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med. 8 (Suppl. 3), 27–33 (2007).
Stopa, E. G. et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J. Neuropathol. Exp. Neurol. 58, 29–39 (1999).
Theofilas, P., Dunlop, S., Heinsen, H. & Grinberg, L. T. Turning on the light within: subcortical nuclei of the isodentritic core and their role in Alzheimer's disease pathogenesis. J. Alzheimers Dis. 46, 17–34 (2015).
Coban, A. et al. Reduced orexin-A levels in frontotemporal dementia: possible association with sleep disturbance. Am. J. Alzheimers Dis. Other Demen. 28, 606–611 (2013).
Yasui, K. et al. CSF orexin levels of Parkinson's disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degeneration. J. Neurol. Sci. 250, 120–123 (2006).
Warren, J. D. & Clark, C. N. A new hypnic paradigm of neurodegenerative proteinopathies. Sleep Med. 32, 282–283 (2017). This article proposes a new hypnic paradigm for neurodegeneration.
Vucic, S. & Kiernan, M. C. Transcranial magnetic stimulation for the assessment of neurodegenerative disease. Neurotherapeutics 14, 91–106 (2017). This paper provides a good overview of TMS.
Cantone, M. et al. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin. Neurophysiol. 125, 1509–1532 (2014).
Vucic, S., Nicholson, G. A. & Kiernan, M. C. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 131, 1540–1550 (2008).
Geevasinga, N. et al. Cortical function in asymptomatic carriers and patients with C9orf72 amyotrophic lateral sclerosis. JAMA Neurol. 72, 1268–1274 (2015).
Menon, P., Kiernan, M. C. & Vucic, S. Cortical hyperexcitability precedes lower motor neuron dysfunction in ALS. Clin. Neurophysiol. 126, 803–809 (2015).
Eisen, A., Kim, S. & Pant, B. Amyotrophic lateral sclerosis (ALS): a phylogenetic disease of the corticomotoneuron? Muscle Nerve 15, 219–224 (1992).
Liepert, J., Bar, K. J., Meske, U. & Weiller, C. Motor cortex disinhibition in Alzheimer's disease. Clin. Neurophysiol. 112, 1436–1441 (2001).
Kuhn, A. A. et al. Patterns of abnormal motor cortex excitability in atypical parkinsonian syndromes. Clin. Neurophysiol. 115, 1786–1795 (2004).
Wittstock, M. et al. Interhemispheric inhibition in different phenotypes of progressive supranuclear palsy. J. Neural Transm. 120, 453–461 (2013).
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L. & Greicius, M. D. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52 (2009). This paper is a good overview of network involvement in neurodegeneration.
Kullmann, S. et al. Resting-state functional connectivity of the human hypothalamus. Hum. Brain Mapp. 35, 6088–6096 (2014). This is a good overview of the cortical connections of the hypothalamus.
Seeley, W. W. Anterior insula degeneration in frontotemporal dementia. Brain Struct. Funct. 214, 465–475 (2010).
Bonthius, D. J., Solodkin, A. & Van Hoesen, G. W. Pathology of the insular cortex in Alzheimer disease depends on cortical architecture. J. Neuropathol. Exp. Neurol. 64, 910–922 (2005).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007). This article provides a good overview of the salience network.
Zhou, J. et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 133, 1352–1367 (2010).
Rosen, H. J. et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58, 198–208 (2002).
Day, G. S. et al. Salience network resting-state activity: prediction of frontotemporal dementia progression. JAMA Neurol. 70, 1249–1253 (2013).
Farb, N. A. S. et al. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex 49, 1856–1873 (2013).
Lewis, J. et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25, 402–405 (2000).
Ittner, L. M. et al. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc. Natl Acad. Sci. USA 105, 15997–16002 (2008).
Ke, Y. D. et al. Short-term suppression of A315T mutant human TDP-43 expression improves functional deficits in a novel inducible transgenic mouse model of FTLD-TDP and ALS. Acta Neuropathol. 130, 661–678 (2015).
Walker, A. K. et al. Functional recovery in new mouse models of ALS/FTLD after clearance of pathological cytoplasmic TDP-43. Acta Neuropathol. 130, 643–660 (2015).
Ittner, L. M., Ke, Y. D. & Gotz, J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J. Biol. Chem. 284, 20909–20916 (2009).
van Eersel, J. et al. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer's disease models. Proc. Natl Acad. Sci. USA 107, 13888–13893 (2010).
Platt, B. et al. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLOS ONE 6, e27068 (2011).
Holth, J. K., Mahan, T. E., Robinson, G. O., Rocha, A. & Holtzman, D. M. Altered sleep and EEG power in the P301S Tau transgenic mouse model. Ann. Clin. Transl Neurol. 4, 180–190 (2017).
Koss, D. J. et al. Mutant Tau knock-in mice display frontotemporal dementia relevant behaviour and histopathology. Neurobiol. Dis. 91, 105–123 (2016).
Cantero, J. L. et al. Tau protein role in sleep-wake cycle. J. Alzheimers Dis. 21, 411–421 (2010).
Liu, R., Sheng, Z. F., Cai, B., Zhang, Y. H. & Fan, D. S. Increased orexin expression promotes sleep/wake disturbances in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Chin. Med. J. 128, 239–244 (2015).
Rothman, S. M., Herdener, N., Frankola, K. A., Mughal, M. R. & Mattson, M. P. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer's disease. Brain Res. 1529, 200–208 (2013).
Di Meco, A., Joshi, Y. B. & Pratico, D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer's disease with plaques and tangles. Neurobiol. Aging 35, 1813–1820 (2014).
Ishii, M., Wang, G., Racchumi, G., Dyke, J. P. & Iadecola, C. Transgenic mice overexpressing amyloid precursor protein exhibit early metabolic deficits and a pathologically low leptin state associated with hypothalamic dysfunction in arcuate neuropeptide Y neurons. J. Neurosci. 34, 9096–9106 (2014).
Joly-Amado, A. et al. Metabolic changes over the course of aging in a mouse model of tau deposition. Neurobiol. Aging 44, 62–73 (2016).
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science 316, 750–754 (2007).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 (2010).
Ittner, A. et al. Site-specific phosphorylation of tau inhibits amyloid-beta toxicity in Alzheimer's mice. Science 354, 904–908 (2016).
Leboucher, A. et al. Detrimental effects of diet-induced obesity on tau pathology are independent of insulin resistance in tau transgenic mice. Diabetes 62, 1681–1688 (2013).
Brownlow, M. L. et al. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res. 271, 79–88 (2014).
Ahmed, R. M. et al. Systemic metabolism in frontotemporal dementia. Neurology 83, 1812–1818 (2014).
Erro, R., Barone, P., Moccia, M., Amboni, M. & Vitale, C. Abnormal eating behaviors in progressive supranuclear palsy. Eur. J. Neurol. 20, e47–48 (2013).
Acknowledgements
This work was supported by funding received by ForeFront (a collaborative research group dedicated to the study of frontotemporal dementia and amyotrophic lateral sclerosis) from the National Health and Medical Research Council of Australia (NHMRC) programme (grant 1037746 to L.M.I., J.R.H. G.H. and M.C.K.) and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program (CE110001021 to J.R.H. and O.P.) and other grants and/or sources (NHMRC project grants 1003139 and 1081916, and a Royal Australasian College of Physicians Vincent Fairfax family fellowship). The authors are grateful to the research participants involved with the ForeFront research studies. R.M.A. is an NHMRC Early Career Fellow (1120770). Y.D.K. is an NHMRC R. D. Wright Biomedical Career Development Fellow (112564). O.P. is an NHMRC Senior Research Fellow (1103258). G.H. is an NHMRC Senior Principal Research Fellow (1079679).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ahmed, R., Ke, Y., Vucic, S. et al. Physiological changes in neurodegeneration — mechanistic insights and clinical utility. Nat Rev Neurol 14, 259–271 (2018). https://doi.org/10.1038/nrneurol.2018.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2018.23
This article is cited by
-
Natural flavonoids as potential therapeutics in the management of Alzheimer’s disease: a review
3 Biotech (2024)
-
Small fiber neuropathy for assessment of disease severity in amyotrophic lateral sclerosis: corneal confocal microscopy findings
Orphanet Journal of Rare Diseases (2022)
-
Hypermetabolism associated with worse prognosis of amyotrophic lateral sclerosis
Journal of Neurology (2022)
-
Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition
Nature Neuroscience (2021)
-
Pathophysiology and Treatment of Non-motor Dysfunction in Amyotrophic Lateral Sclerosis
CNS Drugs (2021)