Abstract
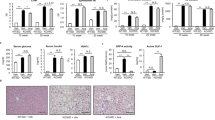
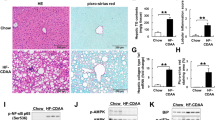
Epidemiological studies have demonstrated a close association of type 2 diabetes and hepatocellular carcinoma (HCC). Exenatide (Ex-4), a potent diabetes drug targeting glucagon-like peptide-1 receptor (GLP-1R), is protective against non-alcoholic fatty liver disease (NAFLD). However, the Ex-4 function and GLP-1R status have yet been explored in HCC. Herein we investigated the effect of Ex-4 in diethylnitrosamine (DEN)-treated mice consuming control or high-fat high-carbohydrate diet. Administration of Ex-4 significantly improved obesity-induced hyperglycemia and hyperlipidemia and reduced HCC multiplicity in obese DEN-treated mice, in which suppressed proliferation and induced apoptosis were confined to tumor cells. The tumor suppression effects of Ex-4 were associated with high expression of GLP-1R and activation of cyclic AMP (cAMP) and protein kinase A (PKA). Importantly, Ex-4 also downregulated epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 3 (STAT3), which lie downstream of cAMP-PKA signaling, resulting in suppression of multiple STAT3-targeted genes including c-Myc, cyclin D1, survivin, Bcl-2 and Bcl-xl. The growth inhibitory effects of Ex-4 were consistent in GLP-1R-abundant hepatoma cell lines and xenograft mouse model, wherein both PKA and EGFR had obligatory roles in mediating Ex-4 functions. In addition, Ex-4 also effectively suppressed inflammatory and fibrotic phenotypes in mice fed with methionine–choline-deficient (MCD) diet and choline-deficient ethionine-supplemented (CDE) diet, respectively. In summary, Ex-4 elicits protective functions against NAFLD and obesity-associated HCC through cAMP-PKA-EGFR-STAT3 signaling, suggesting its administration as a novel approach to reduce HCC risk in diabetic patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378: 31–40.
El-Serag HB, Hampel H, Javadi F . The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4: 369–380.
Farrell GC, Wong VW, Chitturi S . NAFLD in Asia–as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013; 10: 307–318.
Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007; 30: 1212–1218.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A . Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108.
Wang P, Kang D, Cao W, Wang Y, Liu Z . Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev 2012; 28: 109–122.
Hosokawa T, Kurosaki M, Tsuchiya K, Matsuda S, Muraoka M, Suzuki Y et al. Hyperglycemia is a significant prognostic factor of hepatocellular carcinoma after curative therapy. World J Gastroenterol 2013; 19: 249–257.
Drucker DJ . The biology of incretin hormones. Cell Metab 2006; 3: 153–165.
Xu G, Stoffers DA, Habener JF, Bonner-Weir S . Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999; 48: 2270–2276.
Nauck M . Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016; 18: 203–216.
Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 2011; 31: 1285–1297.
Ding X, Saxena NK, Lin S, Gupta NA, Anania FA . Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006; 43: 173–181.
Nauck MA, Friedrich N . Do GLP-1-based therapies increase cancer risk? Diabetes Care 2013; 36: S245–S252.
Fan R, Li X, Gu X, Chan JC, Xu G . Exendin-4 protects pancreatic beta cells from human islet amyloid polypeptide-induced cell damage: potential involvement of AKT and mitochondria biogenesis. Diabetes Obes Metab 2010; 12: 815–824.
Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC . Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011; 141: 150–156.
Feng X, Dong K, Chaing W, Bhutada NS, Inciardi J, Woldemariam T et al. Assessing pancreatic cancer risk associated with dipeptidyl peptidase 4 inhibitors: data mining of FDA adverse event reporting system (FAERS). J Pharmacovigilance 2013; 1: 110.
Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T et al. Pancreatic safety of incretin-based drugs–FDA and EMA assessment. N Engl J Med 2014; 370: 794–797.
Koehler JA, Drucker DJ . Activation of glucagon-like peptide-1 receptor signaling does not modify the growth or apoptosis of human pancreatic cancer cells. Diabetes 2006; 55: 1369–1379.
Koehler JA, Kain T, Drucker DJ . Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology 2011; 152: 3362–3372.
Ligumsky H, Wolf I, Israeli S, Haimsohn M, Ferber S, Karasik A et al. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res Treat 2012; 132: 449–461.
Nomiyama T, Kawanami T, Irie S, Hamaguchi Y, Terawaki Y, Murase K et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014; 63: 3891–3905.
Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010; 52: 934–944.
Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet 2004; 36: 1306–1311.
Delghandi MP, Johannessen M, Moens U . The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal 2005; 17: 1343–1351.
Barbier AJ, Poppleton HM, Yigzaw Y, Mullenix JB, Wiepz GJ, Bertics PJ et al. Transmodulation of epidermal growth factor receptor function by cyclic AMP-dependent protein kinase. J Biol Chem 1999; 274: 14067–14073.
Absood A, Hu B, Bassily N, Colletti L . VIP inhibits human HepG2 cell proliferation in vitro. Regul Pept 2008; 146: 285–292.
Hsuan JJ . Oncogene regulation by growth factors. Anticancer Res 1993; 13: 2521–2532.
Shao H, Cheng HY, Cook RG, Tweardy DJ . Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res 2003; 63: 3923–3930.
Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS . Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol 1997; 8: 1197–1206.
Carpenter RL, Lo HW . STAT3 target genes relevant to human cancers. Cancers (Basel) 2014; 6: 897–925.
Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 2013; 62: 606–615.
Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM, Chan KA . Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology 2012; 55: 1462–1472.
Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010; 51: 1584–1592.
Korner M, Stockli M, Waser B, Reubi JC . GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 2007; 48: 736–743.
Kovach SJ, Price JA, Shaw CM, Theodorakis NG, McKillop IH . Role of cyclic-AMP responsive element binding (CREB) proteins in cell proliferation in a rat model of hepatocellular carcinoma. J Cell Physiol 2006; 206: 411–419.
Otsuka M, Kato N, Shao RX, Hoshida Y, Ijichi H, Koike Y et al. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology 2004; 40: 243–251.
Mayoral R, Fernandez-Martinez A, Bosca L, Martin-Sanz P . Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis 2005; 26: 753–761.
Caretta A, Mucignat-Caretta C . Protein kinase A in cancer. Cancers (Basel) 2011; 3: 913–926.
Ito Y, Takeda T, Sakon M, Tsujimoto M, Higashiyama S, Noda K et al. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer 2001; 84: 1377–1383.
Hopfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherubl H . Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol 2004; 41: 1008–1016.
Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 2005; 41: 307–314.
Ito Y, Matsuura N, Sakon M, Miyoshi E, Noda K, Takeda T et al. Expression and prognostic roles of the G1-S modulators in hepatocellular carcinoma: p27 independently predicts the recurrence. Hepatology 1999; 30: 90–99.
Watanabe J, Kushihata F, Honda K, Sugita A, Tateishi N, Mominoki K et al. Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery 2004; 135: 604–612.
Yang Y, Zhu J, Gou H, Cao D, Jiang M, Hou M . Clinical significance of Cox-2, Survivin and Bcl-2 expression in hepatocellular carcinoma (HCC). Med Oncol 2011; 28: 796–803.
Boehm AL, Sen M, Seethala R, Gooding WE, Freilino M, Wong SM et al. Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-X(L) enhances antitumor effects in squamous cell carcinoma of the head and neck. Mol Pharmacol 2008; 73: 1632–1642.
Kang ZF, Deng Y, Zhou Y, Fan RR, Chan JC, Laybutt DR et al. Pharmacological reduction of NEFA restores the efficacy of incretin-based therapies through GLP-1 receptor signalling in the beta cell in mouse models of diabetes. Diabetologia 2013; 56: 423–433.
Yu Z, Gao YQ, Feng H, Lee YY, Li MS, Tian Y et al. Cell cycle-related kinase mediates viral-host signalling to promote hepatitis B virus-associated hepatocarcinogenesis. Gut 2014; 63: 1793–1804.
Acknowledgements
This study is supported by the Collaborative Research Fund (C4017-14G) and the General Research Fund (14120816) of the Research Grants Council of Hong Kong, National Natural Science Foundation of China (81272305, 81170722, 81270438, 81302167 and 81501210) and Focused Investments Scheme B (1907301) of the Chinese University of Hong Kong.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Zhou, M., Mok, M., Sun, H. et al. The anti-diabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcinogenesis through cAMP–PKA–EGFR–STAT3 axis. Oncogene 36, 4135–4149 (2017). https://doi.org/10.1038/onc.2017.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.38
This article is cited by
-
cAMP-PKA/EPAC signaling and cancer: the interplay in tumor microenvironment
Journal of Hematology & Oncology (2024)
-
Semaglutide reduces tumor burden in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH-HCC with advanced fibrosis
Scientific Reports (2023)
-
MAFLD: an optimal framework for understanding liver cancer phenotypes
Journal of Gastroenterology (2023)
-
DPP-4 inhibitors and GLP-1RAs: cardiovascular safety and benefits
Military Medical Research (2022)
-
GLP-1 mimetics as a potential therapy for nonalcoholic steatohepatitis
Acta Pharmacologica Sinica (2022)