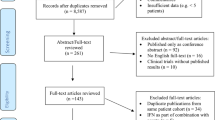
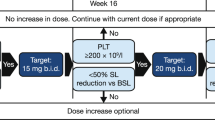
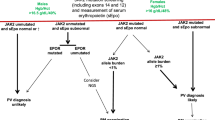
Abstract
Interferon-alpha (rIFNα) is the only disease-modifying treatment for polycythemia vera (PV), but whether or not it prolongs survival is unknown. This large single center retrospective study of 470 PV patients compares the myelofibrosis-free survival (MFS) and overall survival (OS) with rIFNα to two other primary treatments, hydroxyurea (HU) and phlebotomy-only (PHL-O). The median age at diagnosis was 54 years (range 20–94) and the median follow-up was 10 years (range 0–45). Two hundred and twenty-nine patients were women (49%) and 208 were high-risk (44%). The primary treatment was rIFNα in 93 (20%), HU in 189 (40%), PHL-O in 133 (28%) and other cytoreductive drugs in 55 (12%). The treatment groups differed by ELN risk score (p < 0.001). In low-risk patients, 20-year MFS for rIFNα, HU, and PHL-O was 84%, 65% and 55% respectively (p < 0.001) and 20-year OS was 100%, 85% and 80% respectively (p = 0.44). In high-risk patients, 20-year MFS for rIFNα, HU, and PHL-O was 89%, 41% and 36% respectively (p = 0.19) and 20-year OS was 66%, 40%, 14% respectively (p = 0.016). In multivariable analysis, longer time on rIFNα was associated with a lower risk of myelofibrosis (HR: 0.91, p < 0.001) and lower mortality (HR: 0.94, p = 0.012). In conclusion, this study supports treatment of PV with rIFNα to prevent myelofibrosis and potentially prolong survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Passamonti F, Giorgino T, Mora B, Guglielmelli P, Rumi E, Maffioli M, et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia. 2017;31:2726–31.
Spivak JL. Myeloproliferative neoplasms. N Engl J Med. 2017;376:2168–81.
Masarova L, Yin CC, Cortes JE, Konopleva M, Borthakur G, Newberry KJ, et al. Histomorphological responses after therapy with pegylated interferon α-2a in patients with essential thrombocythemia (ET) and polycythemia vera (PV). Exp Hematol Oncol. 2017;6:30.
Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107:451–8.
Kiladjian J-J, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–72.
Silver RT, Hasselbalch HC. Optimal therapy for polycythemia vera and essential thrombocythemia: Preferred use of interferon therapy based on phase 2 trials. Hematology. 2016;21:387–91.
Tashi T, Swierczek S, Kim SJ, Salama ME, Song J, Heikal N, et al. Pegylated interferon Alfa-2a and hydroxyurea in polycythemia vera and essential thrombocythemia: differential cellular and molecular responses. Leukemia. 2018;32:1830–3.
Sacchi S, Leoni P, Liberati M, Riccardi A, Tabilio A, Tartoni P, et al. A prospective comparison between treatment with phlebotomy alone and with interferon-alpha in patients with polycythemia vera. Ann Hematol. 1994;68:247–50.
Silver RT, Vandris K, Goldman JJ. Recombinant interferon-α may retard progression of early primary myelofibrosis: a preliminary report. Blood. 2011;117:6669–72.
Silver RT, Kiladjian JJ, Hasselbalch HC. Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev Hematol. 2013;6:49–58.
Silver RT, Barel AC, Lascu E, Ritchie EK, Roboz GJ, Christos PJ, et al. The effect of initial molecular profile on response to recombinant interferon-α (rIFNα) treatment in early myelofibrosis. Cancer. 2017;123:2680–7.
Bose P, Deininger MW, Dunbar A, George T, Gojo I, Gundabolu K et al. NCCN Guidelines Panel Disclosures NCCN Guidelines Version 1.2020 Myeloproliferative Neoplasms. 2020.
Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European. LeukemiaNet Leuk. 2018;32:1057–69.
Knudsen TA, Hansen DL, Ocias LF, Bjerrum OW, Brabrand M, El Fassi D, et al. Long-term efficacy and safety of recombinant interferon alpha-2 vs. hydroxyurea in polycythemia vera: preliminary results from the three-year analysis of the Daliah trial - a randomized controlled phase III clinical trial. Blood. 2018;132:580.
Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7:e196–208.
Mascarenhas J, Kosiorek HE, Prchal JT, Rambaldi A, Berenzon D, Yacoub A, et al. Results of the Myeloproliferative Neoplasms - Research Consortium (MPN-RC) 112 Randomized Trial of Pegylated Interferon Alfa-2a (PEG) Versus Hydroxyurea (HU) Therapy for the Treatment of High Risk Polycythemia Vera (PV) and High Risk Essential Thrombocythemia (ET). Blood. 2018;132:577.
Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol. 1975;12:339–51.
Silver RT, Chow W, Orazi A, Arles SP, Goldsmith SJ. Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood. 2013;122:1881–6.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular Events and Intensity of Treatment in Polycythemia Vera. N. Engl J Med. 2013;368:22–33.
Cancer Institute N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017 https://www.meddra.org/ (Accessed 18 May 2019).
Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the international working group for myelofibrosis research and treatment. Leukemia. 2008;22:437–438.
Barbui T, Vannucchi AM, De Stefano V, Masciulli A, Carobbio A, Ghirardi A et al. Phase II randomized clinical trial comparing ropeginterferon versus phlebotomy in low-risk patients with polycythemia vera. results of the pre-planned interim analysis. Hematologica; 303391; LB. https://library.ehaweb.org/eha/2020/eha25th/303391/tiziano.barbui.phase.ii.randomized.clinical.trial.comparing.ropeginterferon.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dlb2602 (Accessed 5 Jul 2020).
Hasselbalch HC, Silver RT. Interferon in polycythemia vera and related neoplasms. Can it become the treatment of choice without a randomized trial? Expert Rev Hematol. 2015;8:439–45.
Verstovsek S, Silver RT, Cross NCP, Tefferi A. JAK2V617F mutational frequency in polycythemia vera: 100%, >90%, less? Leukemia. 2006;20:2067.
Wang YL, Vandris K, Jones A, Cross NCP, Christos P, Adriano F, et al. JAK2 Mutations are present in all cases of polycythemia vera. Leukemia 2008;22:1289.
Silver RT. Recombinant interferon-alpha for treatment of polycythaemia vera. Lancet (Lond, Engl). 1988;2:403.
Silver RT. Interferon-α2b: A new treatment for polycythemia vera. Ann Intern Med. 1993;119:1091–2.
Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European leukemiaNet. J Clin Oncol. 2011;29:761–70.
Srour SA, Devesa SS, Morton LM, Check DP, Curtis RE, Linet MS, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol. 2016;174:382–96.
Szuber N, Vallapureddy RR, Penna D, Lasho TL, Finke C, Hanson CA, et al. Myeloproliferative neoplasms in the young: Mayo Clinic experience with 361 patients age 40 years or younger. Am J Hematol. 2018;93:1474–84.
Karantanos T, Chaturvedi S, Braunstein EM, Spivak J, Resar L, Karanika S, et al. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood Adv. 2020;4:2567–76.
Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32.
Alvarez-Larr an A, Kerguelen A, Hern andez-Boluda JC, erez-Encinas MP, Ferrer-Mar ın F, arez AB, et al. Frequency and prognostic value of resistance/intolerance to hydroxycarbamide in 890 patients with polycythaemia vera. Br J Haematol. 2016;172:786–93.
Acknowledgements
We acknowledge Paul J Christos, PhD for his review of the statistical methodology and results.
This study was supported by grants from the David L. Johns Family of the Cancer Research & Treatment Fund (CR&T), the Myeloproliferative Neoplasms Research Foundation (MPN-RF), the Clinical & Translational Science Center (CTSC) of Weill Cornell and the National Center for Advancing Translational Sciences (NCATS) (grant # 1 UL1 TR002384-04).
Data sharing statement
Data will not be publicly available due to HIPAA regulations. Similarly, individual patient data will not be shared. However, a request for sharing de-identified data can be made to the corresponding author and approval will be sought from the IRB.
Author information
Authors and Affiliations
Contributions
GAZ – designed the study, collected data, analyzed data, examined patients and wrote the paper. SK – collected data, extracted data and analyzed data. TC, GH, DJ, NS, CS – collected data. EKR – examined patients and reviewed manuscript. JMS – conceived and designed the study, examined patients, and reviewed manuscript. RTS – conceived and designed the study, examined patients and wrote the paper. All authors approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
GAZ – Consultancy, PharmaEssentia. SK, TC, GH, DJ, NS, CS, EKR, and JMS – No conflicts of interest to disclose. RTS – Speaker bureau and consultancy, PharmaEssentia.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented in part at the annual meetings of the American Society of Hematology in Atlanta, GA, 2018; Orlando, FL, 2019, and San Diego, CA, 2020.
Rights and permissions
About this article
Cite this article
Abu-Zeinah, G., Krichevsky, S., Cruz, T. et al. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia 35, 2592–2601 (2021). https://doi.org/10.1038/s41375-021-01183-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01183-8
This article is cited by
-
Splenomegaly (SPML) in polycythemia vera (PV): its clinical significance and its relation to symptoms, post-polycythemic myelofibrosis (PPMF) and survival
Leukemia (2023)
-
Clinicohematologic and molecular response of essential thrombocythemia patients treated with pegylated interferon-α: a multi-center study of the German Study Group-Myeloproliferative Neoplasms (GSG-MPN)
Leukemia (2023)
-
Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment
Leukemia (2023)
-
Clonal architecture evolution in Myeloproliferative Neoplasms: from a driver mutation to a complex heterogeneous mutational and phenotypic landscape
Leukemia (2023)
-
Inhibition of ERK1/2 signaling prevents bone marrow fibrosis by reducing osteopontin plasma levels in a myelofibrosis mouse model
Leukemia (2023)