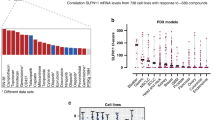
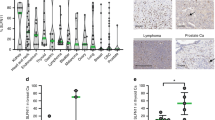
Abstract
Background
Although unresectable or recurrent gastric cancers (GC) are frequently treated with platinum-based chemotherapy, response to treatment remains unpredictable. Because Schlafen 11 (SLFN11) is recently identified as a critical determinant of platinum sensitivity, we investigated the potential clinical utility of SLFN11 in the treatment of GC.
Methods
We analysed the correlation between SLFN11 expression and overall survival in 169 GC patients by our established immunohistochemical approach. The impact of SLFN11 expression on the response to platinum and transition of SLFN11 expression upon long-term treatment with platinum were examined using GC cell lines and organoids.
Results
GC patients with high-SLFN11 expression exhibited significantly better survival than those with low-SLFN11 expression, and the significance increased when we selected patients treated with platinum-based chemotherapy. Knockout of SLFN11 and reactivation of SLFN11 in GC cells conferred resistance and sensitivity to platinum, respectively. In GC cells and organoids, long-term treatment with oxaliplatin suppressed SLFN11 expression while imparting drug resistance. The acquired resistance to oxaliplatin was reversed by reactivation of SLFN11 with epigenetic modifying drugs.
Conclusions
This is the first report revealing definitive clinical implications of SLFN11 in the treatment of GC patients and providing novel strategies for the drug selection based on SLFN11 expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J. Cancer 136, E359–E386 (2015).
Davidson, M., Okines, A. F. & Starling, N. Current and future therapies for advanced gastric cancer. Clin. Colorectal Cancer 14, 239–250 (2015).
Takahari, D., Chin, K., Ishizuka, N., Takashima, A., Minashi, K., Kadowaki, S. et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naive, HER2-positive advanced gastric cancer. Gastric Cancer 22, 1238–1246 (2019).
Yamada, Y., Higuchi, K., Nishikawa, K., Gotoh, M., Fuse, N., Sugimoto, N. et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann. Oncol. 26, 141–148 (2015).
Murai, J. & Targeting, D. N. A. repair and replication stress in the treatment of ovarian cancer. Int J. Clin. Oncol. 22, 619–628 (2017).
Murai, J., Thomas, A., Miettinen, M. & Pommier, Y. Schlafen 11 (SLFN11), a restriction factor for replicative stress induced by DNA-targeting anti-cancer therapies. Pharm. Ther. 201, 94–102 (2019).
Li, M., Kao, E., Malone, D., Gao, X., Wang, J. Y. J. & David, M. DNA damage-induced cell death relies on SLFN11-dependent cleavage of distinct type II tRNAs. Nat. Struct. Mol. Biol. 25, 1047–1058 (2018).
Barretina, J., Caponigro, G., Stransky, N., Venkatesan, K., Margolin, A. A., Kim, S. et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Zoppoli, G., Regairaz, M., Leo, E., Reinhold, W. C., Varma, S., Ballestrero, A. et al. Putative DNA/RNA helicase Schlafen-11 (SLFN11) sensitizes cancer cells to DNA-damaging agents. Proc. Natl Acad. Sci. USA 109, 15030–15035 (2012).
Tang, S. W., Thomas, A., Murai, J., Trepel, J. B., Bates, S. E., Rajapakse, V. N. et al. Overcoming resistance to DNA-targeted agents by epigenetic activation of Schlafen 11 (SLFN11) expression with class I histone deacetylase inhibitors. Clin. Cancer Res. 24, 1944–1953 (2018).
Rathkey, D., Khanal, M., Murai, J., Zhang, J., Sengupta, M., Jiang, Q. et al. Sensitivity of mesothelioma cells to PARP inhibitors is not dependent on BAP1 but Is enhanced by temozolomide in cells with high-Schlafen 11 and low-O6-methylguanine-DNA methyltransferase expression. J. Thorac. Oncol. 15, 843–859 (2020).
Murai, J., Feng, Y., Yu, G. K., Ru, Y., Tang, S. W., Shen, Y. et al. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 7, 76534–76550 (2016).
Marzi, L., Agama, K., Murai, J., Difilippantonio, S., James, A., Peer, C. J. et al. Novel fluoroindenoisoquinoline non-camptothecin topoisomerase I inhibitors. Mol. Cancer Ther. 17, 1694–1704 (2018).
Lok, B. H., Gardner, E. E., Schneeberger, V. E., Ni, A., Desmeules, P., Rekhtman, N. et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin. Cancer Res 23, 523–535 (2017).
Iwasaki, J., Komori, T., Nakagawa, F., Nagase, H., Uchida, J., Matsuo, K. et al. Schlafen11 expression is associated with the antitumor activity of trabectedin in human sarcoma cell lines. Anticancer Res. 39, 3553–3563 (2019).
Gardner, E. E., Lok, B. H., Schneeberger, V. E., Desmeules, P., Miles, L. A., Arnold, P. K. et al. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 Axis. Cancer Cell 31, 286–299 (2017).
Coussy, F., El-Botty, R., Chateau-Joubert, S., Dahmani, A., Montaudon, E., Leboucher, S. et al. BRCAness, SLFN11, and RB1 loss predict response to topoisomerase I inhibitors in triple-negative breast cancers. Sci. Transl. Med. 12, aax2625 (2020).
Conteduca, V., Ku, S. Y., Puca, L., Slade, M., Fernandez, L., Hess, J. et al. SLFN11 expression in advanced prostate cancer and response to platinum-based chemotherapy. Mol. Cancer Ther. 19, 1157–1164 (2020).
Pietanza, M. C., Waqar, S. N., Krug, L. M., Dowlati, A., Hann, C. L., Chiappori, A. et al. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J. Clin. Oncol. 36, 2386–2394 (2018).
Winkler, C., Armenia, J., Jones, G. N., Tobalina, L., Sale, M. J., Petreus, T. et al. SLFN11 informs on standard of care and novel treatments in a wide range of cancer models. Br. J. Cancer https://doi.org/10.1038/s41416-020-01199-4 (2020).
Kagami, T., Yamade, M., Suzuki, T., Uotani, T., Tani, S., Hamaya, Y. et al. The first evidence for SLFN11 expression as an independent prognostic factor for patients with esophageal cancer after chemoradiotherapy. BMC Cancer 20, 1123 (2020).
Murai, J., Tang, S. W., Leo, E., Baechler, S. A., Redon, C. E., Zhang, H. et al. SLFN11 Blocks stressed replication forks independently of ATR. Mol. Cell 69, 371–384.e376 (2018).
Murai, J., Zhang, H., Pongor, L., Tang, S. W., Jo, U., Moribe, F. et al. Chromatin remodeling and immediate early gene activation by SLFN11 in response to replication stress. Cell Rep. 30, 4137–4151 e4136 (2020).
Nogales, V., Reinhold, W. C., Varma, S., Martinez-Cardus, A., Moutinho, C., Moran, S. et al. Epigenetic inactivation of the putative DNA/RNA helicase SLFN11 in human cancer confers resistance to platinum drugs. Oncotarget 7, 3084–3097 (2016).
Peng, Y., Wang, L., Wu, L., Zhang, L., Nie, G. & Guo, M. Methylation of SLFN11 promotes gastric cancer growth and increases gastric cancer cell resistance to cisplatin. J. Cancer 10, 6124–6134 (2019).
Takashima, T., Sakamoto, N., Murai, J., Taniyama, D., Honma, R., Ukai, S. et al. Immunohistochemical analysis of SLFN11 expression uncovers potential non-responders to DNA-damaging agents overlooked by tissue RNA-seq. Virchows Arch https://doi.org/10.1007/s00428-020-02840-6 (2020).
Namikawa, T. & Hanazaki, K. Mucin phenotype of gastric cancer and clinicopathology of gastric-type differentiated adenocarcinoma. World J. Gastroenterol. 16, 4634–4639 (2010).
Ishikawa, A., Sakamoto, N., Honma, R., Taniyama, D., Fukada, K., Hattori, T. et al. Annexin A10 is involved in the induction of pancreatic duodenal homeobox1 in gastric cancer tissue, cells and organoids. Oncol. Rep. 43, 581–590 (2020).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Yoo, C. H., Noh, S. H., Shin, D. W., Choi, S. H. & Min, J. S. Recurrence following curative resection for gastric carcinoma. Br. J. Surg. 87, 236–242 (2000).
D'Angelica, M., Gonen, M., Brennan, M. F., Turnbull, A. D., Bains, M. & Karpeh, M. S. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann. Surg. 240, 808–816 (2004).
Seidlitz, T., Merker, S. R., Rothe, A., Zakrzewski, F., von Neubeck, C., Grutzmann, K. et al. Human gastric cancer modelling using organoids. Gut 68, 207–217 (2019).
Narasimhan, V., Wright, J. A., Churchill, M., Wang, T., Rosati, R., Lannagan, T. R. M. et al. Medium-throughput drug screening of patient-derived organoids from colorectal peritoneal metastases to direct personalized therapy. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-20-0073 (2020).
Yao, Y., Xu, X., Yang, L., Zhu, J., Wan, J., Shen, L. et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 26, 17–26 e16 (2020).
Dombret, H., Seymour, J. F., Butrym, A., Wierzbowska, A., Selleslag, D., Jang, J. H. et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126, 291–299 (2015).
Fenaux, P., Mufti, G. J., Hellstrom-Lindberg, E., Santini, V., Finelli, C., Giagounidis, A. et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 10, 223–232 (2009).
Ryan, Q. C., Headlee, D., Acharya, M., Sparreboom, A., Trepel, J. B., Ye, J. et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J. Clin. Oncol. 23, 3912–3922 (2005).
Ivanova, T., Zouridis, H., Wu, Y., Cheng, L. L., Tan, I. B., Gopalakrishnan, V. et al. Integrated epigenomics identifies BMP4 as a modulator of cisplatin sensitivity in gastric cancer. Gut 62, 22–33 (2013).
Kimura, A., Ogata, K., Altan, B., Yokobori, T., Ide, M., Mochiki, E. et al. Nuclear heat shock protein 110 expression is associated with poor prognosis and chemotherapy resistance in gastric cancer. Oncotarget 7, 18415–18423 (2016).
Ye, Y., Li, J., Jiang, D., Li, J., Xiao, C., Li, Y. et al. FGFR4 Gly388Arg polymorphism affects the progression of gastric cancer by activating STAT3 pathway to induce epithelial to mesenchymal transition. Cancer Res. Treat. https://doi.org/10.4143/crt.2020.138 (2020).
Ye, Y., Li, X., Yang, J., Miao, S., Wang, S., Chen, Y. et al. MDM2 is a useful prognostic biomarker for resectable gastric cancer. Cancer Sci. 104, 590–598 (2013).
Fan, Y., Ying, H., Wu, X., Chen, H., Hu, Y., Zhang, H. et al. The mutational pattern of homologous recombination (HR)-associated genes and its relevance to the immunotherapeutic response in gastric cancer. Cancer Biol. Med. 17, 1002–1013 (2020).
Mu, Y., Lou, J., Srivastava, M., Zhao, B., Feng, X. H., Liu, T. et al. SLFN11 inhibits checkpoint maintenance and homologous recombination repair. EMBO Rep. 17, 94–109 (2016).
Murai, J. & Pommier, Y. PARP trapping beyond homologous recombination and platinum sensitivity in cancers. Annu Rev. Cancer Biol. 3, 131–150 (2019).
Acknowledgements
The authors thank Mr. Shinichi Norimura (Technical Center, Hiroshima University) for his excellent technical assistance. We appreciate Dr. Eric Smith at the University of Cincinnati for the professional editing. We also thank the Analysis Center of Life Science of Hiroshima University for the use of their facilities.
Author information
Authors and Affiliations
Contributions
T.T., N.S. and J.M. designed the study. T.T., D.T., N.S., R.A., R.H., U.K., K.K., K.T. and H.O. collected and analysed the patient clinical data. T.T., D.T, M.Y., R.A., T.H., R.H., P.Q.T., S.U., R.M. and K.H. performed the experiments and collected and analysed the data. K.K., H.O., AT.S., E.M. and W.Y. interpreted and analysed the results. T.T., D.T., N.S., AT.S. and J.M. drafted and edited the paper. All of the authors read and approved the final paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kure Medical Center and Chugoku Cancer Center, Kure, Japan (No. 2019-36), Human Genome Research of Hiroshima University, Hiroshima (E 597 01) and conformed to the ethical guidelines of the Declaration of Helsinki. All patients provided written informed consent to participate in this study.
Consent to publish
Not applicable.
Data availability
All the data supporting the findings of this study are available within the Supplementary Information files and from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
This work was supported by Grants-in-Aid for Scientific Research (JP15H04713 and JP16K08691 to W.Y., JP16H06999 to N.S. and JP19H03505 to J.M.), Challenging Exploratory Research (26670175, JP16K15247 to W.Y.) from the Japan Society for the Promotion of Science, the National Institute of Health (NIH) (R01NS089815 to A.T.S.) and a research grant from The Uehara Memorial Foundation (to J.M.). This work was supported in part by research funds from the Yamagata prefectural government and the City of Tsuruoka.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Takashima, T., Taniyama, D., Sakamoto, N. et al. Schlafen 11 predicts response to platinum-based chemotherapy in gastric cancers. Br J Cancer 125, 65–77 (2021). https://doi.org/10.1038/s41416-021-01364-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01364-3
This article is cited by
-
Transcriptome analysis of an AKT inhibitor-resistant endometrial cancer cell line
Pharmacological Reports (2024)
-
Schlafen 12 restricts HIV-1 latency reversal by a codon-usage dependent post-transcriptional block in CD4+ T cells
Communications Biology (2023)
-
Structural, molecular, and functional insights into Schlafen proteins
Experimental & Molecular Medicine (2022)
-
Circulating tumor cell heterogeneity in neuroendocrine prostate cancer by single cell copy number analysis
npj Precision Oncology (2021)
-
A wake-up call for cancer DNA damage: the role of Schlafen 11 (SLFN11) across multiple cancers
British Journal of Cancer (2021)