Abstract
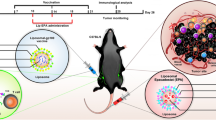
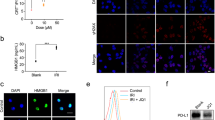
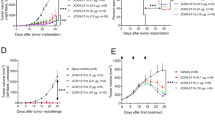
Programmed cell death protein-1 (PD-1), as an immune checkpoint molecule, attenuates T-cell activity and induces T-cell exhaustion. Although siRNA has a great potential in cancer immunotherapy, its delivery to target cells is the main limitation of using siRNA. This study aimed to prepare a liposomal formulation as a siRNA carrier to silence PD-1 expression in T cells and investigate it’s in vivo antitumor efficacy. The liposomal siRNA was prepared and characterized by size, zeta potential, and biodistribution. Following that, the uptake assay and mRNA silencing were evaluated in vitro at mRNA and protein levels. siRNA-PD-1 (siPD-1)-loaded liposome nanoparticles were injected into B16F0 tumor-bearing mice to evaluate tumor growth, tumor-infiltrating lymphocytes, and survival rate. Liposomal siPD-1 efficiently silenced PD-1 mRNA expression in T cells (P < 0.0001), and siPD-1-loaded liposomal nanoparticles enhanced the infiltration of T-helper 1 (Th 1) and cytotoxic T lymphocytes into the tumor tissue (P < 0.0001). Liposome-PD-1 siRNA monotherapy and PD-1 siRNA-Doxil (liposomal doxorubicin) combination therapy improved the survival significantly, compared to the control treatment (P < 0.001). Overall, these findings suggest that immunotherapy with siPD-1-loaded liposomes by enhancing T-cell-mediated antitumor immune responses could be considered as a promising strategy for the treatment of melanoma cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Mullen CA, Schreiber H. Tumor growth and evasion of immune destruction: UV-induced tumors as a model. Surv immunologic Res. 1985;4:264–70.
Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–72.
He X, Xu C. PD-1: a driver or passenger of T cell exhaustion? Mol Cell. 2020;77:930–1.
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8.
Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13.
Konstantinidou M, Zarganes-Tzitzikas T, Magiera-Mularz K, Holak TA, Domling A. Immune checkpoint PD-1/PD-L1: is there life beyond antibodies? Angew Chem. 2018;57:4840–8.
Buchbinder EI, Desai ACTLA-4. and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98.
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–72.
Bertucci F, Finetti P, Mamessier E, Pantaleo MA, Astolfi A, Ostrowski J, et al. PDL1 expression is an independent prognostic factor in localized GIST. Oncoimmunology. 2015;4:e1002729.
Sponaas A-M, Moharrami NN, Feyzi E, Standal T, Rustad EH, Waage A, et al. PDL1 expression on plasma and dendritic cells in myeloma bone marrow suggests benefit of targeted anti PD1-PDL1 therapy. PLoS ONE. 2015;10:e0139867.
Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1 + CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc Natl Acad Sci USA. 2017;114:4993–8.
Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–45.
Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; anti–PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286–93.
Falchook GS, Leidner R, Stankevich E, Piening B, Bifulco C, Lowy I, et al. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J Immunother Cancer. 2016;4:1–5.
Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004;432:173.
Jagani H, Rao JV, Palanimuthu VR, Hariharapura RC, Gang S. A nanoformulation of siRNA and its role in cancer therapy: in vitro and in vivo evaluation. Cell Mol Biol Lett. 2013;18:120.
Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J. 2011;6:1130–46.
Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38.
Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123:1183–9.
Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–6.
Tsermentseli SK, Kontogiannopoulos KN, Papageorgiou VP, Assimopoulou AN. Comparative study of PEGylated and conventional liposomes as carriers for shikonin. Fluids. 2018;3:36.
Howard FB, Levin IW. Lipid vesicle aggregation induced by cooling. Int J Mol Sci. 2010;11:754–61.
Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Tropical J Pharm Res. 2013;12:265–73.
Mirzaaghasi A, Han Y, Ahn S-H, Choi C, Park J-H. Biodistribution and pharmacokinectics of liposomes and exosomes in a mouse model of sepsis. Pharmaceutics 2021;13:427.
Koning GA, Storm G. Targeted drug delivery systems for the intracellular delivery of macromolecular drugs. Drug Discov Today. 2003;8:482–3.
Metselaar JM, Storm G. Liposomes in the treatment of inflammatory disorders. Expert Opin Drug Deliv. 2005;2:465–76.
Faisal SM, Chen J-w, McDonough SP, et al. Y-F. Immunostimulatory and antigen delivery properties of liposomes made up of total polar lipids from non-pathogenic bacteria leads to efficient induction of both innate and adaptive immune responses. Vaccine. 2011;29:2381–91.
Stecher C, Battin C, Leitner J, Zettl M, Grabmeier-Pfistershammer K, Höller C, et al. PD-1 blockade promotes emerging checkpoint inhibitors in enhancing T cell responses to allogeneic dendritic cells. Front Immunol. 2017;8:572.
Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH Jr, et al. Tumor infiltrating Foxp3+ regulatory T‐cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006;107:2866–72.
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39.
Cai J, Wang D, Zhang G, Guo X. The role of PD-1/PD-L1 axis in Treg development and function: implications for cancer immunotherapy. OncoTargets Ther. 2019;12:8437.
Wang C, Yi T, Qin L, Maldonado RA, von Andrian UH, Kulkarni S, et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Investig. 2013;123:1677–93.
Yoshida K, Okamoto M, Sasaki J, Kuroda C, Ishida H, Ueda K, et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer. 2020;20:1–10.
Hu J, Sun C, Bernatchez C, Xia X, Hwu P, Dotti G, et al. T-cell homing therapy for reducing regulatory T cells and preserving effector T-cell function in large solid tumors. Clin Cancer Res. 2018;24:2920–34.
De La Cruz LM, Nocera NF, Czerniecki BJ. Restoring anti-oncodriver Th1 responses with dendritic cell vaccines in HER2/neu-positive breast cancer: progress and potential. Immunotherapy. 2016;8:1219–32.
Liudahl SM, Coussens L. To help or to harm: dynamic roles of CD4+ T helper cells in solid tumor microenvironments. In Immunology: Immunotoxicology, Immunopathology, and Immunotherapy. Elsevier. 2017;1:97-116. https://doi.org/10.1016/B978-0-12-809819-6.00008-3.
Nie X, Chen W, Zhu Y, Huang B, Yu W, Wu Z, et al. B7-DC (PD-L2) costimulation of CD4+ T-helper 1 response via RGMb. Cell Mol Immunol. 2018;15:888–97.
Yamamura M, Modlin RL, Ohmen JD, Moy RL. Local expression of antiinflammatory cytokines in cancer. J Clin Investig. 1993;91:1005–10.
Oshikawa K, Yanagisawa K, Ohno S, Tominaga S-I, Sugiyama Y. Expression of ST2 in helper T lymphocytes of malignant pleural effusions. Am J Respiratory Crit Care Med. 2002;165:1005–9.
Chen Y-M, Yang W-K, Whang-Peng J, Tsai C-M, Perng R-P. An analysis of cytokine status in the serum and effusions of patients with tuberculous and lung cancer. Lung Cancer. 2001;31:25–30.
Chtanova T, Mackay CR. T cell effector subsets: extending the Th1/Th2 paradigm. Adv Immunol. 2001;78:233–66.
Park JY, Jang MJ, Chung YH, Kim KY, Kim SS, Lee WB, et al. Doxorubicin enhances CD4+ T-cell immune responses by inducing expression of CD40 ligand and 4-1BB. Int Immunopharmacol. 2009;9:1530–9.
Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. 2020;124:359–67.
Inozume T, Hanada K-i, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, et al. Selection of CD8+ PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T-cells. J Immunother. 2010;33:956.
Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer. 2018;65:e27227.
Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in tumor microenvironment. Computat Struct Biotechnol J. 2019;17:1–13.
Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306.
Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Investig. 2014;124:2246–59.
Li C, Han X. Melanoma cancer immunotherapy using PD-L1 siRNA and imatinib promotes cancer-immunity cycle. Pharm Res. 2020;37:1–10.
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286.
Morris K, Castanotto D, Al-Kadhimi Z, Jensen M, Rossi J, Cooper LJ. Enhancing siRNA effects in T cells for adoptive immunotherapy. Hematology. 2005;10:461–7.
Funding
We are thankful for the financial support from the Nanotechnology Research, Mashhad University of medical sciences, Mashhad, Iran (grant number: 971960). We would also like to thank Dr. Amir Abbas Momtazi-Borojeni, a post-doctoral researcher of Iran’s National Elites Foundation at the Mashhad University of Medical Sciences, for the final scientific polishing and edition of the present paper.
Author information
Authors and Affiliations
Contributions
MB conceived the presented idea, wrote the manuscript, performed the experiments, and analyzed data. He also designed the study. FM assisted with measurements. ARN gave consultation. MS gave consultation with the model preparation. HNA helped with the edition of manuscript. AS contributed to sample preparation. MM assisted with measurements. JTA helped with his laboratory facilities. MM supervised the study and gavec onsultation with the writing of the manuscript. MRJ supervised the project and financially supported the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barati, M., Mirzavi, F., Nikpoor, A.R. et al. Enhanced antitumor immune response in melanoma tumor model by anti-PD-1 small interference RNA encapsulated in nanoliposomes. Cancer Gene Ther 29, 814–824 (2022). https://doi.org/10.1038/s41417-021-00367-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-021-00367-9
This article is cited by
-
Immune checkpoint inhibition mediated with liposomal nanomedicine for cancer therapy
Military Medical Research (2023)
-
Advances in PD-1 signaling inhibition-based nano-delivery systems for tumor therapy
Journal of Nanobiotechnology (2023)
-
CD73 downregulation by EGFR-targeted liposomal CD73 siRNA potentiates antitumor effect of liposomal doxorubicin in 4T1 tumor-bearing mice
Scientific Reports (2022)
-
Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy
Acta Pharmacologica Sinica (2022)