Abstract
Regulatory T (Treg) cells derived from the thymus (tTreg) and periphery (pTreg) have central and distinct functions in immunosuppression, but mechanisms for the generation and activation of Treg subsets in vivo are unclear. Here, we show that mechanistic target of rapamycin (mTOR) unexpectedly supports the homeostasis and functional activation of tTreg and pTreg cells. mTOR signaling is crucial for programming activated Treg-cell function to protect immune tolerance and tissue homeostasis. Treg-specific deletion of mTOR drives spontaneous effector T-cell activation and inflammation in barrier tissues and is associated with reduction in both thymic-derived effector Treg (eTreg) and pTreg cells. Mechanistically, mTOR functions downstream of antigenic signals to drive IRF4 expression and mitochondrial metabolism, and accordingly, deletion of mitochondrial transcription factor A (Tfam) severely impairs Treg-cell suppressive function and eTreg-cell generation. Collectively, our results show that mTOR coordinates transcriptional and metabolic programs in activated Treg subsets to mediate tissue homeostasis.
Similar content being viewed by others
Introduction
Regulatory T (Treg) cells expressing the transcription factor Foxp3 suppress conventional T-cell responses to establish self-tolerance, prevent autoimmunity, and maintain tissue homeostasis1,2. Foxp3 deficiency eliminates Treg-cell development and function, leading to autoimmune diseases characterized by excessive T helper 1 (TH1), TH2, or TH17 responses, and germinal center (GC) B-cell reactions driven by T follicular helper (TFH) cells3,4,5. Thymic-derived Treg (tTreg) cells exit the thymus and populate peripheral tissues, where resting Treg cells [also called central Treg (cTreg) cells] are activated in response to antigen and inflammatory cues6,7,8,9. These activation signals increase effector molecule expression and induce transcription factors that define the selective suppressive functions and tissue localization of activated Treg cells [also known as effector Treg (eTreg) cells]5,10,11,12,13,14,15. Peripherally-derived Treg (pTreg) cells are a developmentally distinct population of activated Treg cells that arises from the naive CD4+ T-cell pool and inhibit TH2 or TH17 responses at mucosal sites6,16,17,18,19. The transcription factor interferon regulatory factor 4 (IRF4) is expressed in both eTreg and pTreg cells in vivo and is an essential positive regulator of their homeostasis and function7,15,17,20,21,22. IRF4 expression and function are induced by TCR signals in Treg cells by incompletely understood mechanisms7,8,22.
Metabolic rewiring is important for T-cell fate decisions, but the metabolic programs regulating Treg-cell activation and specialization remain uncertain23. The activation of the mechanistic target of rapamycin (mTOR) induces metabolic reprogramming necessary for conventional T-cell activation and differentiation23,24. In contrast, mTOR appears to antagonize Treg-cell differentiation and expansion in vitro and suppressive activity in vivo23,25,26. Mechanistically, inhibition of mTOR upregulates fatty acid oxidation, which supports mitochondrial respiration important for Treg-cell differentiation, proliferation, and survival in vitro27,28. Moreover, low levels of mTOR activation are needed to prevent excessive glycolysis that can impair Treg-cell survival and lineage stability23. Although the prevailing model is that mTOR activation hinders Treg-cell function, Treg cells have higher basal levels of mTORC1 activation than conventional T cells29,30, which is essential for Treg-cell function in vivo30. Thus, mTOR-dependent metabolic programming might have context-dependent roles in different Treg-subsets or under distinct physiological conditions.
Here, we show that mTOR orchestrates activation-induced transcriptional and metabolic signatures that are essential for Treg-cell activation and function. We find that either acute or chronic inhibition of mTOR disrupts Treg-cell suppressive activity and leads to uncontrolled conventional T-cell activation. In line with this observation, mucosal CD4+ T-cell responses, including TH2 responses, are increased when Treg cells lose mTOR, associated with a loss of eTreg and pTreg cells in mucosal sites. Mechanistically, mTOR mediates Treg-cell activation and suppressive activity by promoting IRF4 expression and mitochondrial metabolism. Indeed, disruption of mitochondrial metabolism severely impairs the suppressive function of activated Treg cells and their homeostasis in tissues. Collectively, our results show that mTOR controls peripheral tolerance by integrating transcriptional and metabolic programs critical for the homeostasis and suppressive activity of activated Treg cells.
Results
mTOR promotes activated Treg-cell suppressive activity
Treg cells activated in vivo have enhanced suppressive activity critical for immune homeostasis7,8,31,32, yet the molecular events controlling Treg-cell activation remain to be fully defined. To identify pathways associated with increased suppressive function of Treg cells, we mined a published dataset of activated Treg cells isolated from diphtheria toxin (DT)-treated Foxp3DTR mice (DTR, diphtheria toxin receptor)32. Gene set enrichment analysis (GSEA) revealed that the hallmark mTORC1 and PI3K-Akt-mTOR signaling pathways were among the most significantly (false discovery rate, FDR < 0.05) upregulated gene sets in activated vs. resting Treg cells (Fig. 1a). Thus, increased Treg-cell suppressive activity is correlated with enhanced mTOR signaling. To rigorously test the function of mTOR for the suppressive activity of activated Treg cells, we activated Treg cells in vitro in the presence or absence of the mTOR inhibitor, PP242. We found that acute inhibition of mTOR diminished the ability of activated Treg cells to suppress conventional T-cell proliferation (Fig. 1b) and to express the immunosuppressive molecule CTLA4 (Fig. 1c), indicating a kinase-dependent function of mTOR in Treg-cell function. Accordingly, the suppressive activity of Treg cells isolated from Cd4CreMtorfl/fl mice was dampened (Fig. 1d). Thus, mTOR is essential for the suppressive function of Treg cells in vitro.
mTOR is essential for activated Treg cell function. a Enrichment plots of the Hallmark mTORC1 (left) and Hallmark PI3K-Akt-mTOR (right) signaling pathways in activated Treg (aTreg) compared to resting Treg (rTreg) cells, identified by gene set enrichment analysis (GSEA). The top 20 enriched genes in each pathway (position indicated by the vertical black line) are listed to the right of each plot. b In vitro suppressive activity of Treg cells activated in the presence or absence of PP242. TN: naive CD4+ T cells. c Flow cytometry analysis of CTLA4 expression in Treg cells activated in the presence or absence of PP242. d In vitro suppressive activity of Treg cells isolated from Cd4CreMtor+/+ or +/fl or Cd4CreMtorfl/fl mice. e Representative image of lymphadenopathy in Foxp3Cre/DTRMtorfl/fl mice after DT treatment (left). Right, cell numbers of the spleen and peripheral lymph nodes (pLN) of Foxp3Cre/DTRMtor+/+ or +/fl or Foxp3Cre/DTRMtorfl/fl mice. f Flow cytometry analysis of naive and effector/memory CD4+Foxp3-YFP− in pLN. g, h Cells from the spleen and pLN of Foxp3Cre/DTRMtor+/+ or +/fl or Foxp3Cre/DTRMtorfl/fl mice that received DT treatments were stimulated using PMA and ionomycin for 4–5 h. g Flow cytometry analysis of IL-4-producing CD4+ T cells in the spleen and pLN. h Quantification of IFN-γ+ CD4+Foxp3− and CD8+ T cells or IL-4+ and IL-17A+ CD4+Foxp3− T cells in the spleen. Error bars show mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; unpaired, two-tailed Student’s t-test. Data are representative of three (c) or four (e–g) biological replicates from three (c) or two (e−g) independent experiments. Data are quantified from three technical replicates representative of three independent experiments (b, d) or four or five biological replicates per group as indicated, compiled from two independent experiments (e, h). Numbers indicate percentage of cells in gates
To establish a role for mTOR in Treg-cell function in vivo, we generated female Foxp3Cre/DTRMtorfl/fl mosaic mice. These mice express a floxed Mtor allele24, whose expression can be deleted by Cre recombinase driven under the Foxp3 promoter (denoted as Foxp3Cre)33, resulting in the deletion of mTOR within Treg cells after they have expressed Foxp3. Acute depletion of DTR-expressing Treg cells with DT forces the remaining Foxp3Cre-expressing Treg cells to become activated, expand, and control immune homeostasis in adult mice34. Upon DT treatment, Foxp3Cre/DTRMtorfl/fl mosaic mice, but not their respective Foxp3Cre/DTRMtor+/+ or +/fl controls, developed inflammation associated with increased organ size and cell number, especially peripheral lymph nodes (Fig. 1e). Further, there were increased frequencies of CD44hiCD62Llo effector/memory CD4+ in the peripheral lymph nodes (Fig. 1f). After DT treatment, Foxp3Cre/DTRMtorfl/fl mice had a profound enrichment for IL-4-producing and small but significant increase in IL-17A-producing, but not IFN-γ-producing, CD4+ T cells in the spleen (Fig. 1g, h). Similar observations were found in peripheral lymph nodes (Fig. 1g). Therefore, mTOR signaling is essential for the suppressive function of activated Treg cells, and its acute deletion in Treg cells leads to loss of immune homeostasis and the activation of TH2, and to a lesser extent, TH17 cells.
Treg cells require mTOR to prevent spontaneous autoimmunity
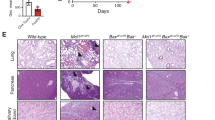
To determine the effects of long-term deletion of Mtor on Treg-cell suppressive function in vivo, we next generated mice bearing a conditional deletion of Mtor within all committed Foxp3+ Treg cells (denoted as Foxp3CreMtorfl/fl mice). As anticipated, Mtor was efficiently deleted within Foxp3-YFP+ Treg cells from Foxp3CreMtorfl/fl mice (Supplementary Fig. 1a). In contrast to their littermate controls that remained healthy, Foxp3CreMtorfl/fl mice developed an early-onset lymphoproliferative and autoimmune disease, indicated by reduced body size and hunched posture, enlargement of peripheral lymphoid organs, and extensive lymphocyte and/or myeloid cell infiltration in multiple organs, such as the skin and lung (Fig. 2a–c). This disease ultimately led to the early death of Foxp3CreMtorfl/fl mice (Fig. 2d). These mice had reduced frequencies of CD44loCD62Lhi naive CD4+ and CD8+ T cells and increased frequencies of CD44hiCD62Llo effector/memory phenotype CD4+ and CD8+ T cells (Fig. 2e). There were also significant increases in IFN-γ-, IL-4-, IL-10-, IL-13-, and IL-17A-producing CD4+ T cells and IFN-γ-producing CD8+ T cells in mice with mTOR-deficient Treg cells (Fig. 2f and Supplementary Fig. 1b). Foxp3CreMtorfl/fl mice had 5–10-fold and 10–15-fold increases in the frequencies of cells producing TH2- or TH17-associated cytokines, respectively, while IFN-γ-producing cells were increased by ~5-fold (Supplementary Fig. 1c). Within Treg cells, the frequency of IFN-γ-producing cells was also increased in Foxp3CreMtorfl/fl mice (Supplementary Fig. 1d). We also found that the frequencies and total numbers of PD-1+CXCR5+ TFH cells (Fig. 2g) and CD95+GL7+ GC B cells (Fig. 2h) were increased in Foxp3CreMtorfl/fl mice. We, therefore, performed immunohistochemistry analysis of GCs, B cells, and T cells in whole tissue sections. This analysis revealed that PNA+ cells were diffusely distributed in extrafollicular regions, while T and B cells were markedly increased, in mesenteric lymph nodes (Supplementary Fig. 1e). Foxp3CreRptorfl/fl mice, which have impaired mTORC1 signaling30, also had elevated TFH and GC B-cell responses (Supplementary Fig. 1f), indicating that mTORC1 is essential for the Treg-cell-mediated suppression of spontaneous GC reactions. To determine if TFH cells produce elevated levels of IL-4 and/or IL-21 to promote GC reactions35,36, we isolated CD4+Foxp3-YFP−CD44hiCXCR5−PD-1− non-TFH cells and CD4+Foxp3-YFP−CD44hiCXCR5+PD-1+ TFH cells from Foxp3CreMtorfl/fl mice and their littermate controls, and measured the expression of Il4 and Il21. TFH cells from Foxp3CreMtorfl/fl mice had increased expression of Il4, but not Il21, while non-TFH cells had increased expression of both Il4 and Il21 (Supplementary Fig. 1g, h). Thus, constitutive depletion of mTOR revealed its essential role for Treg cell-mediated suppression of conventional T-cell responses in vivo.
Disruption of mTOR in Treg cells results in fatal autoimmunity. a Representative image of 47-day-old Foxp3CreMtor+/fl and Foxp3CreMtorfl/fl littermates. b Representative image of lymphadenopathy in 47-day-old Foxp3CreMtorfl/fl mice (left). Right, cell numbers of the spleen and peripheral lymph nodes (pLN) of Foxp3CreMtor+/+, Foxp3CreMtor+/fl, or Foxp3CreMtorfl/fl mice. The numbers of mice per group are indicated. c Representative hematoxylin and eosin staining of the indicated tissues from 6-week-old Foxp3CreMtor+/fl and Foxp3CreMtorfl/fl mice. The magnifications are indicated above the respective images for each tissue. d Survival curve of Foxp3CreMtor+/fl and Foxp3CreMtorfl/fl mice. The numbers of mice per group are indicated. e Flow cytometry analysis of naive and effector/memory CD4+Foxp3-YFP– (depicted as CD4+) or CD8+ T-cell populations. f Splenocytes from Foxp3CreMtor+/+ or +/fl and Foxp3CreMtorfl/fl mice were stimulated using PMA and ionomycin for 4–5 h. Cytokine production by CD4+ and CD8+ T cells was assessed by flow cytometry and quantified. g Flow cytometry analysis of PD-1+CXCR5+ TFH cells. Right, frequency and number of TFH cells in Foxp3CreMtor+/+ or +/fl and Foxp3CreMtorfl/fl mice. h Flow cytometry analysis of CD95+GL7+ GC B cells. Right, frequency and number of GC B cells in Foxp3CreMtor+/+ or +/fl and Foxp3CreMtorfl/fl mice. Error bars show mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; unpaired, two-tailed Student’s t-test. Data are representative of at least twelve (a, b, e) or three (c) biological replicates per group. Data are quantified from the numbers of mice as indicated in the legend key (b, d) or from ten or eleven (f, IFN-γ+ and IL-17A+ cells from Foxp3CreMtor+/+ or +/fl or Foxp3CreMtorfl/fl mice, respectively), nine or ten (f, IL-4+ cells from Foxp3CreMtor+/+ or +/fl or Foxp3CreMtorfl/fl mice, respectively), eight (g), or nine (h) biological replicates per group, compiled from more than eight independent experiments (f−h). Numbers indicate percentage of cells in gates
mTOR supports Treg-cell suppression of mucosal TH2 responses
Treg cells regulate T-cell responses important for tissue homeostasis, especially at barrier surfaces like the lung, intestines, and skin1,2. We found that in the lung of Foxp3CreMtorfl/fl mice, there were respective 5–10-fold and 10–15-fold increases of IL-4- and IL-13-producing CD4+ T cells, while the increases in TH1 and TH17 responses were less pronounced (2–3-fold increased) (Fig. 3a and Supplementary Fig. 2a). TH2 and TH17 responses were also more elevated than TH1 responses in the colon lamina propria (Fig. 3b). Because we found a consistent increase of TH2 cytokines in both the lung and colon lamina propria and acute deletion of mTOR led to a profound increase of TH2 responses (Fig. 1h), we next performed comprehensive immunohistochemistry analyses of multiple organs in Foxp3CreMtorfl/fl mice. Elevated TH2 responses are associated with an accumulation of eosinophils, alternatively activated M2 macrophages, and neutrophils in target tissues16. Indeed, MBP+ eosinophils were increased in the lung (Fig. 3c), as well as the dermis of the skin (Supplementary Fig. 2b) of mice-bearing mTOR-deficient Treg cells. Additionally, CD163+ macrophages were expanded, including Ym1+ M2 macrophages present in the alveolar space and interstitium of the lung (Fig. 3d). Increased M2 macrophage activation was also evident in the skin (Supplementary Fig. 2c). We also observed an increase of cells positive for iNOS2, which primarily stains for neutrophils, in the lung (Fig. 3e) and skin (Supplementary Fig. 2d). TH2 inflammation is also associated with the accumulation of mucosal mast cells (MMCs) in the intestines37. Foxp3CreMtorfl/fl mice had an increase of MCPT1+ interepithelial MMCs and MCPT4+ lamina propria MMCs in the large and small intestines (Fig. 3f). Altogether, these results underscore an important role for mTOR in mediating Treg-cell-dependent suppression of effector T-cell responses, especially TH2-associated events, within mucosal tissues.
Treg cells require mTOR for the suppression of mucosal TH2 responses. a, b Flow cytometry analysis of cytokine-producing CD4+ T cells isolated from the lung (a) or colon lamina propria (LP) (b) of Foxp3CreMto+/fl or Foxp3CreMtorfl/fl mice. c Representative images of major basic protein (MBP) staining for eosinophils in the lung of Foxp3CreMtor+/fl and Foxp3CreMtorfl/fl mice. d Representative images for M2 macrophages in the lung of Foxp3CreMtor+/fl or Foxp3CreMtorfl/fl mice by CD163 and Ym1 staining. e Representative images for neutrophils, as indicated by inducible nitric oxide synthease 2 (iNOS2) staining, in the lung of Foxp3CreMtor+/fl and Foxp3CreMtorfl/fl mice. f Representative immunohistochemistry of ieMMCs and lpMMCs in the large intestines (left) and small intestines (right) of Foxp3CreMtorfl/+ or Foxp3CreMtorfl/fl mice. Data are representative of four independent experiments (a, b) or three biological replicates per group (c−f). Numbers indicate percentage of cells in gates
mTOR enforces mucosal tTreg- and pTreg-cell homeostasis
Recent work shows that tTreg cells present in tissues exhibit TH2-biased gene signatures and express transcription factors essential for the suppression of TH2 responses12,38,39. Additionally, the absence of pTreg cells drives elevated TH2 responses in mucosal tissues16,17,18. Therefore, we hypothesized that reduced abundance of tTreg and/or pTreg cells might account for increased TH2 responses in the lung and colon of Foxp3CreMtorfl/fl mice. Neuropilin-1 (Nrp1) and Helios are expressed at higher levels in tTreg than pTreg cells40,41. We found that there was a significant decrease in the frequency of Nrp1+ tTreg and Nrp1− pTreg cells in the lung of Foxp3CreMtorfl/fl mice (Fig. 4a). Helios staining revealed a similar reduction in tTreg and pTreg cells in the lung of Foxp3CreMtorfl/fl mice (Fig. 4b). We tested if these effects were cell-intrinsic by adoptively transferring an equal ratio of CD45.1+ wild-type bone marrow cells and CD45.2+ Foxp3CreMtor+/fl or Foxp3CreMtorfl/fl bone marrow cells into irradiated Rag1–/–-recipient mice. This inflammation-free system confirmed that the reduction of these lung Treg-cell populations was cell-intrinsic (Fig. 4c). We next examined pTreg and tTreg cell populations in the colon lamina propria of Foxp3CreMtorfl/fl mice by staining for either Helios or RORγt, a transcription factor selectively enriched in pTreg cells isolated from the intestines17,19. Similar to our observations in the lung, pTreg cells, as well as Helios+ or RORγt– tTreg cells, were reduced in the colon lamina propria of Foxp3CreMtorfl/fl mice (Fig. 4d, e). The reduction of RORγt+ pTreg cells may also contribute to the increased TH17 cell activation in the colon lamina propria of Foxp3CreMtorfl/fl mice (Fig. 3b)19. Analysis of Helios+ and Helios− Treg-cell populations in the colon lamina propria from mixed bone marrow chimeras verified cell-intrinsic effects (Fig. 4f). Altogether, these results indicate that the accumulation of mucosal tissue tTreg and pTreg cells is disrupted in the absence of mTOR.
Mucosal tTreg- and pTreg-cell homeostasis is altered in the absence of mTOR. a, b Quantification of the frequencies of Nrp1+ and Nrp1– (a) or Helios+ and Helios– Treg cells (b) in the lung of Foxp3CreMtor+/+ or +/fl and Foxp3CreMtorfl/fl mice, respectively. c Quantification of the frequencies of Helios+ and Helios– Treg cells among the CD45.2+CD4+TCRβ+ T cells in the lung of mixed bone marrow chimeras. d Quantification of the frequencies of Helios+ and Helios– Treg cells in the colon lamina propria of Foxp3CreMtor+/+ or +/fl and Foxp3CreMtorfl/fl mice. e Flow cytometry analysis of Foxp3 vs. RORγt expression (left) and quantification of the frequencies of RORγt+ and RORγt– Treg cells (right) in the colon lamina propria of Foxp3CreMtor+/+ or +/fl and Foxp3CreMtorfl/fl mice. f Quantification of the frequencies of Helios+ and Helios– Treg cells among the CD45.2+CD4+TCRβ+ T cells in the colon lamina propria of mixed bone marrow chimeras. g Experimental schematic for in vivo pTreg maintenance assay. h, i Quantification of frequency and/or number of donor-derived Foxp3-YFP+ pTreg cells (h) or total CD4+ T cells (i) in Rag1–/– mice 4 weeks after adoptive transfer of naive T cells isolated from Foxp3CreMtor+/fl and Foxp3CreMtorfl/fl mice. j Quantification of GATA3 expression in Treg cells stimulated under various conditions (with TGF-β and IL-6 included in all the conditions) for 3 days in the presence or absence of Torin 1. k Quantification of GATA3+ Treg cells (Foxp3+GATA3+ in CD4+TCRβ+) from the colon lamina propria of Foxp3CreMtor+/+ or +/fl and Foxp3CreMtorfl/fl mice. Error bars show mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; unpaired, two-tailed Student’s t-test. Data are quantified from five (a, b), eight (c), ten (d), eleven (e), seven (f), three or five (h, i; as indicated), three (j), or six (k) biological replicates, compiled from five (a, b), four (c, f), eight (d), nine (e), two (h, i), three (j), or six (k) independent experiments. Numbers indicate percentage of cells in quadrants
To determine the role for mTOR in pTreg-cell maintenance in vivo, we purified naive Foxp3-YFP−CD4+ T cells from either Foxp3CreMtor+/fl or Foxp3CreMtorfl/fl mice and adoptively transferred these cells into Rag1–/– mice (Fig. 4g). In this system, naive T cells can acquire Foxp3 expression42, and the concomitant expression of the Cre transgene induces Mtor deletion in pTreg cells generated in vivo. The frequency and number of mTOR-deficient pTreg cells were reduced in mesenteric lymph nodes (Fig. 4h), while the numbers of donor-derived total CD4+ T cells were comparable (Fig. 4i). These results indicate that mTOR promotes the maintenance of pTreg cells in vivo.
Activated Treg cells express GATA3, which is required to suppress TH2 responses1,2,11,12,16,38. Therefore, we next examined if mTOR regulates GATA3 expression in activated Treg cells. We established an in vitro system where Treg cells from wild-type mice were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β and IL-6 to mimic the environmental signals at mucosal sites1,2,12. As expected, compared with IL-2 stimulation, IL-4 strongly upregulated GATA3 expression under these conditions12. However, IL-4-induced GATA3 upregulation was diminished upon inhibition of mTOR activity (Fig. 4j). The frequency of GATA3+ Treg cells was also significantly reduced in the colon lamina propria (Fig. 4k), a site where these cells are enriched under steady state11,12. These in vitro and in vivo results highlight the requirement of mTOR signaling for GATA3 expression in Treg cells.
mTOR promotes eTreg-cell generation
After thymic development, peripheral cTreg cells undergo antigen and inflammation-driven activation and differentiate into eTreg cells that are enriched in tissues, including the lung and colon lamina propria1,2,7,8,21,22. Although eTreg cells are crucial for immune homeostasis, the molecular requirements driving their activation and function are still poorly defined. Our above data indicated that mTOR-deficient tTreg cells were reduced in the lung and colon. Moreover, unbiased GSEA showed that mTORC1 signaling was enriched in CD44hiCD62Llo eTreg cells compared to CD44loCD62Lhi cTreg cells (Fig. 5a). Given these results, we next tested whether mTOR regulates eTreg-cell generation. Because Treg cells isolated from inflammatory environments could undergo secondary phenotypic changes, we analyzed cell-intrinsic effects of mTOR deficiency in cTreg and eTreg cells isolated from healthy, female mosaic mice (designated as Foxp3Cre/+). There was an increase in the frequency but not number of Foxp3-YFP+CD44loCD62Lhi cTreg cells and a reduction in the frequency and number of Foxp3-YFP+CD44hiCD62Llo eTreg cells in the spleen of Foxp3Cre/+Mtorfl/fl mosaic mice (Fig. 5b). We also confirmed the reduction of eTreg cells in the spleen of mixed bone marrow chimeras (Supplementary Fig. 3a). Consistent with elevated mTORC1 signaling in eTreg cells (Fig. 5a), the frequency and number of eTreg cells were reduced in the absence of Rptor (Supplementary Fig. 3b). The number of KLRG1+ Treg cells was also reduced in absence of mTOR, consistent with a reduction of eTreg cells (Fig. 5c and Supplementary Fig. 3c)9. The expression of CD25, a marker expressed at higher levels on cTreg cells than eTreg cells9, was increased on mTOR-deficient Treg cells (Fig. 5d and Supplementary Fig. 3d). Moreover, eTreg-cell-associated molecules like ICOS and CTLA4 were expressed at lower levels in the absence of mTOR, while the expression of TIGIT or Foxp3 was equivalent between the control and mTOR-deficient Treg cells (Fig. 5d and Supplementary Fig. 3d)1,2,9. Activated Treg cells also differentiate into specialized or tissue-resident Treg-cell populations, including CXCR5+PD1+Foxp3+ TFR cells that express Bcl65,10,13. Both TFR cells and Bcl6 expression were reduced in the absence of mTOR (Fig. 5e and Supplementary Fig. 3e). Consistent with our earlier analysis of mixed bone marrow chimeras, there was nearly a complete loss of colon Treg cells in Foxp3CreMtorfl/fl mosaic mice (Fig. 5f). Thus, mTOR is essential for maintaining eTreg cells in vivo.
mTOR is essential for eTreg cell differentiation. a Enrichment plot of the Hallmark mTORC1 pathway activated in eTreg cells compared to cTreg cells, identified by gene set enrichment analysis (GSEA). The top 15 enriched genes (position indicated by the vertical black line) are listed below the plot. b Flow cytometry analysis (left) and quantification of frequencies and cell numbers (right) of CD4+Foxp3-YFP+CD44loCD62Lhi cTreg cells and CD4+Foxp3-YFP+CD44hiCD62Llo eTreg cells in Foxp3Cre/+Mtor+/+ or +/fl and Foxp3Cre/+Mtorfl/fl mosaic mice. c Quantification of the number of KLRG1+ Treg cells in the spleen of mixed bone marrow chimeras. d Quantification of CD25, ICOS, CTLA4, TIGIT, and Foxp3 expression in Treg cells from mixed bone marrow chimeras. e Quantification of the frequency of TFR cells (CD4+Foxp3-YFP+CXCR5+PD-1+ Treg cells, left) and Bcl6 expression in total Foxp3+ Treg cells (right) in mixed bone marrow chimeras. f Flow cytometry analysis (left) and quantification of the frequency and number (right) of Foxp3-YFP+ Treg cells in the colon lamina propria of Foxp3Cre/+Mtor+/+ or +/fl and Foxp3Cre/+Mtorfl/fl mosaic mice. g Quantification of active caspase-3 in CD45.2+CD4+Foxp3-YFP+ Treg cells from mixed bone marrow chimeras. h Flow cytometry analysis of CD44 vs. CD62L expression on cTreg cells activated under the indicated conditions for 3 days. US: unstimulated. i Quantification of CD25 and FOXP3 expression in human CD4+CD25+CD45RA+CD45RO– naive Treg cells activated for 3 days in the presence or absence of Torin 1 or PP242. Error bars show mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; unpaired, two-tailed Student’s t-test. Data are representative of at least six (b), six (f), or three (h) biological replicates per group or are quantified from eleven (b), fifteen (c), seven or eight (d; CD45.2+Foxp3CreMtor+/+ or +/+ chimera or CD45.2+Foxp3CreMtor+/+ or +/fl mice, respectively; CD25 and Foxp3), ten (d; ICOS, CTLA4, and TIGIT; e), six (f), three (g), or five (i) biological replicates, compiled from seven (b), eight (c), four (d, TIGIT; f), five (d; CD25 and Foxp3), six (d; ICOS and CTLA4; e), or two (g, i) independent experiments. Numbers indicate percentage of cells in gates
Mechanistically, the loss of eTreg cells in the absence of mTOR could be due to defective survival or reduced activation-induced differentiation. To test the former, we analyzed the expression of active caspase-3 in control and mTOR-deficient Treg cells isolated from mixed bone marrow chimeras and found normal survival of mTOR-deficient Treg cells (Fig. 5g). Also, the frequency of 7AAD+ cells was similar or reduced in purified CD44loCD62Lhi cTreg cells activated in the presence of the mTOR inhibitors (Supplementary Fig. 3f), further indicating that mTOR is not essential for cell survival.
To test the role of mTOR in activation-induced differentiation, we purified CD44loCD62Lhi cTreg cells from wild-type mice and activated them for 3 days in the presence or absence of the mTOR inhibitors, Torin 1 and PP24243. We found that cTreg cells differentiation into CD44hiCD62Llo eTreg-like cells was impaired by mTOR inhibition (Fig. 5h). Similarly, the frequency of mTOR-deficient Treg cells in the spleen and peripheral lymph nodes of DT-treated Foxp3Cre/DTRMtorfl/fl mice was reduced relative to the controls (Supplementary Fig. 3g, left panel), but total numbers of Treg cells were not significantly different (Supplementary Fig. 3g, right panel), likely due to the increased organ size (Fig. 1e). Thus, mTOR-deficient Treg cells also fail to appropriately respond to activation-induced signals in vivo. We also investigated if these regulatory pathways applied to human cells. In the presence of the mTOR inhibitors, activated human CD45RAhiCD45ROlo naive Treg cells had impaired upregulation of CD25 and FOXP3 (Fig. 5i), which are expressed more abundantly in human CD45RAloCD45ROhi activated Treg cells than naive Treg cells44. Thus, mTOR activity represents an evolutionarily conserved pathway for driving eTreg-cell generation.
mTOR links activation signals to IRF4 upregulation
IRF4 is induced by TCR signals to promote eTreg-cell differentiation and regulates Treg-cell-mediated suppression of TH2 responses in vivo7,8,15,21,22. To determine if mTOR induces IRF4 expression upon activation, we purified control and mTOR-deficient cTreg cells and activated them for 24 and 48 h before analyzing IRF4 expression by flow cytometry. IRF4 expression was reduced at 24 and 48 h after activation (Fig. 6a). Acute mTOR inhibition with Torin 1 or PP242 also significantly reduced activation-induced upregulation of IRF4 in cTreg cells (Fig. 6b). Mechanistically, mTOR controls IRF4 expression at the post-transcriptional level, because cTreg cells activated in the presence of the mTOR inhibitors for 24 or 48 h had increased Irf4 expression compared to the internal controls (Fig. 6c). To show that the ~20–30% reduction of IRF4 expression was biologically important, we analyzed gene expression profiles in cTreg cells activated in the presence or absence of Torin 1 or PP242. Among the genes that were consistently altered by both inhibitors were 124 IRF4 target genes, including Ccr8 and Eea18,15,21,22 (Fig. 6d). Also, the loss of IRF4 expression likely accounted for the impairment of mTOR-deficient Treg cells to express ICOS (Fig. 5d and Supplementary Fig. 3d), which is induced by IRF4-dependent mechanisms to drive eTreg-cell differentiation in vivo9,15. Altogether, these data indicate that mTOR promotes eTreg-cell differentiation, in part, by modulating IRF4 expression, which also helps explain how TH2 responses become elevated in Foxp3CreMtorfl/fl mice.
mTOR links activation signals to upregulation of IRF4 expression and downstream targets. a Wild-type (WT) and mTOR-deficient CD4+Foxp3-YFP+CD44loCD62Lhi cTreg cells were purified from mixed bone marrow chimeras and activated under the indicated conditions for 24 and 48 h. IRF4 expression was assessed by flow cytometry. b CD4+Foxp3-YFP+CD44loCD62Lhi cTreg cells were purified from Foxp3Cre mice and activated as in a in the presence or absence of Torin 1 and PP242. IRF4 expression was assessed by flow cytometry. c cTreg cells were purified and activated as in b and Irf4 mRNA expression was analyzed by qPCR. d Heat map of IRF4 target genes differentially expressed in resting cTreg cells or cTreg cells activated in the presence of DMSO, Torin 1, or PP242 for 20 h. e Enrichment plot of the Hallmark glycolysis pathway in cTreg cells activated for 20 h in the presence of either Torin 1 or DMSO control, identified by gene set enrichment analysis (GSEA). The top 30 enriched genes (position indicated by the vertical black line) are listed. f Functional enrichment of the IRF4 target genes shown in d. Error bars show mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; unpaired, two-tailed Student’s t-test. Data are representative of three independent experiments (b) or are quantified from five (a) and four (b, c) biological replicates, compiled from two independent experiments (a, c)
mTOR orchestrates mitochondrial metabolism in Treg cells
Metabolism is a crucial determinant of Treg-cell biology23, but the mechanisms controlling metabolic rewiring required for the function of activated Treg cells are not clear. GSEA showed that cTreg cells upregulated mTORC1 signaling and several metabolic pathways, including glycolysis, upon activation (Supplementary Fig. 4a), while mTOR inhibitor-treated cTreg cells had a significant downregulation of genes in the glycolytic pathway (Fig. 6e and Supplementary Table 1), including Hk2 (Fig. 6d). Because IRF4 also promotes metabolic reprograming of conventional CD4+ and CD8+ T cells45, we determined if mTOR signals via IRF4 to regulate Treg-cell metabolism. We performed functional enrichment analysis of IRF4 targets that were differentially expressed in cTreg cells activated in the presence of mTOR inhibitors (Fig. 6d). This analysis revealed enrichments for glycolytic and nucleotide metabolism and upstream regulators of these pathways, including Myc and mTORC130,46,47 (Fig. 6f). Thus, the mTOR-IRF4 axis supports the upregulation of glycolytic and nucleotide metabolism during cTreg-cell activation.
Besides glycolysis, cTreg cells upregulated genes in the oxidative phosphorylation pathway in an mTOR-dependent manner (Fig. 7a and Supplementary Table 1). Indeed, 366 mitochondrial genes (identified from the MitoCarta 2.0 database48) were differentially expressed in activated cTreg cells (vs. unstimulated cells), and 158 of these genes were mTOR targets (Fig. 7b). Twenty-one mitochondrial genes were putative IRF4 gene targets (based on IRF4 ChIP-seq analysis22), but only six of these genes were induced upon cTreg-cell activation. Only one of these IRF4 targets (Mrps28) was upregulated in an mTOR-dependent manner during cTreg-cell activation (Fig. 7b). These data, combined with the functional enrichment analysis above, suggest that mTOR promotes mitochondrial gene expression in a largely IRF4-independent manner. To further test the effects of mTOR on the metabolic pathways, we performed metabolomics profiling using high-resolution mass spectrometry on resting and activated Treg cells. We found that 54 metabolites were differentially expressed between Treg cells activated in the presence of Torin 1 vs. DMSO (Fig. 7c). For instance, the expression of the TCA cycle intermediates isocitrate/citrate, malate, and succinate and the electron acceptor NAD+ were significantly decreased in Treg cells activated in the presence of Torin 1 (Fig. 7c). Unbiased metabolite set enrichment analysis (MSEA) revealed that activated Treg cells significantly upregulated metabolic pathways associated with mitochondria-dependent energy production and the biosynthesis of proteins and nucleotides, such as the citric acid cycle, the mitochondrial electron transport chain, and pyrimidine biosynthesis49 (Supplementary Fig. 4b). The upregulation of these mitochondria-related metabolic pathways was impaired when Treg cells were activated in the presence of Torin 1 (Fig. 7d). Consistent with this observation, Treg cells activated in the presence of Torin 1 had lower mitochondrial membrane potential (TMRM), whereas mitochondria number as indicated by Mitotracker staining was comparable (Fig. 7e). Thus, mTOR links activation signals to IRF4-dependent and -independent transcriptional programs to induce metabolic reprogramming during Treg-cell activation.
mTOR controls metabolic reprogramming upon Treg-cell activation. a Enrichment plot of the Hallmark oxidative phosphorylation pathway in cTreg cells activated for 20 h in the presence of either Torin 1 or DMSO control, identified by gene set enrichment analysis (GSEA). The top 30 enriched genes (position indicated by the vertical black line) are listed. b Venn-diagram depicting mitochondria-related genes (defined in MitoCarta 2.0 database) that are IRF4 targets (blue circle), or differentially expressed in activated cTreg cells (vs. unstimulated cells; black circle) or activated cTreg cells treated with mTOR inhibitors (vs. those treated with DMSO; red circle). The numbers indicate the shared and independent genes in each category. c Heat map of differentially expressed intracellular metabolites in freshly isolated Treg cells or Treg cells activated for 16 h in the presence of DMSO or Torin 1. d Metabolite set enrichment analysis (MSEA) of KEGG metabolic pathways downregulated in Torin 1-treated activated Treg cells compared to vehicle-treated activated Treg cells. e Flow cytometry of TMRM and Mitotracker staining in Treg cells activated for 20 h in the presence of Torin 1 or vehicle control. Data are representative of three (Mitotracker) or four (TMRM) biological replicates from two (Mitotracker) or three (TMRM) independent experiments (e)
Tfam is essential for eTreg-cell homeostasis and function
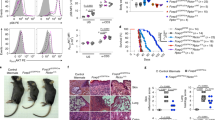
We next genetically defined the importance of mitochondrial metabolism in Treg-cell function in vivo. We conditionally deleted mitochondrial transcription factor A (Tfam), a nuclear-encoded transcription factor essential for efficient electron transport chain activity50,51, in Treg cells by breeding Foxp3Cre transgenic mice with mice-bearing floxed alleles for Tfam51. Tfam-deficient Treg cells isolated from Foxp3Cre/+Tfamfl/fl mosaic mice had comparable mitochondrial content but less mitochondria-derived reactive oxygen species (ROS) (Fig. 8a), consistent with reduced mitochondrial respiratory chain function. We found that Tfam is critical for Treg-cell function, as Foxp3CreTfamfl/fl mice developed a severe inflammatory disease associated with smaller body size, skin inflammation, alopecia (Fig. 8b), early lethality (Fig. 8c), and enlargement of the peripheral lymph nodes (Fig. 8d). Further, there were increased effector/memory T cells (Fig. 8e) and significantly enhanced IFN-γ-producing CD8+ T cell and TH1, TH2, and TH17-cell activation (Fig. 8f) in diseased mice-bearing Tfam-deficient Treg cells. Tfam-deficient Treg cells also had a propensity for increased IFN-γ production (Supplementary Fig. 5a). Moreover, the frequency and number of TFH cells were increased (Fig. 8g). Only the frequency, not the number, of GC B cells was increased, likely due to a reduction of total B220+ B cells in Foxp3CreTfamfl/fl mice (Fig. 8h and Supplementary Fig. 5b). Thus, Tfam deficiency in Treg cells leads to altered immune homeostasis and development of autoimmunity.
Mitochondrial metabolism is critical for Treg-cell function in vivo. a Quantification of Mitotracker (left) and MitoSOX (right) in Treg cells from Foxp3Cre/+Tfam+/fl and Foxp3Cre/+Tfamfl/fl mosaic mice. b Image of 8-week-old mice. c Survival curve of Foxp3CreTfam+/+ (n = 14) and Foxp3CreTfamfl/fl mice (n = 19). d Representative image of lymphadenopathy (left) and cell numbers in the spleen and peripheral lymph nodes (pLN) of Foxp3CreTfam+/fl and Foxp3CreTfamfl/fl mice. e Flow cytometry analysis of naive and effector/memory CD4+Foxp3-YFP– (depicted as CD4+) or CD8+ T cells in Foxp3CreTfam+/fl and Foxp3CreTfamfl/fl mice. f Quantification of cytokine production by CD4+Foxp3– and CD8+ T cells. g Flow cytometry analysis (left) and frequency and number (right) of TFH cells. h Flow cytometry analysis (left) and frequency and number (right) of GC B cells. i, j Quantification of frequencies and numbers of cTreg cells and eTreg cells (i) or number of KLRG1+ Treg cells (j) in the spleen of mixed bone marrow chimeras. k Quantification of ICOS, CTLA4, and TIGIT expression in Treg cells from mixed bone marrow chimeras. l Quantification of the frequency of TFR cells (left) and Bcl6 expression in total Foxp3+ Treg cells (right) in mixed bone marrow chimeras. m, n Quantification of frequency of Treg cells in the colon lamina propria and lung of Foxp3CreTfam+/+ or +/fl or Foxp3CreTfamfl/fl mice (m) or mixed bone marrow chimeras (n). Error bars show mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; unpaired, two-tailed Student’s t-test. Data are representative of nine (b, d, e), twelve (g), or ten (h) biological replicates per group, or are quantified from five (a), eight or nine (d, f; Foxp3CreTfamfl/fl or Foxp3CreTfam+/+ or +/fl mice, respectively), twelve (g), ten (h), nine or ten (i−l, n; CD45.2+Foxp3CreTfam+/+ chimeras and CD45.2+Foxp3CreTfam+/fl chimeras, respectively), five (m, colon), or eight (m, lung) biological replicates per group, compiled from four (a), six (d, f), eight (g), seven (h), four (i−l, n), three (m; colon), or five (m; lung) independent experiments. Numbers indicate percentage of cells in gates
We next analyzed Treg-cell populations in mixed bone marrow chimeras to determine the cell-intrinsic role of Tfam-dependent mitochondrial metabolism in eTreg-cell accumulation and homeostasis. There was a reduction in the frequency and number of CD44hiCD62Llo eTreg cells (Fig. 8i) and KLRG1+ Treg cells (Fig. 8j) in the absence of Tfam. Further, Tfam-deficient Treg cells had reduced expression of ICOS and CTLA4 (Fig. 8k), but not Foxp3 (Supplementary Fig. 5c). However, unlike mTOR-deficient Treg cells, CD25 expression was not increased on Tfam-deficient Treg cells (Supplementary Fig. 5c), and TIGIT expression was reduced (Fig. 8k). Tfam deficiency also reduced TFR-cell generation and Bcl6 expression (Fig. 8l). Further, there was a cell-intrinsic reduction of Tfam-deficient Treg cells within the colon lamina propria and the lung (Fig. 8m, n). Collectively, these results indicate that Tfam-dependent mitochondrial metabolism is critical for the function and homeostasis of activated Treg cells in vivo.
Discussion
Activated tTreg and pTreg cells are crucial for peripheral T-cell tolerance and tissue homeostasis. Here, we show that activated Treg-cell populations have increased mTOR signaling necessary for Treg-cell activation and tissue Treg-cell homeostasis. Mechanistically, mTOR tunes IRF4-dependent transcriptional programming and mitochondrial metabolism. In the absence of mTOR, activated tTreg and pTreg cells are severely decreased in mucosal tissues, associated with excessive TH2, and to a lesser extent TH1 and TH17 responses, and disrupted tissue homeostasis. Further, the homeostasis and suppressive activity of activated Treg cells is impaired by the loss of mitochondrial metabolism and consequently leads to autoimmunity (Supplementary Fig. 5d). Thus, our data identify and establish a critical mTOR-dependent metabolic node that regulates the homeostasis and suppressive function of activated Treg cells in vivo.
TCR-dependent signals coupled with co-stimulation and inflammatory cues drive Treg-cell activation and differentiation into specialized or tissue-resident Treg-cell subsets1,2,7,8,9,21,22. Several transcriptional programs are essential for the differentiation and function of activated Treg cells7,20,21,22,43,52,53. However, the upstream signaling pathways driving eTreg-cell homeostasis are unknown. Here, we show that activation signals through mTOR are required for Treg-cell activation and function, thereby establishing the first kinase pathway, to our knowledge, that links TCR signals and transcriptional programs necessary for Treg-cell activation. Mechanistically, mTOR promotes the expression of IRF4 and GATA3, transcription factors that are essential for Treg-cell-dependent suppression of TH2 responses7,11,12,15. Moreover, IRF4 also enforces eTreg-cell differentiation and pTreg-cell homeostasis to limit mucosal TH2 responses16,17,20,21,22. Therefore, by promoting the expression of IRF4 and GATA3, mTOR maintains activated Treg-cell populations that facilitate tissue homeostasis. The mTOR-dependent induction of IRF4 expression in cTreg cells was not transcriptionally regulated, and, in conventional CD4+ T cells54, occurs independently of the mTOR-4EBP1 translation axis. Therefore, mTOR likely regulates IRF4 expression at the post-translational level, such as via SUMOlyation55. A key question that remains is, why are mucosal tissues more sensitive to the upregulation of TH2 responses than secondary lymphoid organs in mice-bearing mTOR-deficient Treg cells? One possibility is that these sites are enriched for activated tTreg- and pTreg-cell populations and hence their loss more readily increases TH2 responses at these sites than in the peripheral lymphoid organs1,2,56. Further, recent work shows that tissue Treg cells express high levels of ST2 (IL-33 receptor)38,39, which induces GATA3 activation that biases Treg cells toward the TH2 suppressive program39. Thus, mTOR deficiency and other conditions that decrease GATA3 expression will impair this feed forward loop and disrupt TH2-like Treg-cell suppressive responses.
Metabolic reprogramming contributes to cell fate decisions. However, the metabolic programs promoting the homeostasis and function of activated Treg cells are not completely understood23. Despite the previous work suggesting an inhibitory role of mTOR for mitochondrial oxidative metabolism in induced Treg cells in vitro27, we show here that mitochondrial metabolism is highly induced during Treg-cell activation in an mTOR-dependent manner, and is essential for activated Treg-cell function and tissue homeostasis in vivo. Indeed, Treg-specific deletion of Tfam impairs Treg-cell function, leading to the hyperactivation of conventional T cells and autoimmunity. Mechanistically, mitochondrial metabolism could affect eTreg-cell proliferation or survival within tissues as has been reported in vitro27,28. Of note, Raptor-deficient Treg cells have reduced mitochondria-related gene expression30, and Foxp3CreTfamfl/fl and Foxp3CreRptorfl/fl mice have similar elevations in activated T-cell responses, disease pathologies, and survival kinetics30. Additionally, deficiency of Tfam and Raptor impairs TFR cell accumulation57. Thus, our data suggest a key role for Raptor-mTORC1-induced mitochondrial metabolism in establishing the fate and function of activated Treg cells in different microenvironments.
Treg cells must adapt to different environmental cues to acquire unique suppressive functions, and the appropriate balance of mTOR and metabolic signaling appears to be linked to this process. Inactivation of mTORC1 alone or combined with mTORC2 disrupts Treg-cell suppressive activity30; however, whether mTORC1-independent functions of Raptor58 or Raptor/Rictor-independent mTOR complexes play roles in Treg-cell biology remained unclear. Our results here suggest that mTOR does act through Raptor and Rictor to promote Treg-cell function. Compared to Raptor deficiency alone, loss of mTOR or Raptor and Rictor in Treg cells leads to similar extensions in lifespan30, which may be due to more limited tissue damage, such as in the intestines. The balance of mTORC1- and/or mTORC2-induced metabolic programs may also tune Treg-cell suppression of effector T-cell responses. For instance, Raptor-deficient Treg cells have increased mTORC2-Akt activity30, which can upregulate glycolysis at the expense of mitochondrial metabolism and lead to inappropriate suppression of TH1 and TFH responses59,60,61. Despite being dispensable for Treg-cell suppressive activity30,61, mTORC2 can promote Treg-cell trafficking to sites of inflammation and non-lymphoid tissues via upregulating glycolysis or suppressing Foxo1 activity43,62. Thus, gain of mTORC1 and concomitant loss of mTORC2 activity could reduce Treg-cell trafficking to TH1 and TH17 inflammatory sites63,64,65. mTOR may also promote trafficking to sites of TH2 inflammation by modulating Ccr8 expression and/or CCL22/CCR4-induced chemotaxis62,66. We, therefore, propose that Treg-cell function is finely tuned by graded nature of mTOR signaling and metabolic programs, which are likely influenced by local environmental signals. This tunable nature of mTOR signaling in Treg cells may offer a therapeutic strategy to modulate Treg-cell responses to selectively alter the conventional T-cell responses in autoimmunity, infectious diseases, and cancer.
Methods
Mice
C57BL/6, CD45.1+, Cd4Cre, Foxp3DTR, Rag1–/–, Mtorfl, and Tfamfl mice were purchased from The Jackson Laboratory. Foxp3Cre mice, from Dr. Alexander Rudensky, have been described previously33. All genetic models used in this study were on the C57BL/6 background, and both male and female mice were used for quantification and analysis, except for histological analysis where only male mice were used. Mice were generally 4–6-weeks-old unless otherwise indicated. The number of animals in each group are provided in the figures and/or figure legends. All mice were kept in specific pathogen-free conditions within the Animal Resource Center at St. Jude Children’s Research Hospital. The animal protocols were approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital. Mixed bone marrow chimeras were generated by adoptive transfer of CD45.1+ bone marrow cells mixed 1:1 with CD45.2+ bone marrow cells from Foxp3CreMtor+/fl or Foxp3CreMtorfl/fl mice into sub-lethally irradiated Rag1–/– mice as described30. For the pTreg cell in vivo maintenance model, CD4+Foxp3-YFP−CD44loCD62Lhi naive T cells from the spleens and peripheral lymph nodes of Foxp3CreMtor+/fl or Foxp3CreMtorfl/fl mice were purified on a Synergy or Reflection fluorescence activated cell sorter (Sony Biotechnology). Then, 0.75 × 106 cells were transferred via retroorbital injection into sex-matched Rag1–/– mice. The presence of Foxp3-YFP+ Treg cells was evaluated in the mesenteric lymph nodes 4 weeks later42. Foxp3Cre/DTR mosaic mice were treated with DT (50 μg kg−1) i.p. three times per week, for a total of four injections. The mice were euthanized, and tissues were harvested for flow cytometry analysis 11 days following the first DT treatment. Sample sizes were chosen based upon previous data generated within the laboratory and were selected to maximize the chance of uncovering statistically significant differences of the mean. No animals were excluded from analysis.
Flow cytometry
Lymphocytes were harvested form the peripheral lymphoid tissues by manual disruption or the colon lamina propria as previously described30,67. For surface marker analyses, cells were stained in PBS containing 2% (wt/vol) BSA and the appropriate antibodies. The following fluorescent-conjugate-labeled antibodies, purchased from various commercial sources (Biolegend, BD Biosciences, Thermo Fisher Scientific, and Sony Biotechnology), were used: anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), anti-B220 (clone RA3-6B2), anti-CD62L (clone MEL-14), anti-CD44 (clone IM7), anti-CD95 (clone Jo2), anti-GL7 (clone GL-7), anti-CD279 (PD-1) (Clone J43), and anti-TCRβ (clone H57-597) antibodies. Biotin-conjugated anti-CXCR5 antibody (clone 2G8) and PE-labeled streptavidin from BD Biosciences were used for TFH cell staining. Intracellular staining was performed using the Foxp3/Transcription Factor Staining buffers (Cat #00-5523-00, Thermo Fisher Scientific) per the manufacturer’s instructions. The following antibodies were used: anti-CD152 (CTLA4) (clone UC10-4B9), anti-Foxp3 (clone NRRF-30), anti-RORγt (clone B2D), anti-GATA3 (clone TWAJ), anti-IRF4 (clone 3E4), anti-Helios (clone 22F6), anti-IL-4 (clone 11B11), anti-IL-10 (clone JES5-16E3), anti-IL-13 (clone eBio13A), anti-IFN-γ (clone XMG1.2), anti-IL-17A (clone TC11-18H10.1), anti-human CD25 (clone BC96), anti-human CD45RA (clone HI100), anti-human CD45RO (clone UCHL1), anti-human CD4 (clone A161A1), and anti-human FOXP3 (clone 236 A/E7). For intracellular cytokine staining, total splenocytes were stimulated for 4–5 h with phrobol 12-myristate 13-acetate (PMA) and ionomycin in the presence of monensin (BD Biosciences). Surface and intracellular staining was then performed as above. For active caspase-3 staining, surface molecules were stained before cells were fixed, permeabilized, and stained for intracellular active caspase-3 using the BD Biosciences active caspase-3 apoptosis kit per the manufacturer’s instructions (Cat # 550914). Staining for mitochondrial dyes (MitoSOX, TMRM, and Mitotracker Deep Red; Thermo Fisher Scientific) was performed as previously described30.
Histology and immunohistochemistry
Tissues were fixed in 10% (vol/vol) neutral buffered formalin solution, embedded in paraffin, section, and stained with hematoxylin and eosin. Blinded samples were analyzed by an experienced pathologist (P.V.) for the presence of lesions indicative of autoimmune disease. For the identification of ieMMCs or lpMMCs, tissue sections from the small intestines and large intestines were respectively stained with primary rat anti-MCPT1 monoclonal antibody (Cat # 14-55303-82, Thermo Fisher Scientific) or goat anti-MCPT4 antibody (LS-B5958, LifeSpan Biosciences) as described previously37. GCs were identified by staining with anti-CD3 antibody and peanut agglutinin as described61.
In vitro Treg-cell suppression assays
For analysis of Treg-cell suppression in vitro, CD4+CD25hi Treg cells or CD4+Foxp3-YFP+ Treg cells, isolated from the lymphoid organs of the respective Cd4Cre- or Foxp3Cre-expressing mice, were co-cultured with naive CD4+ T cells and irradiated splenocytes as antigen presenting cells as previously described30. For suppression assays using in vitro activated Treg cells, CD25hi Treg cells were sorted from the lymphoid organs of C57BL/6 mice, resuspended in complete Click’s medium containing IL-2 (200 U ml−1), and activated using anti-CD3 (10 μg ml−1) and anti-CD28 (10 μg ml−1) antibodies for 3 days in the presence of PP242 (500 nM, Tocris Bioscience) or vehicle control. The live cells were then isolated using Lymphocyte Separation medium and co-cultured with naive CD4+ T cells and irradiated splenocytes for 3 days, and the incorporation of [3H]-thymidine was assessed as described30.
Treg-cell cultures
CD4+Foxp3-YFP+CD44loCD62Lhi cTreg cells were purified and activated with anti-CD3 and anti-CD28 antibodies in the presence of recombinant IL-2 for various times in the presence of vehicle, Torin 1 (50 nM) or PP242 (500 nM) before total RNA was harvested using the Qiagen RNeasy micro kit per the manufacturer’s instructions. Alternatively, cTreg cells were stimulated as above for 1–3 days and analyzed by flow cytometry as previously described43. For analysis of GATA3 protein expression, CD4+CD25+ Treg cells were isolated from the mesenteric lymph nodes using the CD25+ Treg-cell enrichment kit (Miltenyi). The cells were then activated with anti-CD3 (5 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies for 3 days in the presence of various stimuli and/or Torin 1 (50 nM) as indicated in the figure: TGF-β (2 ng ml−1), IL-2 (200 U ml−1), IL-4 (20 ng ml−1), IL-6 (20 ng ml−1), and butyrate (125 μM). The expression of GATA3 in Foxp3+ Treg cells was assessed by flow cytometry.
Human Treg-cell cultures
All human studies were in compliance with the Declaration of Helsinki. Blood donors were recruited by the Blood Donor Center at St. Jude Children’s Research Hospital, where they provided written consent for their discarded blood products to be used for research. This consent form has been reviewed and approved by the Institutional Review Board at St. Jude Children’s Research Hospital. We were provided with apheresis rings containing peripheral blood mononuclear cells (PBMCs) isolated from de-identified donors. Human CD4+CD25+CD45RA+CD45RO− naive Treg cells were purified from these human PBMCs, and activated with anti-CD3 (clone OKT3, 5 μg ml−1) and anti-CD28 (clone CD28.2, 5 μg ml−1) for 3 days in the presence of IL-2 and mTOR inhibitors as above. The expression of human CD25 and human FOXP3 was then analyzed by flow cytometry.
Metabolomics
CD4+CD25hi Treg cells, isolated from lymphoid organs of C57BL/6 mice, were purified and resuspended in complete Click’s medium. Then, 1.3 × 106 Treg cells were treated with medium alone or immobilized anti-CD3 antibody (10 μg ml−1) and anti-CD28 antibody (10 μg ml−1) for 16 h in the presence Torin 1 (50 nM) or vehicle control. Intracellular metabolites, isolated using methanol extraction of two technical replicates, were analyzed using the Ultimate 3000 UHPLC (Dionex) coupled to Q Exactive Plus-Mass spectrometer (QE-MS, Thermo Fisher Scientific) for metabolite profiling. Detailed methods were previously described68, except that mobile phase A was replaced with water containing 5 mM ammonium acetate (pH 6.8). Differentially expressed metabolites were identified by Limma (Bioconductor) and the Benjamini-Hochberg method was used to estimate the false discover rate (FDR). MetaboAnalyst was used to analyze range-scale data and provide KEGG pathway analysis of significantly altered metabolic pathways (log2 = 0.5) (www.metaboanalyst.ca/)49.
Gene expression analysis
For mTOR deletion efficiency in Treg cells, quantitative real-time PCR analysis was performed using Mtor Taqman probes (Thermo Fisher Scientific, Cat #4351372). For detection of Il4 and Il21, CD4+Foxp3-YFP–CD44hiCXCR5–PD-1− non-TFH cells or CD4+Foxp3-YFP–CD44hiCXCR5+PD-1+ TFH cells were stimulated for 4 h using plate bound anti-CD3 (5 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies. Quantitative real-time PCR analysis was performed using SyBR Green Real-Time PCR Master Mix (Thermo Fisher Scientific) and primers for Il4 (Forward 5’-GGTCTCAACCCCCAGCTAGT-3’; Reverse 5’-GCCGATGATCTCTCTCAAGTGAT-3’) and Il21 (Forward 5’-GGACCCTTGTCTGTCTGGTAG-3’; Reverse 5’-TGTGGAGCTGATAGAAGTTCAGG-3’). For microarray analysis, RNA samples from unstimulated cTreg cells or cTreg cells activated in the presence of vehicle, Torin 1, or PP242 for 20 h as indicated above were analyzed with the GeneChip Mouse Gene 2.0 ST Array (Thermo Fisher Scientific). Differentially expressed transcripts in biological triplicate samples were identified by ANOVA (Partek Genomics Suite version 6.5), and the Benjamini-Hochberg method was used to estimate the FDR.
GSEA of hallmark pathways in resting vs. activated Treg cells from these microarray samples or published datasets (GSE5575332 or GSE610777) was performed as previously described30. IRF4 targets were identified from ChIP-seq data22 deposited in GSE98263 and compared against genes that were differentially expressed in activated cTreg cells treated with or without mTOR inhibitors as above. IRF4 target genes that were differentially expressed in activated cTreg cells treated with mTOR inhibitors were subjected to functional enrichment analysis of metabolism-related pathways, where significance was determined using the Fisher exact test and Benjamini-Hochberg method (FDR < 0.05).
Statistics
The results in graphs represent the mean ± s.e.m., with the numbers of mice per group and number of experimental replicates indicated in each figure legend. The P-values were calculated with unpaired, two-tailed Student’s t-test assuming equal variance (GraphPad Prism software), where *P < 0.05; **P < 0.01; ***P < 0.001. No specific randomization methods were used in these studies. Investigators were not blinded to samples except where indicated for histological and immunohistochemical analysis.
Data availability
Microarray data that support the findings of this study have been deposited in the Gene Expression Omnibus with the primary accession code GSE104130. Other data are available from the corresponding author upon request.
References
Li, M. O. & Rudensky, A. Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 16, 220–233 (2016).
Ohkura, N., Kitagawa, Y. & Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17, 983–988 (2011).
Abbas, A. K. et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 14, 307–308 (2013).
Levine, A. G., Arvey, A., Jin, W. & Rudensky, A. Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078 (2014).
Vahl, J. C. et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity 41, 722–736 (2014).
Smigiel, K. S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014).
Linterman, M. A. et al. Foxp3+follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Rudra, D. et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat. Immunol. 13, 1010–1019 (2012).
Wohlfert, E. A. et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J. Clin. Invest. 121, 4503–4515 (2011).
Wollenberg, I. et al. Regulation of the germinal center reaction by Foxp3+follicular regulatory T cells. J. Immunol. 187, 4553–4560 (2011).
Yu, F., Sharma, S., Edwards, J., Feigenbaum, L. & Zhu, J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat. Immunol. 16, 197–206 (2015).
Zheng, Y. et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458, 351–356 (2009).
Josefowicz, S. Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012).
Ohnmacht, C. et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349, 989–993 (2015).
Wu, C. et al. The transcription factor musculin promotes the unidirectional development of peripheral Treg cells by suppressing the TH2 transcriptional program. Nat. Immunol. 18, 344–353 (2017).
Sefik, E. et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349, 993–997 (2015).
Dias, S. et al. Effector regulatory T cell differentiation and immune homeostasis depend on the transcription factor Myb. Immunity 46, 78–91 (2017).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Vasanthakumar, A. et al. The TNF receptor superfamily-NF-kappaB axis is critical to maintain effector regulatory T cells in lymphoid and non-lymphoid tissues. Cell Rep. 20, 2906–2920 (2017).
Newton, R., Priyadharshini, B. & Turka, L. A. Immunometabolism of regulatory T cells. Nat. Immunol. 17, 618–625 (2016).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Delgoffe, G. M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Battaglia, M., Stabilini, A. & Roncarolo, M. G. Rapamycin selectively expands CD4+CD25+FoxP3+regulatory T cells. Blood 105, 4743–4748 (2005).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Gerriets, V. A. et al. Metabolic programming and PDHK1 control CD4+T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2015).
Procaccini, C. et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 33, 929–941 (2010).
Zeng, H. et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499, 485–490 (2013).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Arvey, A. et al. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat. Immunol. 15, 580–587 (2014).
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008).
DuPage, M. et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity 42, 227–238 (2015).
Weinstein, J. S. et al. TFH cells progressively differentiate to regulate the germinal center response. Nat. Immunol. 17, 1197–1205 (2016).
Reinhardt, R. L., Liang, H. E. & Locksley, R. M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10, 385–393 (2009).
Vogel, P. et al. Globule leukocytes and other mast cells in the mouse intestine. Vet. Pathol. 55, 76-97 (2018).
Delacher, M. et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat. Immunol. 18, 1160-1172 (2017).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209, 1713–1722 (2012). S1711-1719.
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010).
Liu, G., Yang, K., Burns, S., Shrestha, S. & Chi, H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat. Immunol. 11, 1047–1056 (2010).
Luo, C. T., Liao, W., Dadi, S., Toure, A. & Li, M. O. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature 529, 532–536 (2016).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009).
Man, K. et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 14, 1155–1165 (2013).
Yang, K. et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 39, 1043–1056 (2013).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Calvo, S. E., Clauser, K. R. & Mootha, V. K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucl. Acids Res. 44, D1251–D1257 (2016).
Xia, J. & Wishart, D. S. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26, 2342–2344 (2010).
Larsson, N. G. et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231–236 (1998).
Baixauli, F. et al. Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab. 22, 485–498 (2015).
Grinberg-Bleyer, Y. et al. NF-κB c-rel is crucial for the regulatory T cell immune checkpoint in. Cancer Cell. 170, 1096–1108.e1013 (2017).
Oh, H. et al. An NF-kappaB transcription-factor-dependent lineage-specific transcriptional program promotes regulatory T cell identity and function. Immunity 47, 450–465 e455 (2017).
Yi, W. et al. The mTORC1-4E-BP-eIF4E axis controls de novo Bcl6 protein synthesis in T cells and systemic autoimmunity. Nat. Commun. 8, 254 (2017).
Ding, X. et al. Protein SUMOylation is required for regulatory T cell expansion and function. Cell Rep. 16, 1055–1066 (2016).
Tian, L. et al. Foxp3(+) regulatory T cells exert asymmetric control over murine helper responses by inducing Th2 cell apoptosis. Blood 118, 1845–1853 (2011).
Xu, L. et al. The kinase mTORC1 promotes the generation and suppressive function of follicular regulatory T cells. Immunity 47, 538–551 e535 (2017).
Kim, K. et al. mTORC1-independent Raptor prevents hepatic steatosis by stabilizing PHLPP2. Nat. Commun. 7, 10255 (2016).
Gerriets, V. A. et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 17, 1459–1466 (2016).
Huynh, A. et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 16, 188–196 (2015).
Shrestha, S. et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16, 178–187 (2015).
Kishore, M. et al. Regulatory T cell migration is dependent on glucokinase-mediated glycolysis. Immunity 47, 875–889 e810 (2017).
Apostolidis, S. A. et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat. Immunol. 17, 556–564 (2016).
Park, Y. et al. TSC1 regulates the balance between effector and regulatory T cells. J. Clin. Invest. 123, 5165–5178 (2013).
Wei, J. et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 17, 277–285 (2016).
Soler, D. et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J. Immunol. 177, 6940–6951 (2006).
Hall, J. A. et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 34, 435–447 (2011).
Liu, X., Ser, Z. & Locasale, J. W. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal. Chem. 86, 2175–2184 (2014).
Acknowledgements
We acknowledge Dr. Yongqiang Feng for critical reading of the manuscript, Dr. Alexander Rudensky for Foxp3Cre mice, the St. Jude Immunology FACS core facility for cell sorting, and C. Cloer, M. Hendren, A. KC, B. Rhode, and S. Rankin for animal colony maintenance and technical assistance. This work was supported by The Hartwell Foundation Biomedical Research Fellowship (to N.M.C.) and by NIH AI105887, AI101407, CA176624, NS064599, and CA221290 (to H.C.).
Author information
Authors and Affiliations
Contributions
N.M.C. designed, performed, and analyzed experiments and wrote the manuscript; H.Z., T.M.N. and Y.W. designed, performed, and analyzed experiments; P.V. performed immunohistochemistry analysis and provided histopathology scoring; X.L. and J.W.L. performed metabolomics analysis; Y.D. and G.N. performed bioinformatics analysis; H.C. designed experiments, edited the manuscript, and provided overall direction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chapman, N.M., Zeng, H., Nguyen, TL.M. et al. mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat Commun 9, 2095 (2018). https://doi.org/10.1038/s41467-018-04392-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-04392-5
This article is cited by
-
The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases
Nature Reviews Immunology (2024)
-
Prognostic value of mitochondrial CKMT2 in Pan-cancer and its tumor immune correlation analysis
Scientific Reports (2024)
-
Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
Epithelial cell-derived cytokine TSLP activates regulatory T cells by enhancing fatty acid uptake
Scientific Reports (2023)
-
Metabolic diversity of tumor-infiltrating T cells as target for anti-immune therapeutics
Cancer Immunology, Immunotherapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.