Abstract
There is a persistent and growing clinical need for readily-available substitutes for heart valves and small-diameter blood vessels. In situ tissue engineering is emerging as a disruptive new technology, providing ready-to-use biodegradable, cell-free constructs which are designed to induce regeneration upon implantation, directly in the functional site. The induced regenerative process hinges around the host response to the implanted biomaterial and the interplay between immune cells, stem/progenitor cell and tissue cells in the microenvironment provided by the scaffold in the hemodynamic environment. Recapitulating the complex tissue microstructure and function of cardiovascular tissues is a highly challenging target. Therein the scaffold plays an instructive role, providing the microenvironment that attracts and harbors host cells, modulating the inflammatory response, and acting as a temporal roadmap for new tissue to be formed. Moreover, the biomechanical loads imposed by the hemodynamic environment play a pivotal role. Here, we provide a multidisciplinary view on in situ cardiovascular tissue engineering using synthetic scaffolds; starting from the state-of-the art, the principles of the biomaterial-driven host response and wound healing and the cellular players involved, toward the impact of the biomechanical, physical, and biochemical microenvironmental cues that are given by the scaffold design. To conclude, we pinpoint and further address the main current challenges for in situ cardiovascular regeneration, namely the achievement of tissue homeostasis, the development of predictive models for long-term performances of the implanted grafts, and the necessity for stratification for successful clinical translation.
Similar content being viewed by others
Introduction
Cardiovascular tissues, such as heart valves and blood vessels, are sophisticated dynamic tissues that can grow and adapt their structure according to the hemodynamic environment in which they function. It is this characteristic quality that makes it notoriously challenging to replace these tissues with artificial substitutes in case of end-stage disease or damage. For small-diameter arteries (e.g., peripheral arteries, arteriovenous shunts) and the semilunar heart valves in particular, the development of living, adaptive replacement tissues could greatly improve the underachieving current artificial replacements. Novel cardiovascular tissue engineering (TE) strategies are increasingly moving from an in vitro to an in situ approach. In situ TE is defined as biomaterial-induced endogenous regeneration directly in the tissue’s functional site, or in situ, starting from readily-available, resorbable grafts that gradually transform into an autologous, homeostatic replacement tissue with the ability to repair, remodel, and grow. Grafts for in situ TE can be of biological or synthetic nature and either acellular or on-the-fly preseeded. However, prerequisite for this approach is that the graft is readily-available for implantation and that the graft allows for colonization and remodeling by host cells in order to achieve an adaptive autologous tissue over time. Whereas the traditional TE dogma comprises labor-intensive and lengthy in vitro culture and conditioning phases, in situ TE has been proposed as a more cost-effective and on-demand approach, using relatively simple and shelf-ready grafts.1,2,3
The approach of in situ TE is built on the notion that the natural inflammatory response can be harnessed to induce endogenous tissue regeneration (Fig. 1). The resorbable immunomodulatory scaffold provides a temporary microenvironment, which functions as an instructive road map for endogenous cells to infiltrate and create new, living, and functional tissue. It is hypothesized that, upon implantation, the scaffold provides support for mature tissue formation and adequate mechanical properties to withstand the hemodynamic loads. Over time, the scaffold should slowly resorb, ultimately resulting in a purely biological structure which has the ability to repair, remodel, and grow. Proof-of-concept has been demonstrated by milestone studies describing the endogenous regeneration of small-diameter blood vessels and heart valves using acellular synthetic scaffolds or de novo engineered decellularized extracellular matrix (ECM) (see Table 1). Despite these encouraging reports, we are only just beginning to grasp a more fundamental understanding of the biomaterial-driven regeneration in the complex hemodynamic environment, and many key questions remain to be answered:
-
How can a scaffold recapitulate the complex layered architecture of cardiovascular tissues?
-
What is the influence of biomechanical stimuli on the organization of new tissue?
-
Is it necessary to incorporate exogenous bioactivities to enhance the robustness of the in situ TE approach?
-
How fast and by which mechanism should a scaffold degrade?
-
Is this patient-dependent and should this be personalized?
-
How can we predict inflammatory-driven functional regeneration?
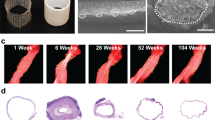
Overview of the different stages of in situ tissue regeneration, going from a synthetic, biodegradable bare construct toward a viable substitute (a). Although many aspects underlying in situ regeneration remain unknown, it is hypothesized that the stages mirror the natural phases of the wound healing response (b), starting with the inflammatory phase, characterized by the infiltration of immune cells and the formation of a preliminary matrix. The subsequent proliferative phase is characterized by a secondary influx of immune and tissue producing cells, extracellular matrix (ECM) deposition, angiogenesis and (re-)endothelialization of the construct. Tissue homeostasis is restored after a remodeling phase of the newly formed ECM and the resolution of inflammation. Photographs of heart valves adapted from;33 photographs of vascular grafts courtesy of Renée Duijvelshoff
To address these questions, this review provides the state-of-the-art across scientific disciplines regarding the physical, biochemical and biomechanical environmental cues involved in biomaterial-driven in situ tissue regeneration; insights that are essential for the rational design of new, robust immunomodulatory scaffolds for in situ cardiovascular TE. Notwithstanding the promising results obtained with decellularized biological scaffolds, we focus our attention primarily on synthetic-based scaffolds. Synthetic scaffolds have the inherent advantage of being produced under controlled processes, and thus bypassing any reproducibility issues associated with biological scaffolds, as well as being fully tailorable. Therein we take an interdisciplinary point of view, taking lessons from regenerative biology, biomaterials science, immunology, and mechanobiology. To conclude we address the imminent challenges in the field of cardiovascular in situ TE: achieving tissue homeostasis, the development of appropriate preclinical models, and the considerations with respect to stratification for clinical translation.
Cardiovascular TE—progressing from in vitro to in situ
Recapitulating the sophisticated biomechanics-dictated cardiovascular microstructures
For both heart valves and blood vessels, the preferred replacement option is a living, adaptive graft. Although the various cardiovascular target tissues (semilunar heart valves, small-diameter arteries, arteriovenous shunts) all come with their own specific requirements, exposure to the hemodynamic environment is the common denominator that poses a major challenge for tissue engineered constructs. Contact with blood implies the need for an endothelium or a surrogate non-thrombogenic layer to prevent thrombus formation, a common complication in synthetic substitutes. Moreover, the hemodynamic loads (e.g., cyclic strains, shear stresses) define the tissue microstructure, leading to a highly-organized and layered structure. For heart valves in particular, it is well-recognized that the valve’s three-layered microstructure (e.g., fibrosa, spongiosa, and ventricularis) is essential to maintain life-long mechanical function.4, 5 Many strategies have been followed to engineer such tissues in vitro, as excellently reviewed in more detail elsewhere.6,7,8 However, reproducing the sophisticated native microstructure and its inherent mechanical function has proven to be highly challenging, resulting in varying success. To avoid the complexity of in vitro culture, cardiovascular TE is increasingly progressing towards in situ strategies, relying on the endogenous regenerative capacity of the body. The approach of in situ TE for cardiovascular application is not necessarily new. Already in the 1970’s, reports by Schoen et al. and Sparks describe the exploitation of the foreign body reaction (FBR) to cylindrical implants to create vascular grafts composed of fibrous capsule tissue.9, 10 Although the regenerative process in this strategy does not occur directly in situ, these studies demonstrate the potential of FBR-driven endogenous tissue regeneration. By now, this notion has evolved into various strategies to induce endogenous regeneration directly in situ, and the proofs-of-concepts for both vascular and valvular replacements have been achieved, as described in the following.
In situ tissue engineering of vascular grafts
Among the first reports of vascular in situ tissue engineering is a series of studies by Van der Lei and colleagues, who attempted the use of a biodegradable polyurethane-based scaffold for direct in situ arterial regeneration.11, 12 These grafts often developed aneurysms due to lack of mechanical strength of the scaffolds and the authors postulate that pre-seeding with either pre-cultured endothelial cells (EC) or smooth muscle cells (SMC) would be beneficial to accelerate the regenerative process, essentially deviating from the in situ approach as we defined it here. The necessity for pre-seeding of cells into synthetic grafts to stimulate in situ regeneration is still a source of active debate, and the underlying mechanisms remain largely unknown. Pioneering work by the groups of Shin’oka and Breuer using on-the-fly preseeding with bone marrow-derived mononuclear cells (BM-MNC) revealed that vascular neotissue arises from the ingrowth of EC and SMC from the neighboring blood vessel wall and that, contrary to the classic tissue engineering paradigm, preseeded cells do not contribute directly to the vascular neotissue, but instead rapidly disappear after implantation.13, 14 Furthermore, vascular neotissue formation was proven to be a host macrophage-mediated regenerative process, with cell seeding being not essential for vascular tissue formation.15 Ultimately, the first clinical trials using BM-MNC were shown to improve neotissue formation for replacements of large-bore venous conduits using biodegradable composite polymer scaffolds.16,17,18 However, seeded BM-MNCs were no longer detectable within a few days of implantation, proving that they primarily ameliorated the regenerative process by paracrine signaling to recruit host immune cells, rather than terminally differentiating into functional tissue cells.19 Despite the promising clinical outcomes described in these clinical studies, stenosis remained the most prevalent graft-related complication in this low-pressure application.18 More recently, Syedain et al. demonstrated proof of somatic growth of an in situ TE pulmonary artery replacement based on decellularized de novo engineered ECM in lambs.20 With respect to acellular synthetic grafts, ongoing clinical trials initiated by Xeltis BV demonstrate promising initial results up to 12 months follow-up, using highly porous electrospun grafts from a resorbable supramolecular polyester for total cavo-pulmonary connection in pediatric patients.21 Although longer follow-up is warranted, these results are indicative of the clinical potential of in situ TE grafts in the low pressure circulation. Application in the less forgiving high-pressure systemic circulation remains challenging, with aneurysm formation being the most common complication. As underlined in the landmark study by Wu et al., scaffold compliance and appropriate mechanotransduction are particularly important properties for the regeneration of arterial grafts.22 Many reports on in situ TE of vascular grafts have contributed to our understanding of the regenerative processes and the influence of the numerous scaffold and host parameters that play a role in this, as summarized in Table 1.
In situ tissue engineering of heart valves
With respect to heart valves, the most compelling results to date have been achieved using acellular biological scaffolds, such as decellularized porcine small-intestine submucosa,23, 24 decellularized allografts,25,26,27 and de novo engineered decellularized ECM.28, 29 Decellularized native grafts have been used in various clinical trials and results indicate that these are suitable base materials for in situ TE.26 Decellularized de novo engineered valves have been proposed as an alternative to negate the need for a donor valve.30 Results by Driessen-Mol et al. demonstrate the feasibility of implanting such valves using minimally invasive delivery techniques leading to extensive recellularization with host cells upon implantation as pulmonary valve replacement in sheep.29 However, shortening of leaflets and progressive insufficiency was reported in this study. The Tranquillo group reported the progressive insufficiency of decellularized in vitro engineered valves when implanted as pulmonary valves in a growing lamb model.31 In this study, the reported insufficiency was attributed to growth of the valvular root and rather than leaflet shortening. A recent study by the same group demonstrates the sustained functionality of similar valves up to 6 months in the aortic position in sheep.28 Interestingly, these valves demonstrated recellularization patterns with vimentin-positive and α-smooth muscle actin (α-SMA)-negative cells, progressing from the root towards the free edge of the valve leaflet, strongly suggesting that these cells originate from the neighboring arterial tissue. Moreover, cellularization mainly occurred on the arterial side of the valve leaflet, while cellularization on the ventricular side was sparse.
To date, experiences of in situ heart valve TE using synthetic-based scaffolds is limited. Weber et al. reported on the implantation of a fast-degrading scaffold based on polyglycolic acid, on-the-fly pre-seeded with BM-MNC, in non-human primates.32 However, the rapid degradation of the scaffold did not allow for sufficient neo-tissue formation to maintain functionality. In collaboration with our clinical partners, we demonstrated the proof-of-concept of using a synthetic scaffold for in situ heart valve TE by using a tunable supramolecular elastomer.33 These valves demonstrated sustained functionality up to 12 months when implanted in the pulmonary position of sheep, and reported preliminary results demonstrate the compatibility with minimally invasive delivery. In this study, we observed spatiotemporal cellularization patterns similar to the aforementioned results of Syedain et al.,28 indicative of gradual regeneration of the valve starting from the hinge region towards the free edge of the leaflet. Prior experience by our group has elucidated that the valve leaflets are acutely colonized with circulatory cells within 1 day of implantation (unpublished data), suggesting that these cells provide the initial cue for neighboring tissue cells to migrate into the valve. Importantly, in our study the degradation of the synthetic scaffold material was observed to be highly localized and was most pronounced in regions with extensive cellularization and neo-tissue formation.33 This suggests that the degradation is cell-driven and correlated to ECM formation, which would mean that the structural integrity of the valves is warranted at all times, although further research is needed to validate this. Using derivative technology, Xeltis BV has recently initiated the first clinical trials using an electrospun resorbable supramolecular polymer as pulmonary valve replacement in pediatric patients (XPlore-I and XPlore-II, NCT numbers: NCT02700100, NCT03022708). However, long-term functionality and growth potential of these in situ TE valves remains to be proven, and translation to the clinically most relevant aortic position is all but trivial given the harsh hemodynamic loads in the systemic circulation. Hence, it is important to get a more fundamental understanding of the endogenous regenerative processes and the cues that can be incorporated in a scaffold to modulate these.
In situ TE - summoning the natural regenerative potential
Inflammation as the driver of regeneration
The in situ TE approach relies on the regenerative capacity of the body. This natural regenerative capacity is highly species-dependent, and, in adult mammals, scarring is the default repair mechanism in response to trauma rather than functional regeneration. Upon synthetic scaffold implantation, disruption of the tissue structure and subsequent cell damage will initiate an inflammatory response by the host. This is the onset of the classical phased wound healing cascade, culminating in an FBR to the material, as expertly reviewed in detail elsewhere.34, 35 Briefly, at the very early stages of implantation, blood-biomaterial interactions lead to the adsorption of endogenous proteins from blood or interstitial fluid to the biomaterial surface. The so formed provisional matrix is rich in mitogens, chemoattractants, cytokines and growth factors which control the subsequent phases of the wound healing and FBR. Following the provisional matrix formation and within the first days after implantation, acute inflammation occurs, with an instantaneous influx of innate immune cells, predominantly neutrophils and monocytes. Chronic inflammation develops as inflammatory stimuli persist at the implant side, with macrophages controlling the microenvironment in cross-talk with lymphocytes, as well as secondary cells, such as fibroblasts, and various stem and progenitor cells, mirroring the cascade of normal wound healing (Fig. 1). Depending on the scaffold properties, the end-stage FBR is characterized either by the accumulation of foreign body giant cells, fibrous encapsulation of the scaffold and eventual graft failure, or by a diminished numbers of macrophages, marked increase in tissue resident cells, and tissue remodeling toward an organized and functional regenerated tissue.
The presence of inflammation can be considered ambivalent; on one hand essential for wound healing, while on the other hand detrimental if any of the phases is disturbed, leading to chronic inflammation, excessive scar tissue formation, and eventual graft failure. Indeed, inflammation is believed to play an all-determining role in biomaterial-driven tissue regeneration. Roh et al. postulated that the in situ regeneration of the inferior vena cava in mice occurred via an inflammation-mediated cascade.19 A recent study by our group applying a shielded porous scaffolds in the abdominal aorta of rats revealed a similar phased regenerative cascade, and it was demonstrated that by modulating the initial inflammatory response in the first days after implantation, late-term tissue development was remarkably enhanced 3 months downstream.36 Using an immunodeficient mouse model, Hibino et al. proposed that platelet activation and the involvement of Natural Killer cells are critical factors for adverse graft remodeling, leading to vascular graft stenosis.37 These results are illustrative of the causality between the initial inflammatory response to an implant and downstream tissue outcome, and show the potential of modulating the long-term regenerative process via the initial scaffold properties.
The plastic macrophage as a target for immunomodulation
Macrophages have been identified as the commanding cellular mediators in the regenerative process. In several animal studies, macrophage depletion resulted in a significant delay of re-epithelialization, decreased collagen deposition, and impaired healing.38 Remarkably, in regenerating species such as salamanders, systemic depletion of macrophages led to failure of growth after limb amputation, while by replenishing the endogenous macrophage population the full regenerative capacity was restored.39 Similarly, in injured arteries of rabbits and rats40 and in tissue-engineered vascular grafts of mouse models,15 systemic macrophage depletion blocked the production of EC, SMC and, ultimately, vascular neotissue.
The functional role of the macrophage is determined by its phenotypic and functional plasticity.41, 42 Rather than being purely “big eaters”, inflammatory cells involved in phagocytosis and pathogen clearance, the current consensus is that macrophages are highly plastic cells that adapt their function depending on the tissue microenvironment. As such they can reversely polarize into functionally distinctly different phenotypes, being the so-called pro-inflammatory “M1” phenotype or the pro-wound healing “M2” phenotype. Within the tissue microenvironment, the complex integration of tissue-specific signals, microbial factors, and soluble mediators determines phenotypic changes, and differential activation of these cells. It is now widely accepted that under the crucial influence of soluble mediators secreted by type 1 or type 2 T helper cells (TH1 and TH2, respectively), macrophages polarize toward the M1 or M2 phenotypes, respectively. More recently a general scheme for macrophage polarization was proposed, based on three different homeostatic activities—host defense, wound healing and immune regulation. It was proposed that M1 and M2a,b,c phenotypes are extremes of a much broader spectrum of functional states43 with overlapping M1–M2 characteristics.44 Depending on their polarization state, macrophages mediate the formation and remodeling of new tissue by secreting essential growth factors and cytokines that either promote or inhibit functional tissue formation (e.g., transforming growth factor-β, TGF-β; tumor necrosis factor-α, TNF-α; matrix metalloproteinases; platelet-derived growth factor, PDGF).42, 45 Besides this regulatory role, it was recently acknowledged that macrophages also directly contribute to tissue formation via producing ECM components themselves, including fibronectin, tropoelastin, various types of collagen and glycosaminoglycans (GAGs) and proteoglycans, such as versican.46,47,48 Moreover, indications for transdifferentiation of macrophages toward the mesenchymal lineage and vice versa can be found in different species and pathologies, but that remains a subject of ample debate.49,50,51 Most important for in situ TE is the notion that the balance of M2/M1 macrophages infiltrating an implanted biomaterial is predictive for long-term tissue outcome.52 Consequently, modulation of the macrophage phenotype has become a valuable target to steer the inflammatory response toward functional regeneration.
Multipotent cells in cross-talk with the inflammatory environment
Supporting the macrophage during the wound healing process, a secondary influx of tissue producing cells, including mature fibroblasts and EC as well as various (circulating) stem/progenitor cells, of both mesenchymal and hematopoietic origin, governs ECM production and remodeling (Fig. 2).49, 53, 54 For cardiovascular in situ TE, the origin of mature tissue cells is unknown so far. Although ingrowth of cells from neighboring tissues has been proposed as the predominant route of cellularization in vascular grafts,14 its significance for the human situation has been contested.55 Given that human cardiovascular tissues have a low natural regenerative potential and a low cellular turnover, regeneration of such tissues is likely to be dependent on the recruitment of cells from high-turnover sites, such as the blood and the bone marrow. Bone marrow-derived progenitors are known to contribute to the valvular interstitial cell population in healthy adult heart valves and homing of progenitors is a normal homeostatic process.56,57,58 The peripheral blood contains various progenitor cell populations, such as endothelial progenitor cells (EPC) and smooth muscle progenitor cells, such as fibrocytes. Although the participation of circulating CD34+ progenitors in regeneration is topic of active debate, blood stream-derived cellularization of vascular grafts was proven in dogs, with patchy endothelial coverage with underlying α-SMA+ SMCs.59, 60 More recently, we demonstrated the regenerative potential of circulatory cells in a rat model of arterial regeneration with a suggested role for CD34+ progenitors.36 Table 2 summarizes the relevant cell types for in situ TE and their proposed role in the regenerative process.
Cartoon of the various (simplified) cell-cell interactions in in situ tissue engineering, as hypothesized based on the state-of-the-art. After the instantaneous response of protein adhesion and platelet activation (not depicted), circulating polymorphonuclear cells and monocytes are recruited to an implanted scaffold in response to various chemokines (e.g., monocyte chemoattractant protein (MCP)-1). Upon activation, the monocytes give rise to macrophages in the scaffold, which are a source of pro-inflammatory factors (e.g., tumor necrosis factor (TNF)-α, Interleukin (IL)‐1β). Depending on the scaffold properties, this is followed by an M1/TH 1 cell dominated response pro-inflammatory response (bottom) of an M2/TH 2 cell dominated pro-regenerative response (top). The former is characterized by the prolonged presence of M1 macrophages, instigated by TH1 cell-secreted pro-inflammatory cytokines, such as interferon-γ. Recruited fibroblasts typically acquire an activated phenotype, producing non-functional cross-linked fibrous scar tissue. In contrast, the pro-regenerative process is dominated by M2 macrophages under influence of TH2 cell secreted cytokines (e.g., interleukin (IL)-4 and -13). Mesenchymal stromal cells play an important immunomodulatory role by inhibiting pro-inflammatory factors, such as TNF-α, as well as secreting numerous trophic factors (e.g., basic fibroblast growth factor, bFGF; vascular endothelial growth factor, VEGF; stromal cell-derived factor-1α, SDF-1α; transforming growth factor β, TGF-β; matrix metalloproteinase 9, MMP-9). This biochemical milieu attracts tissue cells and modulates the formation and remodeling of well-organized functional neotissue. Upon scaffold degradation, T reg cells inhibit the inflammatory process by secretion of, e.g., IL-10. Homing of circulatory CD34+ progenitor cells, such as fibrocytes and endothelial progenitor cells, as well as endothelial-to-mesenchymal transformation may contribute to cellularization and pathophysiological neotissue formation, although these processes are topic of active debate
Irrespective of cell origin, the interactions between cells in the local scaffold microenvironment are pivotal in the regenerative process (Fig. 2). Local inflammatory reactions contribute to successful regeneration, setting the “soil” for colonizing stem cells, either endogenously recruited or seeded, at the site of injury, as recently reviewed by Forbes and Rosenthal.61 In this line, Ballotta et al. described the synergistic expression of trophic factors (e.g., basic fibroblast growth factor, bFGF; stromal cell-derived factor (SDF)-1α) by human mesenchymal stromal cells (MSCs) when activated by peripheral blood mononuclear cells in a 3D electrospun scaffold in hemodynamic conditions.62 Moreover, MSCs have been described to exert strong immunomodulatory functions by inhibiting the secretion of inflammatory factors, such as TNF-α and interferon-γ by TH1 cells, as well as increasing the secretion of pro-regenerative factors, such as IL-4 and IL-10, by TH2 and Treg cells.63 Various studies employing in vitro co-cultures have unraveled paracrine signaling mechanisms between macrophages and fibroblasts or SMCs, both in 2D and in 3D scaffolds.64,65,66,67,68 Interestingly, Song et al. demonstrated that the macrophage phenotype has an effect on the fibrous matrix production of human fibroblasts via the secretion of either pro-fibrogenic (e.g., TGF-β1, PDGF) or anti-fibrogenic and fibrolytic factors (e.g., TNF-α, MMP-7).69 Using 3D in vitro co-culture, McBane et al. proposed that monocyte-derived macrophages induce a shift in SMC phenotype form a more synthetic, migratory phenotype to a resting, contractile phenotype.70
To conclude, an implanted scaffold serves as an artificial microenvironment that can boost the regenerative capacity of tissue sites with a naturally low regenerative potential, by modulating (1) the local inflammatory environment and (2) the recruitment of stem and progenitor cells to the scene.
Modulating the regenerative response in the hemodynamic environment
Rational scaffold design
Recent reviews have elaborately described the fundamental potential of modulating the immune response, and more specifically macrophage polarization, using biomaterial properties.71, 72 To apply these principles to in situ cardiovascular TE, we should consider the heterogeneity of the infiltrating populations and the interplay between these cells in the mechanically and biochemically dynamic environment. Consequently, in-depth insights are required on the single and combined temporal effects of environmental cues on cell behavior. Rational scaffold design requires interdependent, multi-scale considerations, including micro-mechanical and macromechanical properties, architecture, degradation rate, and bioactivity (Fig. 3).2
Schematic representation of the design strategies that can be employed to tailor resident cell behavior inside the graft. The transfer of hemodynamic loads (a) can be tuned via adaptations in material properties, such as the mechanical properties, geometry (b) and microstructure (d). Concurrently, cell behavior is defined by interdependent microstructural parameters (e.g., fiber diameter, alignment, pore size and topography; (d) and biochemical parameters (e.g., surface chemistry and bio-activation); (c). However, local loads and scaffold parameters change in time due to material degradation (e) and new tissue formation. Subfigures b and e are adapted from refs. 107 and 126, respectively
Biomechanical stimuli
The biomechanical environment plays an important role in cardiovascular development. Hemodynamic loads dictate the structural composition of valves and vessels, both in physiology and pathology.73, 74 For artificial grafts, the continuous exposure to cyclic pressures, shear stresses, and strains require an excellent fatigue behavior of a graft, in particular for heart valves. Moreover, the biomechanical environment dominantly influences the process of cell infiltration and tissue regeneration during in situ TE. To illustrate, implantation of a synthetic graft in the abdominal aorta of mice resulted in a well-organized ECM with native-like elastin content, while a similar graft resulted in only very sparse elastin formation after long-term implantation in the venous circulation.75, 76 Transfer of the biomechanical loads (e.g., shear stress, strain) on a cardiovascular scaffold to the infiltrating cells is dependent on the scaffold properties, such as geometry, microstructure, and mechanical properties. Moreover, the local loads change during scaffold degradation and tissue formation. By specifically tailoring these scaffold properties for the aspired clinical application, the hemodynamic load transmission to residing cells can be tuned in order to direct tissue regeneration towards homeostasis (Fig. 3).
Shear stress
Shear stresses play an important role in cardiovascular regeneration by affecting cell adhesion, activation and signaling. Moreover, fluid dynamics strongly influence developmental processes, such endothelial-to-mesenchymal transformation,77, 78 which are highly relevant for in situ TE strategies, as expertly reviewed elsewhere.79 At the cellular level, in vitro studies using 2D fluidic devices have demonstrated shear stress-dependent adhesion of various vascular cells (i.e., SMC, EC, and EPC) to various substrates in controlled shear flow.80,81,82,83 Shear stresses also have profound effects on the immune response. Increasing shear forces are known to influence platelet activation and adhesion,84 as well as the integrin-mediated adhesion and apoptosis of leukocytes.85, 86 Correspondingly, in vitro studies in our lab demonstrated that shear stress overruled the effect of biochemical stimulation on specific human monocyte recruitment into a 3D electrospun scaffold loaded with MCP-1.87
In vivo, the wall shear stress is notorious determinant of adverse remodeling. Aberrant wall shear stresses at the venous-arteriovenous synthetic graft anastomotic side are known to contribute to neointimal hyperplasia and early graft stenosis.88 Interestingly, differential healing of various grafts in dogs was suggested to be implant site-dependent due to variations in fluid dynamics.89 Taken together, these results highlight the importance of shear stress for in situ TE. For artificial heart valves, the shear stress profile depends on the valve’s geometrical design,90, 91 and as such represents an important design criterion.
Cyclic strain
Traditional in vitro TE studies have given us tremendous insights into the causal relationship between strains and ECM formation in 3D scaffolds. Strain is known to be a potent mediator of collagen production and turnover.92, 93 Gupta et al. proposed that GAGs and proteoglycan synthesis by valvular cells is dependent on cyclic strain.94 Recent studies highlight the importance of physiological strains on the synthesis and organization of the fibrous ECM, and specifically the maturation of elastic fibers.95, 96 Importantly, progenitor cells, such as EPCs and MSCs, have been shown to proliferate and differentiate into the cardiovascular cell lineage in response to strain, although the underlying processes of strain-driven differentiation are not well-understood.97, 98
For in situ TE, it is important to consider that strains also have an impact on the immunological environment. Straining of monocytes/macrophages was shown to contribute to monocyte-to-macrophage differentiation, a different inflammatory gene expression profile and the selective augmentation of related cytokines, matrix metalloproteinases, and scavenger receptors.99, 100 Interestingly, Matheson et al. demonstrated that macrophages display a differential response to uniaxial strain compared to biaxial strain.101 Moreover, they showed that cyclic biaxial strain may contribute to biomaterial degradation via augmentation of enzymatic activities (e.g., increased esterase production).102 In 3D culture, Ballotta et al. observed that moderate amounts of biaxial strain (7%) may contribute to a more anti-inflammatory macrophage phenotype (M2) when compared to high strains (12%).103 In a recent study, Battiston et al. demonstrated that 10% cyclic stretch on a co-culture of monocytes/macrophages and vascular SMC in a polyurethane scaffold contributed to synthesis of collagen type I and III and resulted in improved mechanical tissue properties (elastic modulus, tensile strength).48
The fundamental effects of strain on tissue regeneration directly translate to in vivo data of in situ TE vascular grafts. Numerous studies have stressed the importance of graft compliance for successful elastogenesis and tissue regeneration and reorganization.22, 104, 105 For electrospun meshes, the macromechanical properties can be predicted by multi-scale modeling, taking into account microstructural elements such as cross-link density, fiber alignment, and fiber diameter.106 The resulting strain distribution in the scaffold is determined by the microstructural organization in combination with the macroscopic geometry.107, 108 Multi-scale numerical models represent a valuable enabling tool to predict and tune local strains that will be experienced by recruited cells as a result of the hemodynamic loads.
Scaffold microstructural and material properties
Apart from determining the mechanical behavior, the physical properties of the scaffold have a profound influence on the behavior of colonizing cells. The scaffold microstructure is defined by multiple interdependent parameters, such as the fiber diameter and alignment, pore size, and surface topography and chemistry, and these parameters change during degradation of the scaffold. All these factors are known to have imposing effects on the cellular response to the scaffold, for example, in terms of infiltration, adhesion, and the immunogenic response.
Microstructure
The microstructural parameters of fibrous scaffolds provide essential physical cues, such as contact guidance, to infiltrating cells. Using an electrospun scaffold with aligned microfibers, De Jonge et al. demonstrated that newly formed collagen by human myofibroblasts was deposited in the direction of fiber alignment and that the contact-guiding cue overruled the effect of biomechanical stimuli until approximately 80% of the scaffold was resorbed.109 This suggests that the formation and organization of neotissue can be directed from the get-go by incorporating anisotropy in the scaffold, such as proposed by Sohier et al.110 Interestingly, Fioretta et al. observed a differential effect of electrospun microfibers with varying fiber diameter on cell alignment and ECM deposition between human EPC and mature EC, suggesting that the effect of contact guidance is cell type dependent.111
Apart from contact guidance, the microstructure has profound effects on cellular infiltration and the FBR. For electrospun scaffolds, which are most often used in cardiovascular TE, the fiber diameter is linearly correlated to the pore size. As such, fiber diameter and alignment directly influence the cell infiltration depth into the scaffold.112,113,114 Consequently, multi-layered scaffolds have been suggested not only to control the mechanical behavior,115 but also to control cell infiltration116, 117 (see Table 1). Additionally, it has been shown that electrospun nanofibers minimize blood activation and reduce macrophage activation when compared to microfibers.118, 119 Several studies revealed that the mechanism behind which porous materials have improved healing might involve a shift in the polarity of macrophages at the implant site.120, 121 Along this line, a positive correlation has been established between M2 polarization and pore size, with an increased expression of angiogenic factors (vascular endothelial growth factor (VEGF), bFGF, TGF-β) with increasing pore size.122 Madden et al. proposed a specific “sweet spot” in the scaffold’s microstructural pore size (20–40 μm) to optimally promote favorable M2 polarization.120 More specifically, it was demonstrated that this effect is associated with a change in macrophage shape. Upon identifying that M2 macrophages display an elongated, spindle-shaped morphology while M1 macrophages display a round pancake-like shape, McWhorter et al. controlled cell shape and polarization via engineered micro-patterned substrates. Remarkably, shape-induced and cytokine-induced M2 polarization occurred through distinct yet synergizing pathways, suggesting that biochemical cues compliment the effects of geometrical cues.123 Other important physical stimuli that may influence macrophage polarization include substrate stiffness, topology and surface chemistry, as recently reviewed in more detail by McWhorter et al.72
Material degradation
Material degradation will continuously alter the cellular microenvironment, as microstructure and mechanical loading will change in a time-dependent fashion. It is decisive for the success of the scaffold that functional tissue formation and degradation are well-balanced. Prolonged biomaterial presence will result in chronic inflammation and the formation of a tightly cross-linked fibrotic and calcific network that is unable to remodel. Too fast degradation, on the other hand, may result in the loss of structural integrity.
Degradable materials can be degraded within phagosomes after phagocytosis, or eroded via extracellular resorption, with or without the involvement of foreign body giant cells. After degradation, the monomeric components are removed by the natural (metabolic) pathways of the human body.124 The biodegradation mechanisms, i.e., (i) hydrolysis, (ii) oxidation, (iii) enzymatic degradation, and (iv) physical degradation are in part dependent on the chemical composition and the morphology of the biomaterial. Hong et al. described the tailored degradation rate of polyurethane scaffolds by partial substitution of polyester segments with polycarbonate segments in the polymer backbone.125 Brugmans et al. reported that supramolecular polycaprolactone (PCL)-based polymers are more susceptible to oxidative degradation in comparison to conventional PCL, which is more prone to hydrolytic and enzymatic degradation.126
Macrophages have been shown to play a pivotal role in material degradation, via the production of enzymes and reactive oxygen species that can accelerate degradation. Enzymes such as esterases and lipases endogenously produced by macrophages can accelerate the resorption process.126,127,128 Similarly, for the oxidative resorption pathway, macrophages with different polarization states can guide and accelerate the scaffold degradation via secretion of both reactive oxygen species and enzymes.126, 128 Understanding of the mechanisms of scaffold degradation is all but trivial, as it determines the extent to which cells (e.g., macrophages) will actively degrade scaffold material. Most importantly, scaffold degradation should be in balance with neotissue formation to maintain mechanical functionality at all times.
Taken together, there are multiple interdependent scaffold parameters, which affect the inflammatory and regenerative response. Table 3 provides an overview of suggested scaffold design principles to achieve an optimal pro-regenerative scaffold biomechanical and physical microenvironments.
Biochemical stimuli
In several animal studies, implantation of vascular grafts in the form of cell-free, bare polymer led to remarkable formation of fully functional neovessel mimicking the native tissue,22, 129,130,131 posing unanswered questions on the necessity for bioactivation for tissue regeneration purposes. Nevertheless, bioactivated scaffolds which house chemotactic and/or trophic factors provide suitable biochemical and physico-chemical cues which, by mimicking the critical aspects of natural healing processes, might accelerate tissue regeneration (Table 4). Also, in patients with cardiovascular diseases, bioactivated scaffolds might augment the limited self-healing capacity by artificially accelerating the proliferation and differentiation of the recruited or implanted cells. On-the-fly preseeding of autologous bone marrow-derived cell fractions into biodegradable synthetic cardiovascular grafts stimulated in situ regeneration of autologous neovessels and valves.18, 132 Following studies revealed an interdependent effect on the secretion of trophic factors by preseeded MSC and the inflammatory environment created by circulating immune cells in vitro.62 To boost selected signaling molecules or tether endogenously released factors to promote a regenerative microenvironment, biodegradable scaffolds with controlled release of chemoattractants have been developed, which lead to enhanced infiltration of immune cells, de novo tissue formation and reduced fibrosis in several animal models.19, 36, 133, 134 Much research has been devoted to the modulation of the inflammatory response via macrophage polarization, with development of several bioactivated materials leading to successful nerve,135, 136 bone137, 138 and blood vessels regeneration,139 both in vitro and in vivo.
Over the last decades various bioactivation methodologies have been developed, with direct adsorption of growth factors to scaffolds and hydrogels representing the simplest approach and the first to be extensively investigated (Table 4).135, 137, 139,140,141 However, when growth factors are administered in their native form, they are susceptible to biodegradation, inactivation due to a very short half-life, and insufficient delivery at the active site. In the search for methods overcoming these disadvantages, encapsulation of growth factors via non-covalent and covalent immobilization were investigated. These delivery systems hold a great deal of promise for localized administration, providing a solubilising and protective environment, minimizing the release to non-target sites, and serving also as an artificial ECM for cell penetration.130, 140, 142 Natural occurring GAGs have been identified to bind and modify inflammatory factors, with heparan sulfate being the most well- recognized natural binding site for several cytokines.35, 143, 144 In this respect, heparin-conjugated scaffolds145, 146 and heparin-mimetic peptide nanofibers144, 147 have been proven to indirectly boost cellular infiltration and angiogenesis. Ultimately, recent advances in supramolecular polymers allowed for the development of truly “smart”, cell-responsive scaffolds which can interact with their environment and mediate the host response to the biomaterial, reduce inflammation and promote early in situ re-cellularization.148
Current challenges
The primary goal for in situ cardiovascular TE is to recapitulate the complex structure and function of the native tissue, in a state of quiescent homeostasis (Fig. 4). Therein, the challenge is not to induce tissue formation, yet the regeneration of functional tissue. This can be achieved by presenting the optimal inflammatory, physical, and biomechanical microenvironments for the colonizing cells. These local microenvironments are constituted by the initial scaffold parameters in the hemodynamic environment, but will change over time due to scaffold degradation and the formation of new tissue. Temporal understanding and control of these processes is one of the main current challenges for in situ TE for cardiovascular applications. Consequently, the development of appropriate models is required in order to predict and tune the regenerative process, and to assess the robustness of the technique depending on patient demographics (Fig. 4).
a Schematic illustration displaying the three main interdependent challenges faced for successful, robust in-man application of in situ tissue engineered cardiovascular grafts. The development process is represented by a continuous feedback loop between the optimization of the graft design and the development of predictive models to understand and determine long-term in vivo performance, while taking into account graft recipient variability (e.g., age, gender, co-morbidities, and utility). b Optimization of graft design is visualized as a flowchart, in which interchangeable scaffold design parameters together with the hemodynamic loads and cells will determine if tissue homeostasis will be reached. Societal demands, including patient and physician wishes, should be taken into consideration during the (early) stages of graft development to determine the added value of these grafts for health care. HTA: Health Technology Assessment
Achieving tissue homeostasis
Tissue regeneration is considered the restoration of the original tissue function and structure. In contrast, post-natal wound healing is characterized by abundant deposition of collagen in the form of unorganized bundles within a non-functional matrix, which lacks elastic fibers and functionality: repair, rather than regeneration. The disproportionate accumulation of collagen in cardiovascular tissues, in the form of either a reactive or a reparative fibrosis, further increases stiffness. On the other hand, excessive collagen degradation can tether distortion in tissue architecture, excessive reduction in stiffness, and, ultimately, tissue rupture. For several years, research in the field has focused primarily on collagen deposition, and the possibility to develop cardiovascular grafts of suitable strength, with little regard for elasticity. However, the elastic network represents the hallmark factor distinguishing between the regeneration of functional tissue and fibrous scar tissue. Disturbances in elastin homeostasis have been pinpointed as the underlying causes of valvular grafts failure,149, 150 and aneurysma formation.151 Although elastin production during cardiovascular in situ TE has been reported, the homeostatic restoration of the native-like, organized elastic network during by adult endogenous cells is a major challenge. This is due to the proteolytic inflammatory milieu, which inhibits elastin expression,152 as well as the influence of hemodynamic loads on elastic network formation.95, 96, 153 While being essential for the development and remodeling of neotissue, the hemodynamic environment also provides a persistent cue for fibrosis.154 Many signaling proteins that are essential to tissue regeneration, such as MCP-1 and TGF-β, are also stimulatory factors for fibrosis. Similarly, on the cellular level, M2 macrophages and fibrocytes, for example, are correlated to both regeneration and fibrosis.155 This poses a paradoxal challenge, and the difference between physiological de novo tissue formation and excessive fibrosis is dependent on a rather delicate balance of factors. Developmental principles can provide important lessons for in situ TE. The growth factor profiles in embryonic and post-natal healing are very different,156, 157 with embryonic wounds displaying markedly reduced numbers of inflammatory cells compared to adult wounds, due to deficiencies in leukocyte infiltration. Martin et al. proposed that not the macrophage presence itself, but rather the macrophage polarization state influences the balance between scarring and healing, in accordance with previous literature.158
In vivo observations emphasize that timely resolution of inflammation is critical. In a recent study, Naito et al. characterized the time course of ECM development in an in situ TE venous graft in mice.159 They observed an initial surge in the production of fibrillary collagen, which was postulated to be part of an initial cellular FBR to isolate the polymer. This response was alleviated upon degradation of the scaffold, after which other ECM proteins, such as GAGs, elastin, and collagen type IV increased proportionally. As a result, the mechanical properties of the graft converged to those of the native vein.160 This was attributed to the transient changes in mechanotransduction of hemodynamic loads to the cells. In correspondence, delayed degradation and lack of mechanotransduction was pinpointed as the cause of insufficient tissue regeneration in arterial grafts in mice.161 Similarly, appropriate mechanotransduction and timely degradation of the scaffold were hypothesized to be the key factors underlying the successful in situ regeneration of a neoartery in rats.22 Accordingly, long-term follow-up of arterial PCL grafts in rats revealed extensive chondroid metaplasia, which was likely caused by the persistent presence of the PCL scaffold and poor mechanotransduction, up to 18-month follow-up.131 Sugiyura recently demonstrated that calcification of arterial grafts in mice could be avoided in fast-degrading grafts, in contrast to slow-degrading grafts.162 Correspondingly, Wu et al. advocated the importance of rapid resorption and appropriate mechanotransduction for the remodeling of fast degrading synthetic grafts toward physiological-like neoarteries.22
In summary, all these results corroborate that degradation is inextricably correlated to tissue formation and remodeling. The timely resolution of inflammation is a pivotal factor in the process of de novo tissue formation and the prevention of adverse remodeling. This resolution is governed by the biochemical and biomechanical microenvironment, which may be concerted by well-timed degradation of the synthetic scaffold (see also Table 3).
Development of predictive models
Perhaps one of the most underexposed challenges for in situ cardiovascular TE is the development of appropriate models to predict the long-term in vivo performance of grafts. When employed appropriately, animal models are a vital source of information to study and mimic the complex regenerative processes in vivo. Small animals (mice, rats) in particular are being used extensively to answer preliminary research questions in the developmental phase of new prostheses (e.g., regarding biocompatibility, material choice, and device design). However, although the bulk of animal studies for in situ vascular regeneration reports on promising patency and endothelialization rates, both the underlying mechanisms and the timescales are subject to strong interspecies variations, as well as implant-site variations,89, 163 which may mislead their conclusions in terms of clinical application. It is often overlooked that the predictive value of an animal model is only as useful as the context in which it is interpreted.164 For example, the mechanisms governing the cellularization of the graft with functional tissue cells (i.e., EC and SMC) differ between species. Endothelialization of vascular grafts in mice and rats is characterized by a rapid, progressive transanastomic overgrowth,14 which is typically not observed in humans, even after prolonged implantation periods. Therefore, refined animal models have recently been proposed by ourselves36 and others.165 Differences in critical hemodynamic parameters (e.g., shear stress) between different animal models and humans may further decrease the value and predictability of animal models.166
Another important consideration in the use of animal models is the immunological variance between species, especially considering that the process of in situ TE is highly dependent on the immune response to the implanted biomaterial. It has been demonstrated that the genomic response to acute inflammatory stimuli in mice poorly correlates to the human conditions, as well as between different mouse models.167 Genomic comparison of mice and human monocyte subpopulations has revealed that, although general expression patterns are conserved, significant and even opposing functional differences exist between species.168 Similarly, the characterization of the subsets based on marker expression is species-dependent. This calls for species-specific immunological marker panels to study the function of specific leukocyte populations in vivo.
Novel in vitro and in silico models that aim to predict the biomaterial-dependent host response are starting to gain attention as of recent.113, 169,170,171 Various recent studies defined the macrophage response and the resulting cytokine/chemokine profile (e.g., IL-6, TGF-β, TNF-α, MMP-9 secretion) as predictive parameters for the long-term host response on a biomaterial.170, 172, 173 In a recent study, Wolf et al. evaluated the static in vitro response of human peripheral blood mononuclear cells toward various synthetic and biological materials in vitro in terms of macrophage M1/M2 ratio and secretion of a small set of signaling proteins (MMP-2, MMP-9, IL-6, and interferon-γ-induced protein (IP)-10). Coupled with in silico principle component analysis, the in vitro data could be successfully correlated to the long-term tissue outcomes of the same materials implanted subcutaneously in a animal model.170 Enayati et al. highlighted the potential of a fibroblast-macrophage coculture model to increase the predictive value of in vitro models.174 Adding physiological complexity, bioengineered vascular tissues may serve as suitable in vitro models, as recently reviewed by Wolf et al.175
Taken together, advanced in vitro and in silico models mimicking the principal components of the host response to an implanted biomaterial or even “humanized” animal models in synergy with systematic in vitro studies, might represent an excellent and potentially superior alternative to animal models.
Stratification for clinical translation
Apart from aforementioned inter-species differences, patient-to-patient variability poses a challenge for stratification of in situ TE therapies. Even without considering any graft parameters, in situ TE is completely dependent on the natural regenerative potential of the graft recipient. The wound healing response, and thereby the intrinsic regenerative capacity are highly variable between patients, and even between healthy individuals. Mammalian wound healing is prone to genetic variability.176 Khosravi et al. who recently reported significant functional diversity in the long-term remodeling of identical arterial grafts, even in healthy laboratory animals.177 There is a strong natural variability in both the innate and adaptive immune response among humans, which, to a large extent, can be attributed to differences in age or gender.178,179,180,181 Consequently, young people and pre-menopausal women are known to have an increased risk of scarring than men and elderly, which is attributable to variations in the immune system.156 Common comorbidities of cardiovascular patients include for example diabetes and chronic kidney failure, further contributing to the variability of the regenerative capacity and thereby, the applicability of such techniques for specific patient cohorts. Krawiec et al. reported an increased risk of stenosis in vascular grafts engineered from human cells from diabetic patients, which was suggested to be due to a reduced remodeling capacity.182, 183 Wang et al. reported a significantly reduced regenerative capacity of diabetic rats.184 Together, these findings highlight the importance of risk stratification and the potential need for a personalized approach in translating these therapies to the clinic. Moreover, these are important considerations when selecting the appropriate patient cohort for first-in-man studies. Given the limitations of current alternative treatments, in situ TE would be most beneficial for children and young adults. Hence, pulmonary valved conduits for children with complex congenital heart disease would represent a most valuable first-in-man target, as is the case for the currently ongoing clinical trials by Xeltis BV.
Moreover, to translate toward in-man clinical application, it is important to remain focused on the clinical requirements, the wishes and needs of patients and physicians (cardiothoracic surgeons) as well as the societal demands. Given the societal demand for better, sustainable and more efficient health care it is important to search for strategies that have a higher return of investment and a reduced time to the market. Early Health Technology Assessment (HTA) is emerging as a research field focusing on the evaluation of medical, economic, social and ethical implications of a new medical device to determine the added value for health care.185, 186 As an example, conceptual models can be developed to determine the graft requirements to become cost-effective compared to golden standard treatment methods.187 Hence, these assessments can be used to continuously prioritize and guide design choices during development, to optimally introduce and use the grafts in the clinic. For example, patients with a limited natural regenerative capacity might benefit from the local incorporation of bioactive factors into an in situ TE scaffold (Table 4), and/or complementary systemic therapies, such as drugs to initiate stem cell mobilization.188
Conclusion
In situ TE of blood vessels and heart valves using resorbable synthetic grafts is rapidly progressing and first clinical trials are exemplary of its clinical potential. Here we described the underlying principles of biomaterial-driven regeneration, initiated by the host response to the material and governed by the interplay of immune cells (e.g., macrophages), stem/progenitor cells and tissue cells in the scaffold microenvironment. In enhancing our multi-disciplinary understanding of the fundamental processes underlying successful endogenous regeneration, it is important to consider the interdependent role of physical and biochemical cues in this process, hinging around the biomechanical cues exerted by the hemodynamic environment. The development of advanced predictive models will contribute to stratification of in situ cardiovascular TE as a robust clinical therapy.
Data availability
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.
References
Mol, A., Smits, A. I. P. M., Bouten, C. V. C. & Baaijens, F. P. T. Tissue engineering of heart valves: advances and current challenges. Expert. Rev. Med. Devices. 6, 259–275 (2009).
Bouten, C. V. C. et al. Substrates for cardiovascular tissue engineering. Adv. Drug. Deliv. Rev. 63, 221–241 (2011).
van Loon, S. L. M., Smits, A. I. P. M., Driessen-Mol, A., Baaijens, F. P. T. & Bouten, C. V. C. The Immune Response in In Situ Tissue Engineering of Aortic Heart Valves. Calcific Aortic Valve Disease (ed. Aikawa, E.) 207–245 (2013).
MacGrogan, D. et al. How to make a heart valve: from embryonic development to bioengineering of living valve substitutes. Cold Spring Harb. Perspect. Med. 4, a013912 (2014).
Schoen, F. J. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation 118, 1864–1880 (2008).
Fioretta, E. S., Dijkman, P. E., Emmert, M. Y. & Hoerstrup, S. P. The future of heart valve replacement: recent developments and translational challenges for heart valve tissue engineering. J. Tissue Eng. Regen. Med. doi:10.1002/term.2326 (2016).
Cheung, D. Y., Duan, B. & Butcher, J. T. Current progress in tissue engineering of heart valves: multiscale problems, multiscale solutions. Expert Opin. Biol. Ther. 15, 1155–1172. doi:10.1517/14712598.2015.1051527 (2015).
Pashneh-Tala, S., MacNeil, S. & Claeyssens, F. The tissue-engineered vascular graft—past, present, and future. Tissue. Eng. Part. B. Rev. 22, ten.teb.2015.0100 (2015).
Schoen, F. J., Normann, S. J., Brunswick, R. A. & Diacoff, G. R. Can a small blood vessel prosthesis be derived from heterologous foreign body reactive tissue? J. Biomed. Mater. Res. 13, 149–154 (1979).
Sparks, C. H. Die-grown reinforced arterial grafts: observations on long-term animal grafts and clinical experience. Ann. Surg. 172, 787–794 (1970).
van der Lei, B., Nieuwenhuis, P., Molenaar, I. & Wildevuur, C. R. Long-term biologic fate of neoarteries regenerated in microporous, compliant, biodegradable, small-caliber vascular grafts in rats. Surgery. 101, 459–467 (1987).
Wildevuur, C. R., van der Lei, B. & Schakenraad, J. M. Basic aspects of the regeneration of small-calibre neoarteries in biodegradable vascular grafts in rats. Biomaterials. 8, 418–422 (1987).
Harrington, J. K. et al. Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB. J. 25, 4150–4161 (2011).
Hibino, N. et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB. J. 25, 2731–2739 (2011).
Hibino, N. et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB. J. 25, 4253–4263 (2011).
Matsumura, G., Hibino, N., Ikada, Y., Kurosawa, H. & Shin’oka, T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 24, 2303–2308 (2003).
Shinoka, T. et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg 129, 1330–1338 (2005).
Hibino, N. et al. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 139, 431–436 (2010).
Roh, J. D. et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl. Acad. Sci. USA. 107, 4669–4674 (2010).
Syedain, Z. et al. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat. Commun. 7, 12951 (2016).
Bockeria, L. A. et al. Total cavo-pulmonary connection with a new bio-absorbable vascular graft first clinical experience. J. Thorac. Cardiovasc. Surg. 153, 1542–1550 (2017).
Wu, W., Allen, Ra & Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 18, 1148–1153 (2012).
Zafar, F. et al. Physiological growth, remodeling potential, and preserved function of a novel bioprosthetic tricuspid valve: Tubular bioprosthesis made of small intestinal submucosa-derived extracellular matrix. J. Am. Coll. Cardiol. 66, 877–888 (2015).
Ruiz, C. E. et al. Transcatheter placement of a low-profile biodegradable pulmonary valve made of small intestinal submucosa: A long-term study in a swine model. J. Thorac. Cardiovasc. Surg. 130, 477–484 (2005).
Tudorache, I. et al. Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: Haemodynamic and morphological results at 20 months after implantation. Eur. J. Cardio-thoracic Surg 49, 1228–1238 (2016).
Neumann, A., Cebotari, S., Tudorache, I., Haverich, A. & Sarikouch, S. Heart valve engineering: Decellularized allograft matrices in clinical practice. Biomed. Tech 58, 453–456 (2013).
Iop, L. et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS. ONE. 9, e99593 (2014).
Syedain, Z. et al. 6-Month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials. 73, 175–184 (2015).
Driessen-Mol, A. et al. Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: Long-term functionality and rapid in vivo remodeling in sheep. J. Am. Coll. Cardiol. 63, 1320–1329 (2014).
Dijkman, P. E., Driessen-Mol, A., Frese, L., Hoerstrup, S. P. & Baaijens, F. P. T. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials. 33, 4545–4554 (2012).
Reimer, J. et al. Implantation of a tissue-engineered tubular heart valve in growing lambs. Ann. Biomed. Eng. doi:10.1007/s10439-016-1605-7 (2016).
Weber, B. et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur. Heart. J. 32, 2830–2840 (2011).
Kluin, J. et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant - From material design to 12 months follow-up in sheep. Biomaterials. 125, 101–117 (2017).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Franz, S., Rammelt, S., Scharnweber, D. & Simon, J. C. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 32, 6692–6709 (2011).
Smits, A. I. P. M. et al. In situ tissue engineering of functional small-diameter blood vessels by host circulating cells only. Tissue. Eng. Part. A. 21, 2583–2594 (2015).
Hibino, N. et al. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB. J. 29, 2431–2438 (2015).
Mirza, R., DiPietro, La & Koh, T. J. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 175, 2454–2462 (2009).
Godwin, J. W., Pinto, A. R. & Rosenthal, N. A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. U. S. A. 110, 9415–9420 (2013).
Danenberg, H. D. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 106, 599–605 (2002).
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A. & Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. doi:10.1002/path.4133 (2012).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Mosser, D. & Edwards, J. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Porcheray, F. et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489 (2005).
Spiller, K. L. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 35, 4477–4488 (2014).
Schnoor, M. et al. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J. Immunol. 180, 5707–5719 (2008).
Krettek, A., Sukhova, G. K. & Libby, P. Elastogenesis in human arterial disease: A role for macrophages in disordered elastin synthesis. Arterioscler. Thromb. Vasc. Biol. 23, 582–587 (2003).
Battiston, K. G., Labow, R. S., Simmons, C. a. & Santerre, J. P. Immunomodulatory polymeric scaffold enhances extracellular matrix production in cell co-cultures under dynamic mechanical stimulation. Acta Biomater. doi:10.1016/j.actbio.2015.05.038 (2015).
Daniel, J.-M. & Sedding, D. G. Circulating smooth muscle progenitor cells in arterial remodeling. J. Mol. Cell. Cardiol. 50, 273–279 (2011).
Pilling, D. & Gomer, R. H. Differentiation of circulating monocytes into fibroblast-like cells. Methods. Mol. Biol. 904, 191–206 (2012).
Swirski, F. K. & Nahrendorf, M. Do vascular smooth muscle cells differentiate to macrophages in atherosclerotic lesions? Circ. Res. 115, 605–606 (2015).
Brown, B. N. et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 8, 978–987 (2012).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–966 (1997).
Simper, D., Stalboerger, P. G., Panetta, C. J., Wang, S. & Caplice, N. M. Smooth muscle progenitor cells in human blood. Circulation. 106, 1199–1204 (2002).
Zilla, P., Bezuidenhout, D. & Human, P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 28, 5009–5027 (2007).
Deb, A. et al. Bone marrow-derived myofibroblasts are present in adult human heart valves. J. Heart. Valve. Dis. 14, 674–678 (2005).
Hajdu, Z. et al. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J. Mol. Cell. Cardiol. 51, 955–965 (2011).
Visconti, R. P. et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ. Res. 98, 690–696 (2006).
Kouchi, Y. et al. Apparent blood stream origin of endothelial and smooth muscle cells in the neointima of long, impervious carotid-femoral grafts in the dog. Ann. Vasc. Surg. 12, 46–54 (1998).
Shi, Q. et al. Proof of fallout endothelialization of impervious Dacron grafts in the aorta and inferior vena cava of the dog. J. Vasc. Surg. 20, 546–556 (1994).
Forbes, S. J. & Rosenthal, N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 20, 857–869 (2014).
Ballotta, V., Smits, A. I. P. M., Driessen-Mol, A., Bouten, C. V. C. & Baaijens, F. P. T. Synergistic protein secretion by mesenchymal stromal cells seeded in 3D scaffolds and circulating leukocytes in physiological flow. Biomaterials. 35, 9100–9113 (2014).
Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105, 1815–1822 (2005).
Holt, D. J., Chamberlain, L. M. & Grainger, D. W. Cell–cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 31, 9382–9394 (2010).
Chen, L. et al. Interaction of vascular smooth muscle cells and monocytes by soluble factors synergistically enhances IL-6 and MCP-1 production. Am. J. Physiol. Heart. Circ. Physiol. 296, H987–H996 (2009).
Battiston, K. G., Ouyang, B., Labow, R. S. & Simmons, C. a. & Santerre, J. P. Monocyte/macrophage cytokine activity regulates vascular smooth muscle cell function within a degradable polyurethane scaffold. Acta. Biomater. 10, 1146–1155 (2014).
McBane, J. E. et al. The effect of degradable polymer surfaces on co-cultures of monocytes and smooth muscle cells. Biomaterials. 32, 3584–3595 (2011).
Ploeger, D. T. et al. Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell. Commun. Signal. 11, 29 (2013).
Song, E. et al. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 204, 19–28 (2000).
McBane, J. E., Cai, K., Labow, R. S. & Santerre, J. P. Co-culturing monocytes with smooth muscle cells improves cell distribution within a degradable polyurethane scaffold and reduces inflammatory cytokines. Acta Biomater. 8, 488–501 (2012).
Sridharan, R., Cameron, A. R., Kelly, D. J., Kearney, C. J. & O’Brien, F. J. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater. Today 18, 313–325 (2015).
McWhorter, F. Y., Davis, C. T. & Liu, W. F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life. Sci. 72, 1303–1316 (2014).
Wagenseil, J. E. & Mecham, R. P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89, 957–989 (2009).
Chester, A. H. et al. The living aortic valve: From molecules to function. Glob. Cardiol. Sci. Pract 2014, 52–77 (2014).
Mirensky, T. L. et al. Tissue-engineered arterial grafts: long-term results after implantation in a small animal model. J. Pediatr. Surg. 44, 1127-32–3 (2009).
Mirensky, T. L. et al. Tissue-engineered vascular grafts: does cell seeding matter? J. Pediatr. Surg. 45, 1299–1305 (2010).
Mahler, G. J., Frendl, C. M., Cao, Q. & Butcher, J. T. Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol. Bioeng. 111, 2326–2337 (2014).
Monaghan, M. G. et al. A spatiotemporal observation of EndMT and mesenchymal cell colonization at the onset of human cardiac valve development. Development 49, 473–482 (2015).
Muylaert, D. E. P. et al. Environmental influences on endothelial to mesenchymal transition in developing implanted cardiovascular tissue-engineered grafts. Tissue. Eng. Part. B. Rev. 0, ten.teb.2015.0167 (2015).
Angelos, M. G. et al. Dynamic adhesion of umbilical cord blood endothelial progenitor cells under laminar shear stress. Biophys. J. 99, 3545–3554 (2010).
Markway, B. D. et al. Capture of flowing endothelial cells using surface-immobilized anti-kinase insert domain receptor antibody. Tissue. Eng. Part. C. Methods. 14, 97–105 (2008).
Plouffe, B. D., Radisic, M. & Murthy, S. K. Microfluidic depletion of endothelial cells, smooth muscle cells, and fibroblasts from heterogeneous suspensions. Lab. Chip. 8, 462–472 (2008).
Wang, X. & Cooper, S. Adhesion of endothelial cells and endothelial progenitor cells on peptide-linked polymers in shear flow. Tissue. Eng. Part. A. 19, 1113–1121 (2013).
Ruggeri, Z. M. Platelet adhesion under flow. Microcirculation. 16, 58–83 (2009).
Chen, S. & Springer, T. A. Selectin receptor-ligand bonds: Formation limited by shear rate and dissociation governed by the bell model. Proc. Natl. Acad. Sci. USA 98, 950–955 (2001).
Shive, M. S., Brodbeck, W. G., Colton, E. & Anderson, J. M. Shear stress and material surface effects on adherent human monocyte apoptosis. J. Biomed. Mater. Res. 60, 148–158 (2002).
Smits, A. I. P. M., Ballotta, V., Driessen-Mol, A., Bouten, C. V. C. & Baaijens, F. P. T. Shear flow affects selective monocyte recruitment into MCP-1-loaded scaffolds. J. Cell. Mol. Med. 18, 2176–2188 (2014).
Fitts, M. K., Pike, D. B., Anderson, K. & Shiu, Y.-T. Hemodynamic shear stress and endothelial dysfunction in hemodialysis access. Int. Urol. Nephrol. 7, 33–44 (2014).
Lyman, D. J., Stewart, S. F. C., Murray-Wijelath, J. & Wijelath, E. Role of fluid dynamics on the healing of an in vivo tissue engineered vascular graft. J. Biomed. Mater. Res. B. Appl. Biomater. 77, 389–400 (2006).
Claiborne, T. E. et al. In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. J. Biomech. Eng. 135, 21021 (2013).
Gaudio, C. Del, Gasbarroni, P. L. & Romano, G. P. Experimental investigations on the fluid-mechanics of an electrospun heart valve by means of particle image velocimetry. J. Mech. Behav. Biomed. Mater. 64, 229–239 (2016).
Rubbens, M. P. et al. Intermittent straining accelerates the development of tissue properties in engineered heart valve tissue. Tissue. Eng. Part. A. 15, 999–1008 (2009).
van Geemen, D., Driessen-Mol, A., Baaijens, F. P. T. & Bouten, C. V. C. Understanding strain-induced collagen matrix development in engineered cardiovascular tissues from gene expression profiles. Cell. Tissue. Res. 352, 727–737 (2013).
Gupta, V., Tseng, H., Lawrence, B. D. & Jane Grande-Allen, K. Effect of cyclic mechanical strain on glycosaminoglycan and proteoglycan synthesis by heart valve cells. Acta Biomater. 5, 531–540 (2009).
Huang, A. H. et al. Biaxial stretch improves elastic fiber maturation, collagen arrangement and mechanical properties in engineered arteries. Tissue. Eng. Part. C. Methods. 1–51. doi:10.1089/ten.TEC.2015.0309 (2016).
Venkataraman, L., Bashur, C. A. & Ramamurthi, A. Impact of cyclic stretch on induced elastogenesis within collagenous conduits. Tissue. Eng. Part. A. 20, 1403–1415 (2014).
Yamamoto, K. & Ando, J. Differentiation of stem/progenitor cells into vascular cells in response to fluid mechanical forces. Biorheology 24, 1–10 (2010).
Kurpinski, K., Chu, J., Hashi, C. & Li, S. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 103, 16095–16100 (2006).
Yang, J. H., Sakamoto, H., Xu, E. C. & Lee, R. T. Biomechanical regulation of human monocyte/macrophage molecular function. Am. J. Pathol. 156, 1797–1804 (2000).
Ohki, R., Yamamoto, K. & Mano, H. Identification of mechanically induced genes in human monocytic cells by DNA microarrays. Journal of Hypertension. 20, 685–691 (2002).
Matheson, L. A., Maksym, G. N., Santerre, J. P. & Labow, R. S. Differential effects of uniaxial and biaxial strain on U937 macrophage-like cell morphology: Influence of extracellular matrix type proteins. J. Biomed. Mater. Res. Part A 81A, 971–981 (2007).
Matheson, L. A., Maksym, G. N., Santerre, J. P. & Labow, R. S. Cyclic biaxial strain affects U937 macrophage-like morphology and enzymatic activities. J. Biomed. Mater. Res. Part A 76, 52–62 (2006).
Ballotta, V., Driessen-Mol, A., Bouten, C. V. C. & Baaijens, F. P. T. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 35, 4919–4928 (2014).
Wise, S. G. et al. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 7, 295–303 (2011).
Lee, K.-W., Stolz, D. B. & Wang, Y. Substantial expression of mature elastin in arterial constructs. Proc. Natl. Acad. Sci. U. S. A. 108, 2705–2710 (2011).
Argento, G., Simonet, M., Oomens, C. W. J. & Baaijens, F. P. T. Multi-scale mechanical characterization of scaffolds for heart valve tissue engineering. J. Biomech. 45, 2893–2898 (2012).
Loerakker, S., Argento, G., Oomens, C. W. J. & Baaijens, F. P. T. Effects of valve geometry and tissue anisotropy on the radial stretch and coaptation area of tissue-engineered heart valves. J. Biomech. 46, 1792–1800 (2013).
Argento, G. et al. Modeling the impact of scaffold architecture and mechanical loading on collagen turnover in engineered cardiovascular tissues. Biomech. Model. Mechanobiol. doi:10.1007/s10237-014-0625-1 (2014).
de Jonge, N. et al. Degree of scaffold degradation influences collagen (re)orientation in engineered tissues. Tissue. Eng. Part. A. 20, 1747–1757 (2013).
Sohier, J. et al. The potential of anisotropic matrices as substrate for heart valve engineering. Biomaterials. 35, 1833–1844 (2014).
Fioretta, E. S., Simonet, M., Smits, A. I. P. M., Baaijens, F. P. T. T. & Bouten, C. V. C. C. Differential response of endothelial and endothelial colony forming cells on electrospun scaffolds with distinct microfiber diameters. Biomacromolecules. 15, 821–829 (2014).
Balguid, A. et al. Tailoring fiber diameter in electrospun poly(epsilon-caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue. Eng. Part. A. 15, 437–444 (2009).
Smits, A. I. P. M., Driessen-Mol, A., Bouten, C. V. C. & Baaijens, F. P. T. A mesofluidics-based test platform for systematic development of scaffolds for in situ cardiovascular tissue engineering. Tissue. Eng. Part. C. Methods. 18, 475–485 (2012).
Kurpinski, K. T., Stephenson, J. T., Janairo, R. R. R., Lee, H. & Li, S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds. Biomaterials. 31, 3536–3542 (2010).
Vaz, C. M., van Tuijl, S., Bouten, C. V. C. & Baaijens, F. P. T. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 1, 575–582 (2005).
de Valence, S. et al. Advantages of bilayered vascular grafts for surgical applicability and tissue regeneration. Acta Biomater. 8, 3914–3920 (2012).
Pham, Q. P., Sharma, U. & Mikos, A. G. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 7, 2796–2805 (2006).
Milleret, V., Hefti, T., Hall, H., Vogel, V. & Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 8, 4349–4356 (2012).
Saino, E. et al. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 12, 1900–1911 (2011).
Madden, L. R. et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. U. S. A. 107, 15211–15216 (2010).
Sussman, E. M., Halpin, M. C., Muster, J., Moon, R. T. & Ratner, B. D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 42, 1508–1516 (2014).
Garg, K., Pullen, Na, Oskeritzian, Ca, Ryan, J. J. & Bowlin, G. L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 34, 4439–4451 (2013).
McWhorter, F. Y., Wang, T., Nguyen, P., Chung, T. & Liu, W. F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. U. S. A. 110, 17253–17258 (2013).
Generali, M., Dijkman, P. E. & Hoerstrup, S. P. Bioresorbable Scaffolds for Cardiovascular Tissue Engineering. EMJ Interv. Cardiol 1, 91–99 (2014).
Hong, Y. et al. Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials. 31, 4249–4258 (2010).
Brugmans, M. C. P. et al. Hydrolytic and oxidative degradation of electrospun supramolecular biomaterials: In vitro degradation pathways. Acta biomaterialia. 27, 21–31 (2015).
Zhang, H., Zhou, L. & Zhang, W. Control of scaffold degradation in tissue engineering: a review. Tissue. Eng. Part. B. Rev. 20, 492–502 (2014).
Peng, H. et al. Controlled enzymatic degradation of poly(??-caprolactone)-based copolymers in the presence of porcine pancreatic lipase. Polym. Degrad. Stab 95, 643–650 (2010).
Matsumura, G. et al. Long-term results of cell-free biodegradable scaffolds for in situ tissue engineering of pulmonary artery in a canine model. Biomaterials. 34, 6422–6428 (2013).
Mrówczyński, W. et al. Porcine carotid artery replacement with biodegradable electrospun poly-e-caprolactone vascular prosthesis. J. Vasc. Surg. 59, 210–219 (2014).
de Valence, S. et al. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 33, 38–47 (2012).
Weber, B., Zeisberger, S. M. & Hoerstrup, S. P. Prenatally harvested cells for cardiovascular tissue engineering: fabrication of autologous implants prior to birth. Placenta. 32, S316–S319 (2011).
De Visscher, G. et al. The remodeling of cardiovascular bioprostheses under influence of stem cell homing signal pathways. Biomaterials. 31, 20–28 (2010).
Thevenot, P. T. et al. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 31, 3997–4008 (2010).
Mokarram, N., Merchant, A., Mukhatyar, V., Patel, G. & Bellamkonda, R. V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 33, 8793–8801 (2012).
Potas, J. R., Haque, F., Maclean, F. L. & Nisbet, D. R. Interleukin-10 conjugated electrospun polycaprolactone (PCL) nanofibre scaffolds for promoting alternatively activated (M2) macrophages around the peripheral nerve in vivo. J. Immunol. Methods. 420, 38–49 (2015).
Reeves, A. R. D., Spiller, K. L., Freytes, D. O., Vunjak-Novakovic, G. & Kaplan, D. L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials. 73, 272–283 (2015).
Spiller, K. L. et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 37, 194–207 (2015).
Kumar, V. A. et al. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials. 52, 71–78 (2015).
Chen, F.-M., Zhang, M. & Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 31, 6279–6308 (2010).
Jay, S. M. et al. Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials. 31, 3054–3062 (2010).
Szentivanyi, A., Chakradeo, T., Zernetsch, H. & Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug. Deliv. Rev. 63, 209–220 (2011).
Salek-Ardakani, S., Arrand, J. R., Shaw, D. & Mackett, M. Heparin and heparan sulfate bind interleukin-10 and modulate its activity. Blood. 96, 1879–1888 (2000).
Rajangam, K. et al. Heparin binding nanostructures to promote growth of blood vessels. Nano. Lett. 6, 2086–2090 (2006).
Lee, J., Yoo, J. J., Atala, A. & Lee, S. J. The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials. 33, 6709–6720 (2012).
Nillesen, S. T. M. et al. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 28, 1123–1131 (2007).
Mammadov, R., Mammadov, B., Guler, M. O. & Tekinay, A. B. Growth factor binding on heparin mimetic peptide nanofibers. Biomacromolecules. 13, 3311–3319 (2012).
Muylaert, D. E. P. et al. Early in-situ cellularization of a supramolecular vascular graft is modified by synthetic stromal cell-derived factor-1α derived peptides. Biomaterials. 76, 187–195 (2015).
Lee, T. C., Midura, R. J., Hascall, V. C. & Vesely, I. The effect of elastin damage on the mechanics of the aortic valve. J. Biomech. 34, 203–210 (2001).
Schoof, P. H. et al. Degeneration of the pulmonary autograft: an explant study. J. Thorac. Cardiovasc. Surg. 132, 1426–1432 (2006).
Anidjar, S. et al. Elastase-induced experimental aneurysms in rats. Circulation. 82, 973–981 (1990).
Saitow, C. B., Wise, S. G., Weiss, A. S., Castellot, J. J. & Kaplan, D. L. Elastin biology and tissue engineering with adult cells. Biomol. Concepts 4, 173–185 (2013).
Huang, A. H. et al. Design and use of a novel bioreactor for regeneration of biaxially stretched tissue-engineered vessels. Tissue. Eng. Part. C. Methods. 21, 841–851 (2015).
Nejad, S. P., Blaser, M. C., Paul Santerre, J., Caldarone, C. A. & Simmons, C. A. Biomechanical conditioning of tissue engineered heart valves: Too much of a good thing? Adv. Drug. Deliv. Rev. doi:10.1016/j.addr.2015.11.003 (2015).
Keeley, E. C., Mehrad, B. & Strieter, R. M. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int. J. Biochem. Cell. Biol. 42, 535–542 (2010).
Ferguson, M. W. J. & O’Kane, S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359, 839–850 (2004).
Harty, M., Neff, A. W., King, M. W. & Mescher, A. L. Regeneration or scarring: an immunologic perspective. Dev. Dyn. 226, 268–279 (2003).
Martin, P. et al. Wound Healing in the PU.1 Null Mouse—Tissue Repair Is Not Dependent on Inflammatory Cells. Curr. Biol. 13, 1122–1128 (2003).
Naito, Y. et al. Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells. Tissues. Organs. 195, 60–72 (2012).
Naito, Y. et al. Beyond burst pressure: initial evaluation of the natural history of the biaxial mechanical properties of tissue-engineered vascular grafts in the venous circulation using a murine model. Tissue. Eng. Part. A. 20, 346–355 (2014).
Udelsman, B. V. et al. Characterization of evolving biomechanical properties of tissue engineered vascular grafts in the arterial circulation. J. Biomech. 47, 2070–2079 (2014).
Sugiura, T. et al. Fast-degrading bioresorbable arterial vascular graft with high cellular infiltration inhibits calcification of the graft. J. Vasc. Surg. 1–8. doi:10.1016/j.jvs.2016.05.096 (2016).
Wu, M. H. et al. Implant site influence on arterial prosthesis healing: a comparative study with a triple implantation model in the same dog. J. Vasc. Surg. 25, 528–536 (1997).
McGonigle, P. & Ruggeri, B. Animal models of human disease: challenges in enabling translation. Biochem. Pharmacol. 87, 162–171 (2014).
Pennel, T., Zilla, P. & Bezuidenhout, D. Differentiating transmural from transanastomotic prosthetic graft endothelialization through an isolation loop-graft model. J. Vasc. Surg. 58, 1053–1061 (2013).
Byrom, M. J., Bannon, P. G., White, G. H. & Ng, M. K. C. Animal models for the assessment of novel vascular conduits. J. Vasc. Surg. 52, 176–195 (2010).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 110, 3507–3512 (2013).
Ingersoll, M. A. et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 115, e10–e19 (2010).
Miller, K. S., Khosravi, R., Breuer, C. K. & Humphrey, J. D. A hypothesis-driven parametric study of effects of polymeric scaffold properties on tissue engineered neovessel formation. Acta. Biomater. 11, 283–294 (2015).
Wolf, M. T., Vodovotz, Y., Tottey, S., Brown, B. N. & Badylak, S. F. Predicting in vivo responses to biomaterials via combined in vitro and in silico analysis. Tissue. Eng. Part. C. Methods. 21, 148–159 (2015).
Jannasch, M. et al. In vitro chemotaxis and tissue remodeling assays quantitatively characterize foreign body reaction. ALTEX (2016).
Damanik, F. F. R., Rothuizen, T. C., van Blitterswijk, C., Rotmans, J. I. & Moroni, L. Towards an in vitro model mimicking the foreign body response: tailoring the surface properties of biomaterials to modulate extracellular matrix. Sci. Rep 4, 6325 (2014).
Boersema, G. S. A., Grotenhuis, N., Bayon, Y., Lange, J. F. & Bastiaansen-Jenniskens, Y. M. The effect of biomaterials used for tissue regeneration purposes on polarization of macrophages. Biores. Open Access 5, 6–14 (2016).
Enayati, M. et al. Biocompatibility assessment of a new biodegradable vascular graft via in vitro co-culture approaches and in vivo model. Ann. Biomed. Eng. doi:10.1007/s10439-016-1601-y (2016).
Wolf, F., Vogt, F., Schmitz-Rode, T., Jockenhoevel, S. & Mela, P. Bioengineered vascular constructs as living models for in vitro cardiovascular research. Drug. Discov. Today. 0, (2016).
McBrearty, Ba, Clark, L. D., Zhang, X. M., Blankenhorn, E. P. & Heber-Katz, E. Genetic analysis of a mammalian wound-healing trait. Proc. Natl. Acad. Sci. U. S. A. 95, 11792–11797 (1998).
Khosravi, R. et al. Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts two years post-implantation. Tissue. Eng. Part. A. 21, 1529–1538 (2015).
Bouman, A., Schipper, M., Heineman, M. J. & Faas, M. M. Gender difference in the non-specific and specific immune response in humans. Am. J. Reprod. Immunol. 52, 19–26 (2004).
Goetzl, E. J. et al. Gender specificity of altered human immune cytokine profiles in aging. FASEB. J. 24, 3580–3589 (2010).
Hodkinson, C. F. et al. Whole blood analysis of phagocytosis, apoptosis, cytokine production, and leukocyte subsets in healthy older men and women: the ZENITH study. J. Gerontol. A. Biol. Sci. Med. Sci. 61, 907–917 (2006).
Imahara, S. D., Jelacic, S., Junker, C. E. & O’Keefe, G. E. The influence of gender on human innate immunity. Surgery. 138, 275–282 (2005).
Krawiec, J. T. et al. In vivo functional evaluation of tissue-engineered vascular grafts fabricated using human adipose-derived stem cells from high cardiovascular risk populations. Tissue. Eng. Part. A. 22, 765–775 (2016).
Krawiec, J. T. et al. A cautionary tale for autologous vascular tissue engineering: impact of human demographics on the ability of adipose-derived mesenchymal stem cells to recruit and differentiate into smooth muscle cells. Tissue. Eng. Part. A. 21, 426–437 (2015).
Wang, Z. et al. Differences in the performance of PCL-based vascular grafts as abdominal aorta substitutes in healthy and diabetic rats. Biomater. Sci. doi:10.1039/C6BM00178E (2016).
Markiewicz, K., van Til, J. A. & IJzerman, M. J. Medical devices early assessment methods: Systematic literature review. Int. J. Technol. Assess. Health. Care. 30, 137–146 (2014).
IJzerman, M. J. & Steuten, M. G. Early assessment of medical technologies to inform product development and market access. A review of methods and applications. Appl. Health. Econ. Health. Policy 9, 331–347 (2011).
Huygens, S. A. et al. Conceptual model for early health technology assessment of current and novel heart valve interventions. Open Heart 3, e000500 (2016).
Hopman, R. K. & DiPersio, J. F. Advances in stem cell mobilization. Blood. Rev. 28, 31–40 (2014).
Tara, S. et al. Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft. J. Vasc. Surg. 62, 734–743 (2015).
Fukunishi, T. et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/Chitosan scaffolds in a sheep model. PLoS. ONE. 11, e0158555 (2016).
Tara, S. et al. Well-organized neointima of large-pore poly(L-lactic acid) vascular graft coated with poly(L-lactic-co-ε-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(L-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis. 237, 684–691 (2014).
Sugiura, T. et al. Novel bioresorbable vascular graft with sponge-type scaffold as a small-diameter arterial graft. Ann. Thorac. Surg. 1–8. doi:10.1016/j.athoracsur.2016.01.110 (2016).
Wang, K. et al. Three-layered pcl grafts promoted vascular regeneration in a rabbit carotid artery model. Macromol. Biosci. 608–618. doi:10.1002/mabi.201500355 (2016).
Wang, Z. et al. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 35, 5700–5710 (2014).
Zhu, M. et al. Circumferentially aligned fibers guided functional neoartery regeneration in vivo. Biomaterials. 61, 85–94 (2015).
Bergmeister, H. et al. Biodegradable, thermoplastic polyurethane grafts for small diameter vascular replacements. Acta Biomater. 11, 104–113 (2015).
Yang, X., Wei, J., Lei, D., Liu, Y. & Wu, W. Appropriate density of PCL nano-fiber sheath promoted muscular remodeling of PGS/PCL grafts in arterial circulation. Biomaterials. 88, 34–47 (2016).
Khosravi, R. et al. Long-term functional efficacy of a novel electrospun poly(glycerol sebacate)-based arterial graft in mice. Ann. Biomed. Eng. 44, 1–15 (2016).
Pektok, E. et al. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 118, 2563–2570 (2008).
Zavan, B. et al. Neoarteries grown in vivo using a tissue-engineered hyaluronan-based scaffold. FASEB. J. 22, 2853–2861 (2008).
Pandis, L. et al. Hyaluronan-based scaffold for in vivo regeneration of the rat vena cava: Preliminary results in an animal model. J. Biomed. Mater. Res. A. 93, 1289–1296 (2010).
Lepidi, S. et al. In vivo regeneration of small-diameter (2 mm) arteries using a polymer scaffold. FASEB. J. 20, 103–105 (2006).
Rothuizen, T. C. et al. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials. 75, 82–90 (2016).
Rothuizen, T. C. et al. Tailoring the foreign body response for in situ vascular tissue engineering. Tissue. Eng. Part. C. Methods. 21, 436–446 (2015).
Koobatian, M. T. et al. Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials. 76, 344–358 (2016).
Row, S. et al. Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: The role of cells in the vascular wall. Biomaterials. 50, 115–126 (2015).
Dahl, S. L. M. et al. Readily available tissue-engineered vascular grafts. Sci. Transl. Med 3, 68ra9 (2011).
Van Almen, G. C. et al. Development of non-cell adhesive vascular grafts using supramolecular building blocks. Macromol. Biosci. 16, 350–362 (2016).
Shafiq, M., Jung, Y. & Kim, S. H. Covalent Immobilization of Stem Cell Inducing/Recruiting Factor and Heparin on Cell-Free Small-Diameter Vascular Graft for Accelerated In Situ Tissue Regeneration. J. Biomed. Mater. Res. A. doi:10.1002/jbm.a.35666 (2016).
Shafiq, M., Jung, Y. & Kim, S. H. In situ vascular regeneration using substance P-immobilised poly(L-Lactide-co-??-caprolactone) scaffolds: Stem cell recruitment, angiogenesis, and tissue regeneration. Eur. Cells Mater. 30, 282–302 (2015).
Ota, T. et al. Fibronectin-hepatocyte growth factor enhances reendothelialization in tissue-engineered heart valve. Ann. Thorac. Surg. 80, 1794–1801 (2005).
Zhang, H. et al. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials. 34, 2202–2212 (2013).
Kao, W. J. & Lee, D. In vivo modulation of host response and macrophage behavior by polymer networks grafted with fibronectin-derived biomimetic oligopeptides: the role of RGD and PHSRN domains. Biomaterials. 22, 2901–2909 (2001).
Sun, Q. et al. Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharm. Res. 27, 264–271 (2010).
Silva, E. A. & Mooney, D. J. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J. Thromb. Haemost. 5, 590–598 (2007).
Riley, C. M. et al. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials. 27, 5935–5943 (2006).
Peattie, R. A. et al. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials. 27, 1868–1875 (2006).
Dogan, A. K., Gümüşderelioglu, M. & Aksöz, E. Controlled release of EGF and bFGF from dextran hydrogels in vitro and in vivo. J. Biomed. Mater. Res. B. Appl. Biomater. 74, 504–510 (2005).
Lynn, A. D., Kyriakides, T. R. & Bryant, S. J. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A. 93, 941–953 (2010).
Lynn, A. D., Blakney, A. K., Kyriakides, T. R. & Bryant, S. J. Temporal progression of the host response to implanted poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A. 96, 621–631 (2011).
Zaveri, T. D., Lewis, J. S., Dolgova, N. V., Clare-Salzler, M. J. & Keselowsky, B. G. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 35, 3504–3515 (2014).
Gower, R. M. et al. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials 35, 2024–2031 (2014).
Acknowledgements
This project has received funding from the European Union’s Seventh Framework Program for research, technological development and demonstration under grant agreement no 604514. We also acknowledge the support from the Netherlands Cardio Vascular Research Initiative: The Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organization for Health Research and Development and the Royal Netherlands Academy of Sciences.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparation, drafting, critical revision, and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Prof. Bouten is shareholder of Xeltis BV. Ms. Wissing, Ms. Bonito and Dr. Smits declare no potential competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wissing, T.B., Bonito, V., Bouten, C.V.C. et al. Biomaterial-driven in situ cardiovascular tissue engineering—a multi-disciplinary perspective. npj Regen Med 2, 18 (2017). https://doi.org/10.1038/s41536-017-0023-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-017-0023-2
This article is cited by
-
Valvulogenesis of a living, innervated pulmonary root induced by an acellular scaffold
Communications Biology (2023)
-
Evaluation of pliable bioresorbable, elastomeric aortic valve prostheses in sheep during 12 months post implantation
Communications Biology (2023)
-
Computer Model-Driven Design in Cardiovascular Regenerative Medicine
Annals of Biomedical Engineering (2023)
-
How Smart are Smart Materials? A Conceptual and Ethical Analysis of Smart Lifelike Materials for the Design of Regenerative Valve Implants
Science and Engineering Ethics (2023)
-
Animal studies for the evaluation of in situ tissue-engineered vascular grafts — a systematic review, evidence map, and meta-analysis
npj Regenerative Medicine (2022)