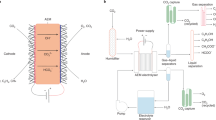
Abstract
To reduce the cost of fuel cell stacks and systems, it is important to create commercial catalysts that are free of platinum group metals (PGMs). To do this, such catalysts must have very high activity, but also have the correct microstructure to facilitate the transport of reactants and products. Here, we show a high-performing commercial oxygen reduction catalyst that was specifically developed for operation in alkaline media and is demonstrated in the cathode of operating anion-exchange membrane fuel cells (AEMFCs). With H2/O2 reacting gases, AEMFCs made with Fe–N–C cathodes achieved a peak power density exceeding 2 W cm−2 (>1 W cm−2 with H2/air) and operated with very good voltage durability for more than 150 h. These AEMFCs also realized an iR-corrected current density at 0.9 V of 100 mA cm−2. Finally, in a second configuration, Fe–N–C cathodes paired with low-loading PtRu/C anodes (0.125 mg PtRu per cm2, 0.08 mg Pt per cm2) demonstrated a specific power of 10.4 W per mg PGM (16.25 W per mg Pt).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and Supplementary Information files.
References
Hydrogen and Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan (Office of Energy Efficiency and Renewable Energy, DOE, 2014); https://www.energy.gov/eere/fuelcells/downloads/fuel-cell-technologies-office-multi-year-research-development-and-22
US DRIVE Fuel Cell Technical Team Roadmap (Office of Energy Efficiency and Renewable Energy, DOE, 2013).
James, B. D., Huya-Kouadio, J. M., Houchins, C. & DeSantis, D. A. Mass Production Cost Estimation of Direct H2 PEM Fuel Cell Systems for Transportation Applications: 2018 Update (Strategic Analysis, 2018); https://www.energy.gov/sites/prod/files/2019/12/f70/fcto-sa-2018-transportation-fuel-cell-cost-analysis.pdf
James, B. 2018 Cost Projections of PEM Fuel Cell Systems for Automobiles and Medium-Duty Vehicles (Office of Energy Efficiency and Renewable Energy, DOE, Strategic Analysis, 2018); https://www.energy.gov/sites/prod/files/2018/04/f51/fcto_webinarslides_2018_costs_pem_fc_autos_trucks_042518.pdf
Satyapal, S. 2017 Annual Progress Report: DOE Hydrogen and Fuel Cells Program (Office of Scientific and Technical Information, DOE, 2018); https://www.osti.gov/servlets/purl/1460682
Huang, G. et al. Composite poly(norbornene) anion conducting membranes for achieving durability, water management and high power (3.4 W/cm2) in hydrogen/oxygen alkaline fuel cells. J. Electrochem. Soc. 166, F637–F644 (2019).
Wang, L., Peng, X., Mustain, W. E. & Varcoe, J. R. Radiation-grafted anion-exchange membranes: the switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance. Energy Environ. Sci. 12, 1575–1579 (2019).
Li, Q. et al. The comparability of Pt to Pt-Ru in catalyzing the hydrogen oxidation reaction for alkaline polymer electrolyte fuel cells operated at 80 °C. Angew. Chem. Int. Ed. 58, 1442–1446 (2019).
Thompson, S. T., Peterson, D., Ho, D. & Papageorgopoulos, D. Perspective—the next decade of AEMFCs: near-term targets to accelerate applied R&D. J. Electrochem. Soc. 167, 84514 (2020).
Mandal, M. et al. The importance of water transport in high conductivity and high-power alkaline fuel cells. J. Electrochem. Soc. 167, 054501 (2020).
Ul Hassan, N. et al. Achieving high-performance and 2000 h stability in anion exchange membrane fuel cells by manipulating ionomer properties and electrode optimization. Adv. Energy Mater. 10, 2001986 (2020).
Setzler, B. P., Zhuang, Z., Wittkopf, J. A. & Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 11, 1020–1025 (2016).
Greeley, J. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1, 552–556 (2009).
Sheng, W., Gasteiger, H. A. & Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc. 157, B1529–B1536 (2010).
Miller, H. A. & Vizza, F. in Anion Exchange Membrane Fuel Cells: Principles, Materials and Systems (eds An, L. & Zhao, T. S.) 79–103 (Springer, 2018).
Omasta, T. J. et al. Strategies for reducing the PGM loading in high power AEMFC anodes. J. Electrochem. Soc. 165, F710–F717 (2018).
Firouzjaie, H. A. & Mustain, W. E. Catalytic advantages, challenges, and priorities in alkaline membrane fuel cells. ACS Catal. 10, 225–234 (2020).
Mustain, W. E. Understanding how high-performance anion exchange membrane fuel cells were achieved: component, interfacial, and cell-level factors. Curr. Opin. Electrochem. 12, 233–239 (2018).
Mustain, W. E., Chatenet, M., Page, M. & Kim, Y. S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. https://doi.org/10.1039/D0EE01133A (2020).
Davydova, E. S., Mukerjee, S., Jaouen, F. & Dekel, D. R. Electrocatalysts for hydrogen oxidation reaction in alkaline electrolytes. ACS Catal. 8, 6665–6690 (2018).
Kabir, S. et al. Platinum group metal-free NiMo hydrogen oxidation catalysts: high performance and durability in alkaline exchange membrane fuel cells. J. Mater. Chem. A 5, 24433–24443 (2017).
Yang, Y. et al. High-loading composition-tolerant Co–Mn spinel oxides with performance beyond 1 W/cm2 in alkaline polymer electrolyte fuel cells. ACS Energy Lett. 4, 1251–1257 (2019).
Martinez, U., Komini Babu, S., Holby, E. F. & Zelenay, P. Durability challenges and perspective in the development of PGM-free electrocatalysts for the oxygen reduction reaction. Curr. Opin. Electrochem. 9, 224–232 (2018).
Gokhale, R., Chen, Y., Serov, A., Artyushkova, K. & Atanassov, P. Direct synthesis of platinum group metal-free Fe-N-C catalyst for oxygen reduction reaction in alkaline media. Electrochem. Commun. 72, 140–143 (2016).
Santori, P. G. et al. High performance FeNC and Mn-oxide/FeNC layers for AEMFC cathodes. J. Electrochem. Soc. 167, 134505 (2020).
Li, Z. et al. Ionic liquids as precursors for efficient mesoporous iron-nitrogen-doped oxygen reduction electrocatalysts. Angew. Chem. Int. Ed. 54, 1494–1498 (2015).
Li, Z. Q., Lu, C. J., Xia, Z. P., Zhou, Y. & Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 45, 1686–1695 (2007).
Xu, X., Shi, C., Li, Q., Chen, R. & Chen, T. Fe–N-doped carbon foam nanosheets with embedded Fe2O3 nanoparticles for highly efficient oxygen reduction in both alkaline and acidic media. RSC Adv. 7, 14382–14388 (2017).
Yan, Z. et al. Nitrogen doped porous carbon with iron promotion for oxygen reduction reaction in alkaline and acidic media. Int. J. Hydrogen Energy 44, 4090–4101 (2019).
Huo, L. et al. 2D layered non-precious metal mesoporous electrocatalysts for enhanced oxygen reduction reaction. J. Mater. Chem. A 5, 4868–4878 (2017).
Garsany, Y., Baturina, O. A., Swider-Lyons, K. E. & Kocha, S. S. Experimental methods for quantifying the activity of platinum electrocatalysts for the oxygen reduction reaction. Anal. Chem. 82, 6321–6328 (2010).
Omasta, T. J. et al. Importance of balancing membrane and electrode water in anion exchange membrane fuel cells. J. Power Sources 375, 205–213 (2018).
Peng, X. et al. Using operando techniques to understand and design high performance and stable alkaline membrane fuel cells. Nat. Commun. 11, 3561 (2020).
Wang, T. et al. High-performance hydroxide exchange membrane fuel cells through optimization of relative humidity, backpressure and catalyst selection. J. Electrochem. Soc. 166, F3305–F3310 (2019).
Uddin, A. et al. High power density platinum group metal-free cathodes for polymer electrolyte fuel cells. ACS Appl. Mater. Interfaces 12, 2216–2224 (2020).
Peng, X. et al. High-performing PGM-free AEMFC cathodes from carbon-supported cobalt ferrite nanoparticles. Catalysts 9, 264–275 (2019).
Wang, L., Bellini, M., Miller, H. A. & Varcoe, J. R. A high conductivity ultrathin anion-exchange membrane with 500+ h alkali stability for use in alkaline membrane fuel cells that can achieve 2 W cm−2 at 80 °C. J. Mater. Chem. A 6, 15404–15412 (2018).
Peng, X. et al. Nitrogen-doped carbon–CoOx nanohybrids: a precious metal free cathode that exceeds 1.0 W cm−2 peak power and 100 h life in anion-exchange membrane fuel cells. Angew. Chem. Int. Ed. 58, 1046–1051 (2019).
Wan, X. et al. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).
Fu, X. et al. In situ polymer graphenization ingrained with nanoporosity in a nitrogenous electrocatalyst boosting the performance of polymer-electrolyte-membrane fuel cells. Adv. Mater. 29, 1604456 (2017).
Shao, Y. Highly Active and Durable PGM-Free ORR Electrocatalysts Through the Synergy of Active Sites (Pacific Northwest National Laboratory, 2018).
Liu, Q., Liu, X., Zheng, L. & Shui, J. The solid-phase synthesis of an Fe-N-C electrocatalyst for high-power proton-exchange membrane fuel cells. Angew. Chem. Int. Ed. 57, 1204–1208 (2018).
Dekel, D. R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 375, 158–169 (2018).
Cheng, J., He, G. & Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: stability issue and addressing strategies. Int. J. Hydrogen Energy 40, 7348–7360 (2015).
Ramaswamy, N. & Mukerjee, S. Alkaline anion-exchange membrane fuel cells: challenges in electrocatalysis and interfacial charge transfer. Chem. Rev. 119, 11945–11979 (2019).
Wang, J. et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 139, 17281–17284 (2017).
DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components (Office of Energy Efficiency and Renewable Energy); https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components
Zhou, R., Zheng, Y., Jaroniec, M. & Qiao, S.-Z. Determination of the electron transfer number for the oxygen reduction reaction: from theory to experiment. ACS Catal. 6, 4720–4728 (2016).
Omasta, T. J. et al. Beyond catalysis and membranes: visualizing and solving the challenge of electrode water accumulation and flooding in AEMFCs. Energy Environ. Sci. 11, 551–558 (2018).
Poynton, S. D. et al. Preparation of radiation-grafted powders for use as anion exchange ionomers in alkaline polymer electrolyte fuel cells. J. Mater. Chem. A 2, 5124–5130 (2014).
Wang, L. et al. Non-fluorinated pre-irradiation-grafted (peroxidated) LDPE-based anion-exchange membranes with high performance and stability. Energy Environ. Sci. 10, 2154–2167 (2017).
Tatus-Portnoy, Z. et al. A low-loading Ru-rich anode catalyst for high-power anion exchange membrane fuel cells. Chem. Commun. 56, 5669–5672 (2020).
Acknowledgements
The authors gratefully acknowledge the financial support of the US Department of Energy Office of Energy Efficiency and Renewable Energy under the Hydrogen and Fuel Cells Technologies Office (HFTO; award no. DE-EE0008433 to H.A. and W.E.M., and award no. DE-EE0008419 to A.Serov and B.Z.). J.R.V. gratefully acknowledges the support of the UK EPSRC (grant no. EP/M014371/1) for the polymer synthesis. J.R.R. also acknowledges the support of the Center of Catalysis for Renewable Fuels (CReF).
Author information
Authors and Affiliations
Contributions
H.A. and W.E.M. were the primary writers of the manuscript. H.A. designed and performed the electrochemical experiments, characterized the catalyst and analysed the data. H.A. and A. Shakouri together performed the electron microscopy imaging and further characterizations. N.U.H. assisted with the AEMFC testing and data analysis. A. Serov and B.Z. contributed to providing the material and characterizing the Fe–N–C catalyst. J.R.V. provided the membrane and assisted with the writing of the manuscript. J.R.R. oversaw the electron microscopy experiments and provided ICDD licences for XRD. W.E.M. supervised the execution of the overall project.
Corresponding author
Ethics declarations
Competing interests
A. Serov and B.Z. are employees at Pajarito Powder, who supplied the commercial catalyst for this work. The other authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Brian Setzler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Adabi, H., Shakouri, A., Ul Hassan, N. et al. High-performing commercial Fe–N–C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat Energy 6, 834–843 (2021). https://doi.org/10.1038/s41560-021-00878-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00878-7
This article is cited by
-
Tuning the apparent hydrogen binding energy to achieve high-performance Ni-based hydrogen oxidation reaction catalyst
Nature Communications (2024)
-
Nanocurvature-induced field effects enable control over the activity of single-atom electrocatalysts
Nature Communications (2024)
-
Research progress of industrial application of membrane electrolysis technology
Ionics (2024)
-
PGM-Free Biomass-Derived Electrocatalysts for Oxygen Reduction in Energy Conversion Devices: Promising Materials
Electrochemical Energy Reviews (2024)
-
Progress and prospect of Pt-based catalysts for electrocatalytic hydrogen oxidation reactions
Nano Research (2024)