Abstract
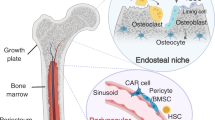
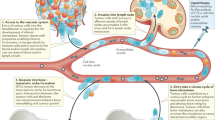
Many cancer types metastasize to bone. This propensity may be a product of genetic traits of the primary tumour in some cancers. Upon arrival, cancer cells establish interactions with various bone-resident cells during the process of colonization. These interactions, to a large degree, dictate cancer cell fates at multiple steps of the metastatic cascade, from single cells to overt metastases. The bone microenvironment may even influence cancer cells to subsequently spread to multiple other organs. Therefore, it is imperative to spatiotemporally delineate the evolving cancer–bone crosstalk during bone colonization. In this Review, we provide a summary of the bone microenvironment and its impact on bone metastasis. On the basis of the microscopic anatomy, we tentatively define a roadmap of the journey of cancer cells through bone relative to various microenvironment components, including the potential of bone to function as a launch pad for secondary metastasis. Finally, we examine common and distinct features of bone metastasis from various cancer types. Our goal is to stimulate future studies leading to the development of a broader scope of potent therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 3 (Suppl. 3), S131–S139 (2008).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Kusumbe, A. P. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384 (2016).
Mendez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Brazill, J. M., Beeve, A. T., Craft, C. S., Ivanusic, J. J. & Scheller, E. L. Nerves in bone: evolving concepts in pain and anabolism. J. Bone Min. Res. 34, 1393–1406 (2019).
McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 16, 717–727 (2014).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Zeng, Z. et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 9, 5395 (2018).
Liu, Y. et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 30, 243–256 (2016).
Tyagi, A. et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat. Commun. 12, 474 (2021).
Catena, R. et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 3, 578–589 (2013).
Granot, Z. et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314 (2011).
Meyer, M. A. et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 9, 1250 (2018).
Cheng, P. et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249 (2008).
Sinha, P. et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 181, 4666–4675 (2008).
Fleming, V. et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front. Immunol. 9, 398 (2018).
Markowitz, J., Wesolowski, R., Papenfuss, T., Brooks, T. R. & Carson, W. E. III Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res. Treat. 140, 13–21 (2013).
Welte, T. et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat. Cell Biol. 18, 632–644 (2016).
Kim, I. S. et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat. Cell Biol. 21, 1113–1126 (2019).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
Kennecke, H. et al. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 (2010).
Smid, M. et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 68, 3108–3114 (2008).
Zhang, X. H., Giuliano, M., Trivedi, M. V., Schiff, R. & Osborne, C. K. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin. Cancer Res. 19, 6389–6397 (2013).
Zhang, X. H. et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16, 67–78 (2009).
Vanharanta, S. & Massague, J. Origins of metastatic traits. Cancer Cell 24, 410–421 (2013).
Zhang, X. H. et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073 (2013).
Awolaran, O., Brooks, S. A. & Lavender, V. Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast 30, 156–171 (2016).
Bellahcene, A. & Castronovo, V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am. J. Pathol. 146, 95–100 (1995).
Tan, C. C. et al. Breast cancer cells obtain an osteomimetic feature via epithelial–mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction. Oncotarget 7, 79688–79705 (2016).
Shiozawa, Y. et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Invest. 121, 1298–1312 (2011).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Price, T. T. et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 8, 340ra373 (2016).
Carlson, P. et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019).
Nobre, A. R. et al. Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGF-β2. Nat. Cancer 2, 327–339 (2021).
Johnson, R. W. et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat. Cell Biol. 18, 1078–1089 (2016).
Gawrzak, S. et al. MSK1 regulates luminal cell differentiation and metastatic dormancy in ER+ breast cancer. Nat. Cell Biol. 20, 211–221 (2018).
Esposito, M. et al. Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639 (2019).
Adams, G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006).
Wei, Q. & Frenette, P. S. Niches for hematopoietic stem cells and their progeny. Immunity 48, 632–648 (2018).
Wang, H. et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210 (2015).
Zheng, H. et al. Therapeutic antibody targeting tumor- and osteoblastic niche-derived jagged1 sensitizes bone metastasis to chemotherapy. Cancer Cell 32, 731–747 e736 (2017).
Wang, D. et al. NPNT promotes early-stage bone metastases in breast cancer by regulation of the osteogenic niche. J. Bone Oncol. 13, 91–96 (2018).
Wang, H. et al. The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell 34, 823–839 e827 (2018).
Bado, I. L. et al. The bone microenvironment increases phenotypic plasticity of ER+ breast cancer cells. Dev. Cell 56, 1100–1117.e1109 (2021).
Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205 (2011).
San Martin, R. et al. Tenascin-C and integrin alpha9 mediate interactions of prostate cancer with the bone microenvironment. Cancer Res. 77, 5977–5988 (2017).
Oskarsson, T. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011).
Lowy, C. M. & Oskarsson, T. Tenascin C in metastasis: a view from the invasive front. Cell Adh. Migr. 9, 112–124 (2015).
Shupp, A. B., Kolb, A. D., Mukhopadhyay, D. & Bussard, K. M. Cancer metastases to bone: concepts, mechanisms, and interactions with bone osteoblasts. Cancers 10, 182 (2018).
Lawson, M. A. et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 6, 8983 (2015).
Guise, T. A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Invest. 98, 1544–1549 (1996).
Guise, T. A. et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 12, 6213s–6216s (2006).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA 95, 3597–3602 (1998).
Kingsley, L. A., Fournier, P. G., Chirgwin, J. M. & Guise, T. A. Molecular biology of bone metastasis. Mol. Cancer Ther. 6, 2609–2617 (2007).
Waning, D. L. & Guise, T. A. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin. Cancer Res. 20, 3071–3077 (2014).
Ell, B. & Kang, Y. SnapShot: bone metastasis. Cell 151, 690–690 e691 (2012).
Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Ross, M. H. et al. Bone-induced expression of integrin beta3 enables targeted nanotherapy of breast cancer metastases. Cancer Res. 77, 6299–6312 (2017).
Welm, A. L. et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc. Natl Acad. Sci. USA 104, 7570–7575 (2007).
Andrade, K. et al. RON kinase: a target for treatment of cancer-induced bone destruction and osteoporosis. Sci. Transl. Med. 9, eaai9338 (2017).
Luo, X. et al. Stromal-initiated changes in the bone promote metastatic niche development. Cell Rep. 14, 82–92 (2016).
Coleman, R. E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 27, 165–176 (2001).
Esposito, M., Guise, T. & Kang, Y. The biology of bone metastasis. Cold Spring Harb. Perspect. Med. 8, a031252 (2018).
Coleman, R. E. et al. Bone metastases. Nat. Rev. Dis. Prim. 6, 83 (2020).
Soni, A. et al. Breast cancer subtypes predispose the site of distant metastases. Am. J. Clin. Pathol. 143, 471–478 (2015).
Cummings, M. C. et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J. Pathol. 232, 23–31 (2014).
Disibio, G. & French, S. W. Metastatic patterns of cancers: results from a large autopsy study. Arch. Pathol. Lab. Med. 132, 931–939 (2008).
Lee, Y. T. Breast carcinoma: pattern of metastasis at autopsy. J. Surg. Oncol. 23, 175–180 (1983).
Banys-Paluchowski, M., Fehm, T., Janni, W., Solomayer, E. F. & Hartkopf, A. Circulating and disseminated tumor cells in breast carcinoma: report from the consensus conference on tumor cell dissemination during the 39th Annual Meeting of the German Society of Senology, Berlin, 27 June 2019. Geburtshilfe Frauenheilkd. 79, 1320–1327 (2019).
Tjensvoll, K. et al. Detection of disseminated tumor cells in bone marrow predict late recurrences in operable breast cancer patients. BMC Cancer 19, 1131 (2019).
Coleman, R. E. & Rubens, R. D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 55, 61–66 (1987).
Coleman, R. E., Smith, P. & Rubens, R. D. Clinical course and prognostic factors following bone recurrence from breast cancer. Br. J. Cancer 77, 336–340 (1998).
Early Breast Cancer Trialists’ Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386, 1353–1361 (2015).
Solomayer, E. F. et al. Influence of zoledronic acid on disseminated tumor cells in primary breast cancer patients. Ann. Oncol. 23, 2271–2277 (2012).
Hartkopf, A. D. et al. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients – results from a large single-centre analysis. Eur. J. Cancer 50, 2550–2559 (2014).
Zhang, W. et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486.e20 (2021).
Kalhor, R., Mali, P. & Church, G. M. Rapidly evolving homing CRISPR barcodes. Nat. Methods 14, 195–200 (2017).
Kalhor, R. et al. Developmental barcoding of whole mouse via homing CRISPR. Science 361, eaat9804 (2018).
Kim, M. Y. et al. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326 (2009).
Nik-Zainal, S. et al. The life history of 21 breast cancers. Cell 149, 994–1007 (2012).
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Chen, Z., Orlowski, R. Z., Wang, M., Kwak, L. & McCarty, N. Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood 123, 2204–2208 (2014).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
da Silva Meirelles, L., Caplan, A. I. & Nardi, N. B. In search of the in vivo identity of mesenchymal stem cells. Stem Cell 26, 2287–2299 (2008).
Muscarella, A. M. Unique cellular protrusions mediate breast cancer cell migration by tethering to osteogenic cells. NPJ Breast Cancer 6, 42 (2020).
Willam, C., Schindler, R., Frei, U. & Eckardt, K. U. Increases in oxygen tension stimulate expression of ICAM-1 and VCAM-1 on human endothelial cells. Am. J. Physiol. 276, H2044–2052 (1999).
Shen, S., Vagner, S. & Robert, C. Persistent cancer cells: the deadly survivors. Cell 183, 860–874 (2020).
Eyre, R. et al. Microenvironmental IL1beta promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat. Commun. 10, 5016 (2019).
Brown, D. et al. Phylogenetic analysis of metastatic progression in breast cancer using somatic mutations and copy number aberrations. Nat. Commun. 8, 14944 (2017).
Ullah, I. et al. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J. Clin. Invest. 128, 1355–1370 (2018).
Sturge, J., Caley, M. P. & Waxman, J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat. Rev. Clin. Oncol. 8, 357–368 (2011).
Wan, X. et al. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 50, 695–703 (2012).
Fournier, P. G. et al. The TGF-beta signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. Cancer Cell 27, 809–821 (2015).
Chen, C. et al. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate 75, 883–895 (2015).
Roudier, M. P. et al. Histological, immunophenotypic and histomorphometric characterization of prostate cancer bone metastases. Cancer Treat. Res. 118, 311–339 (2004).
Furesi, G., Rauner, M. & Hofbauer, L. C. Emerging players in prostate cancer–bone niche communication. Trends Cancer 7, 112–121 (2021).
Knerr, K., Ackermann, K., Neidhart, T. & Pyerin, W. Bone metastasis: osteoblasts affect growth and adhesion regulons in prostate tumor cells and provoke osteomimicry. Int. J. Cancer 111, 152–159 (2004).
Hagberg Thulin, M., Jennbacken, K., Damber, J. E. & Welen, K. Osteoblasts stimulate the osteogenic and metastatic progression of castration-resistant prostate cancer in a novel model for in vitro and in vivo studies. Clin. Exp. Metastasis 31, 269–283 (2014).
Akech, J. et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 29, 811–821 (2010).
Yang, Y. et al. PTEN loss promotes intratumoral androgen synthesis and tumor microenvironment remodeling via aberrant activation of RUNX2 in castration-resistant prostate cancer. Clin. Cancer Res. 24, 834–846 (2018).
Zhang, H. et al. FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res. 71, 3257–3267 (2011).
Dai, J. et al. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 68, 5785–5794 (2008).
Liao, J. et al. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int. J. Cancer 123, 2267–2278 (2008).
Nelson, J. B. et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat. Med. 1, 944–949 (1995).
Lee, Y. C. et al. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol. Cell Proteom. 14, 471–483 (2015).
Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 13, 27–38 (2011).
Nandana, S. et al. Bone metastasis of prostate cancer can be therapeutically targeted at the TBX2–WNT signaling axis. Cancer Res. 77, 1331–1344 (2017).
Lin, S. C. et al. Endothelial-to-osteoblast conversion generates osteoblastic metastasis of prostate cancer. Dev. Cell 41, 467–480 e463 (2017).
Yu-Lee, L. Y. et al. Bone secreted factors induce cellular quiescence in prostate cancer cells. Sci. Rep. 9, 18635 (2019).
Ercole, C. J. et al. Prostatic specific antigen and prostatic acid phosphatase in the monitoring and staging of patients with prostatic cancer. J. Urol. 138, 1181–1184 (1987).
Cramer, S. D., Chen, Z. & Peehl, D. M. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J. Urol. 156, 526–531 (1996).
Dallas, S. L. et al. Preferential production of latent transforming growth factor beta-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J. Cell Physiol. 202, 361–370 (2005).
Cohen, P., Peehl, D. M., Graves, H. C. & Rosenfeld, R. G. Biological effects of prostate specific antigen as an insulin-like growth factor binding protein-3 protease. J. Endocrinol. 142, 407–415 (1994).
Kirschenbaum, A., Liu, X. H., Yao, S., Leiter, A. & Levine, A. C. Prostatic acid phosphatase is expressed in human prostate cancer bone metastases and promotes osteoblast differentiation. Ann. N. Y. Acad. Sci. 1237, 64–70 (2011).
Ishibe, M., Rosier, R. N. & Puzas, J. E. Human prostatic acid phosphatase directly stimulates collagen synthesis and alkaline phosphatase content of isolated bone cells. J. Clin. Endocrinol. Metab. 73, 785–792 (1991).
Santoni, M. et al. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J. Exp. Clin. Cancer Res. 34, 10 (2015).
Wood, S. L. & Brown, J. E. Skeletal metastasis in renal cell carcinoma: current and future management options. Cancer Treat. Rev. 38, 284–291 (2012).
Chen, S. C. & Kuo, P. L. Bone metastasis from renal cell carcinoma. Int. J. Mol. Sci. 17, 987 (2016).
Lin, P. P. et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. J. Bone Jt. Surg. Am. 89, 1794–1801 (2007).
Pan, T. et al. BIGH3 promotes osteolytic lesions in renal cell carcinoma bone metastasis by inhibiting osteoblast differentiation. Neoplasia 20, 32–43 (2018).
Sasaki, H. et al. Beta IGH3, a TGF-beta inducible gene, is overexpressed in lung cancer. Jpn. J. Clin. Oncol. 32, 85–89 (2002).
Yamanaka, M. et al. BIGH3 is overexpressed in clear cell renal cell carcinoma. Oncol. Rep. 19, 865–874 (2008).
Zhang, W., Bado, I., Wang, H., Lo, H. C. & Zhang, X. H. Bone metastasis: find your niche and fit in. Trends Cancer 5, 95–110 (2019).
Keizman, D. et al. Bisphosphonates combined with sunitinib may improve the response rate, progression free survival and overall survival of patients with bone metastases from renal cell carcinoma. Eur. J. Cancer 48, 1031–1037 (2012).
Vrdoljak, E. et al. Bisphosphonates in patients with renal cell carcinoma and bone metastases: a sunitinib global expanded-access trial subanalysis. Future Oncol. 11, 2831–2840 (2015).
Haber, T. et al. Bone metastasis in renal cell carcinoma is preprogrammed in the primary tumor and caused by AKT and integrin alpha5 signaling. J. Urol. 194, 539–546 (2015).
Pan, T. et al. Three-dimensional (3D) culture of bone-derived human 786-O renal cell carcinoma retains relevant clinical characteristics of bone metastases. Cancer Lett. 365, 89–95 (2015).
Satcher, R. L. et al. Cadherin-11 in renal cell carcinoma bone metastasis. PLoS ONE 9, e89880 (2014).
Giuliani, N. et al. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia 26, 1391–1401 (2012).
Riquelme, M. A., Cardenas, E. R. & Jiang, J. X. Osteocytes and bone metastasis. Front. Endocrinol. 11, 567844 (2020).
Terpos, E., Ntanasis-Stathopoulos, I. & Dimopoulos, M. A. Myeloma bone disease: from biology findings to treatment approaches. Blood 133, 1534–1539 (2019).
Pan, T. et al. Cabozantinib reverses renal cell carcinoma-mediated osteoblast inhibition in three-dimensional co-culture in vitro and reduces bone osteolysis in vivo. Mol. Cancer Ther. 19, 1266–1278 (2020).
Choueiri, T. K. et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 17, 917–927 (2016).
Choueiri, T. K. et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN Trial. J. Clin. Oncol. 35, 591–597 (2017).
Escudier, B. et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J. Clin. Oncol. 36, 765–772 (2018).
Betts-Obregon, B. S. et al. TGFbeta induces BIGH3 expression and human retinal pericyte apoptosis: a novel pathway of diabetic retinopathy. Eye 30, 1639–1647 (2016).
Kim, J. E. et al. A TGF-beta-inducible cell adhesion molecule, betaig-h3, is downregulated in melorheostosis and involved in osteogenesis. J. Cell Biochem. 77, 169–178 (2000).
Klamer, S. E. et al. BIGH3 modulates adhesion and migration of hematopoietic stem and progenitor cells. Cell Adh. Migr. 7, 434–449 (2013).
Ivanov, S. V. et al. Two novel VHL targets, TGFBI (BIGH3) and its transactivator KLF10, are up-regulated in renal clear cell carcinoma and other tumors. Biochem. Biophys. Res. Commun. 370, 536–540 (2008).
Thapa, N., Kang, K. B. & Kim, I. S. Beta ig-h3 mediates osteoblast adhesion and inhibits differentiation. Bone 36, 232–242 (2005).
Raggatt, L. J. & Partridge, N. C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 285, 25103–25108 (2010).
Tian, E. et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349, 2483–2494 (2003).
D’Alterio, C. et al. Concomitant CXCR4 and CXCR7 expression predicts poor prognosis in renal cancer. Curr. Cancer Drug Targets 10, 772–781 (2010).
Pan, J. et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol. Cancer 5, 56 (2006).
Wehler, T. C. et al. Strong expression of chemokine receptor CXCR4 by renal cell carcinoma correlates with advanced disease. J. Oncol. 2008, 626340 (2008).
Motzer, R. J., Bander, N. H. & Nanus, D. M. Renal-cell carcinoma. N. Engl. J. Med. 335, 865–875 (1996).
Chambers, A., Kundranda, M., Rao, S., Mahmoud, F. & Niu, J. Anti-angiogenesis revisited: combination with immunotherapy in solid tumors. Curr. Oncol. Rep. 23, 100 (2021).
Geraets, S. E. W., Bos, P. K. & van der Stok, J. Preoperative embolization in surgical treatment of long bone metastasis: a systematic literature review. EFORT Open Rev. 5, 17–25 (2020).
Chan, D. A., Sutphin, P. D., Yen, S. E. & Giaccia, A. J. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol. Cell Biol. 25, 6415–6426 (2005).
Blancher, C., Moore, J. W., Robertson, N. & Harris, A. L. Effects of ras and von Hippel–Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res. 61, 7349–7355 (2001).
Wiesener, M. S. et al. Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1alpha in clear cell renal carcinomas. Cancer Res. 61, 5215–5222 (2001).
Ivanyi, P. et al. Does the onset of bone metastasis in sunitinib-treated renal cell carcinoma patients impact the overall survival? World J. Urol. 34, 909–915 (2015).
Kalra, S. et al. Outcomes of patients with metastatic renal cell carcinoma and bone metastases in the targeted therapy era. Clin. Genitourin. Cancer 15, 363–370 (2017).
Osanto, S. & van der Hulle, T. Cabozantinib in the treatment of advanced renal cell carcinoma in adults following prior vascular endothelial growth factor targeted therapy: clinical trial evidence and experience. Ther. Adv. Urol. 10, 109–123 (2018).
Vanharanta, S. et al. Epigenetic expansion of VHL–HIF signal output drives multiorgan metastasis in renal cancer. Nat. Med. 19, 50–56 (2013).
Atkinson, E. G. & Delgado-Calle, J. The emerging role of osteocytes in cancer in bone. JBMR Plus 3, e10186 (2019).
An, G. et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood 128, 1590–1603 (2016).
Maroni, P. & Bendinelli, P. Bone, a secondary growth site of breast and prostate carcinomas: role of osteocytes. Cancers 12, 1812 (2020).
Zhou, M. et al. KLF10 inhibits cell growth by regulating PTTG1 in multiple myeloma under the regulation of microRNA-106b-5p. Int. J. Biol. Sci. 16, 2063–2071 (2020).
Delgado-Calle, J. et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 76, 1089–1100 (2016).
Terpos, E. et al. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann. Oncol. 23, 2681–2686 (2012).
Roussou, M. et al. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 23, 2177–2181 (2009).
Costa, F. et al. Expression of CD38 in myeloma bone niche: a rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget 8, 56598–56611 (2017).
Chung, C. Role of immunotherapy in targeting the bone marrow microenvironment in multiple myeloma: an evolving therapeutic strategy. Pharmacotherapy 37, 129–143 (2017).
Favaloro, J. et al. Myeloma skews regulatory T and pro-inflammatory T helper 17 cell balance in favor of a suppressive state. Leuk. Lymphoma 55, 1090–1098 (2014).
Pratt, G., Goodyear, O. & Moss, P. Immunodeficiency and immunotherapy in multiple myeloma. Br. J. Haematol. 138, 563–579 (2007).
Gagelmann, N. et al. Development of CAR-T cell therapies for multiple myeloma. Leukemia 34, 2317–2332 (2020).
Mikhael, J. et al. Treatment of multiple myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol. 37, 1228–1263 (2019).
D’Oronzo, S., Coleman, R., Brown, J. & Silvestris, F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J. Bone Oncol. 15, 004 (2019).
Henry, D. H. et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 29, 1125–1132 (2011).
Lipton, A. et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur. J. Cancer 53, 75–83 (2016).
Scagliotti, G. V. et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J. Thorac. Oncol. 7, 1823–1829 (2012).
Body, J. J. et al. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 111, 306–312 (2004).
De Marinis, F. et al. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. J. Thorac. Oncol. 4, 1280–1288 (2009).
Dearnaley, D. P., Mason, M. D., Parmar, M. K., Sanders, K. & Sydes, M. R. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 10, 872–876 (2009).
McKay, R. R. et al. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur. Urol. 66, 502–509 (2014).
Cronin, K. A., Ries, L. A. & Edwards, B. K. The surveillance, epidemiology, and end results (SEER) program of the national cancer institute. Cancer 120 (Suppl. 23), 3755–3757 (2014).
Noone, A. M. et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol. Biomarkers Prev. 26, 632–641 (2017).
Woodward, E. et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 48, 160–166 (2011).
Kinnane, N. Burden of bone disease. Eur. J. Oncol. Nurs. 11 (Suppl. 2), S28–S31 (2007).
Mackiewicz-Wysocka, M., Pankowska, M. & Wysocki, P. J. Progress in the treatment of bone metastases in cancer patients. Expert. Opin. Investig. Drugs 21, 785–795 (2012).
Sato, M. et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Invest. 88, 2095–2105 (1991).
Hughes, D. E. et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Min. Res. 10, 1478–1487 (1995).
Lacey, D. L. et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 11, 401–419 (2012).
Ghajar, C. M. Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer 15, 238–247 (2015).
Risson, E., Nobre, A. R., Maguer-Satta, V. & Aguirre-Ghiso, J. A. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 1, 672–680 (2020).
Barbier, V. et al. Endothelial E-selectin inhibition improves acute myeloid leukaemia therapy by disrupting vascular niche-mediated chemoresistance. Nat. Commun. 11, 2042 (2020).
Wang, H. et al. Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat. Commun. 8, 15045 (2017).
Denefle, T. et al. Thrombospondin-1 mimetic agonist peptides induce selective death in tumor cells: design, synthesis, and structure-activity relationship studies. J. Med. Chem. 59, 8412–8421 (2016).
Allen, M. R. & Burr, D. B. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don’t know. Bone 49, 56–65 (2011).
Garnero, P., Sornay-Rendu, E., Chapuy, M. C. & Delmas, P. D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Min. Res. 11, 337–349 (1996).
Chen, Q. & Massague, J. Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clin. Cancer Res. 18, 5520–5525 (2012).
Nakazawa, Y. et al. Periostin blockade overcomes chemoresistance via restricting the expansion of mesenchymal tumor subpopulations in breast cancer. Sci. Rep. 8, 4013 (2018).
Estey, E. et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J. Clin. Oncol. 12, 671–678 (1994).
Fischer, J. C. et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc. Natl Acad. Sci. USA 110, 16580–16585 (2013).
Boyerinas, B. et al. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 121, 4821–4831 (2013).
Becker, P. S. et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br. J. Haematol. 155, 182–189 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03292536?term=NCT03292536&draw=2&rank=1 (2021).
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Furuyama, N. & Fujisawa, Y. Regulation of collagenolytic protease secretion through c-Src in osteoclasts. Biochem. Biophys. Res. Commun. 272, 116–124 (2000).
Murali, B. et al. Inhibition of the stromal p38MAPK/MK2 pathway limits breast cancer metastases and chemotherapy-induced bone loss. Cancer Res. 78, 5618–5630 (2018).
Nyman, J. S. et al. Combined treatment with a transforming growth factor beta inhibitor (1D11) and bortezomib improves bone architecture in a mouse model of myeloma-induced bone disease. Bone 91, 81–91 (2016).
Cole, L. E., Vargo-Gogola, T. & Roeder, R. K. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv. Drug Deliv. Rev. 99, 12–27 (2016).
Farrell, K. B., Karpeisky, A., Thamm, D. H. & Zinnen, S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 9, 47–60 (2018).
Tian, Z. et al. Harnessing the power of antibodies to fight bone metastasis. Sci. Adv. 7, eabf2051 (2021).
Schmid, P. et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Beer, T. M. et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 35, 40–47 (2017).
Jiao, S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190 e1113 (2019).
Italiano, A. et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 19, 649–659 (2018).
Yoneda, T., Sasaki, A. & Mundy, G. R. Osteolytic bone metastasis in breast cancer. Breast Cancer Res. Treat. 32, 73–84 (1994).
Corey, E. et al. Establishment and characterization of osseous prostate cancer models: intra-tibial injection of human prostate cancer cells. Prostate 52, 20–33 (2002).
Yu, C. et al. Intra-iliac artery injection for efficient and selective modeling of microscopic bone metastasis. J. Vis. Exp. https://doi.org/10.3791/53982 (2016).
Kuchimaru, T. et al. A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries. Nat. Commun. 9, 2981 (2018).
Medina, D. The mammary gland: a unique organ for the study of development and tumorigenesis. J. Mammary Gland Biol. Neoplasia 1, 5–19 (1996).
Sflomos, G. et al. A preclinical model for ERalpha-positive breast cancer points to the epithelial microenvironment as determinant of luminal phenotype and hormone response. Cancer Cell 29, 407–422 (2016).
Sato, N. et al. A metastatic and androgen-sensitive human prostate cancer model using intraprostatic inoculation of LNCaP cells in SCID mice. Cancer Res. 57, 1584–1589 (1997).
Valta, M. P. et al. Development of a realistic in vivo bone metastasis model of human renal cell carcinoma. Clin. Exp. Metastasis 31, 573–584 (2014).
Kashtan, H. et al. Intra-rectal injection of tumour cells: a novel animal model of rectal cancer. Surg. Oncol. 1, 251–256 (1992).
Sakamoto, S. et al. New metastatic model of human small-cell lung cancer by orthotopic transplantation in mice. Cancer Sci. 106, 367–374 (2015).
Martin, C. K. et al. Zoledronic acid reduces bone loss and tumor growth in an orthotopic xenograft model of osteolytic oral squamous cell carcinoma. Cancer Res. 70, 8607–8616 (2010).
Pinho, S. & Frenette, P. S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20, 303–320 (2019).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Mendez-Ferrer, S. et al. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 20, 285–298 (2020).
Sivaraj, K. K. & Adams, R. H. Blood vessel formation and function in bone. Development 143, 2706–2715 (2016).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Park, D. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 (2012).
Logan, M. et al. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77–80 (2002).
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Pinho, S. et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 210, 1351–1367 (2013).
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Acar, M. et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 (2015).
Bruns, I. et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 20, 1315–1320 (2014).
Heazlewood, S. Y. et al. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. 11, 782–792 (2013).
Olson, T. S. et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, 5238–5249 (2013).
Zhao, M. et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 20, 1321–1326 (2014).
Quarta, G., Cattaneo, C. & D’Elia, M. Determining 14C content in different human tissues: implications for application of 14C bomb-spike dating in forensic medicine. Radiocarbon 55, 1845–1849 (2013).
Rodan, G. A. Bone homeostasis. Proc. Natl Acad. Sci. USA 95, 13361–13362 (1998).
Ribot, C. et al. Obesity and postmenopausal bone loss: the influence of obesity on vertebral density and bone turnover in postmenopausal women. Bone 8, 327–331 (1987).
Compston, J. E. et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 124, 1043–1050 (2011).
Krakauer, J. C. et al. Bone loss and bone turnover in diabetes. Diabetes 44, 775–782 (1995).
Einhorn, T. A. & Gerstenfeld, L. C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11, 45–54 (2015).
Schell, H. et al. The haematoma and its role in bone healing. J. Exp. Orthop. 4, 5 (2017).
Bidwell, B. N. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Dobrolecki, L. E. et al. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev. 35, 547–573 (2016).
Hoffman, R. M. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer 15, 451–452 (2015).
Wang, D. et al. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Min. Res. 14, 893–903 (1999).
Qiao, H. & Tang, T. Engineering 3D approaches to model the dynamic microenvironments of cancer bone metastasis. Bone Res. 6, 3 (2018).
Acknowledgements
The authors thank H. Chan and L. Michie for editing the manuscript. X.H.-F.Z. is supported by US Department of Defense DAMD W81XWH-16-1-0073, DAMD W81XWH-20-1-0375, NCI R01CA183878, NCI R01CA251950, NCI R01CA221946, NCI R01CA227904, NCI U01CA252553, Breast Cancer Research Foundation and McNair Foundation. R.L.S. is supported by US DOD KCRP W81XWH-20-1-0895, and UT MD Anderson Cancer Center Knowledge Gap Award and Institutional Research Grant.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Osteoblasts
-
Cells that are responsible for synthesis and mineralization of new bones during development and bone remodelling. They are derived from mesenchymal linage, usually localize at the surface of bone matrix and can differentiate into osteocytes.
- Osteocytes
-
Cells that are derived from osteoblasts and become embedded in the bone matrix.
- Osteoclasts
-
Cells that are responsible for resorption of bones. They are derived from myeloid cell lineage. Matured osteoclasts are multinuclear and function in close coordination with osteoblasts.
- Mesenchymal stem cells
-
(MSCs). Cells that are multipotent and responsible for the production of mesenchymal cells, including osteoblasts, chondrocytes, adipocytes and fibroblasts.
- Haematopoietic stem cells
-
(HSCs). Cells that are multipotent and responsible for production of all blood cells.
- Type H capillaries
-
Vascular networks in the bone marrow that continue from arterioles and precede sinusoid vein vessels. They are surrounded by osteoprogenitor cells and couple osteogenesis and angiogenesis.
- Type L capillaries
-
Sinusoid veins that continue from the H-type capillaries and converge on central vein in the medullar cavity of bone marrow.
- Premetastatic niche
-
Potential destination of metastasis in distant organs before the actual arrival of metastatic cells. It is different from normal tissue because of interactions with bone marrow-derived cells stimulated by primary tumours.
- Endosteal niche
-
The microenvironmental location at the endosteal surface. It is enriched with osteoblasts and osteoprogenitors as well as osteoclasts. Transplanted haematopoietic stem cells often adhere to this niche.
- Epithelial-to-mesenchymal transition
-
(EMT). A process through which epithelial cells lose cell–cell adhesions and other epithelial traits but acquire mesenchymal characteristics, including migration and invasion. Recent studies demonstrate that EMT is a continuum and there exists a hybrid status with both epithelial and mesenchymal features. The hybrid EMT phenotype has been linked to cancer stemness, or the ability to regenerate a tumour.
- Metastatic organotropism
-
The observations that metastasis does not occur randomly to all organs but rather preferentially affects a specific set of distant organs.
- Darwinian selection
-
A process of evolution whereby individuals with greatest fitness among a population survive the selective pressure exerted by the environment. In cancer biology, it was adopted to understand how cancer cells with the most enabling genetic traits progress and expand over other cancer cells under the selective pressure from the microenvironment.
- Perivascular niche
-
The microenvironmental location adjacent to a blood vessel. The components include endothelial cells, pericytes and haematopoietic stem cells. The pericytes exhibit mesenchymal stem cell activities.
- Osteogenic niche
-
The microenvironmental locations including endosteum and trabecular bones, where osteogenesis occurs. It is enriched with osteoblasts and precursor cells. It overlaps with the endosteal niche, but also includes trabecular bones while lacking the osteoclast component by definition.
- Phenotypic plasticity
-
The potential of a cell to alter its phenotypic characteristics in response to environmental stimuli. The ability to switch between epithelial and mesenchymal phenotypes is considered one example of phenotypic plasticity.
- Osteogenesis
-
The process of osteoblast and osteocyte differentiation and formation of new bones.
- Cabozantinib
-
A small-molecule inhibitor of the tyrosine kinases Met, VEGFR2, AXL and RET. It is approved to treat medullary thyroid cancer, renal cell carcinoma and hepatocellular carcinoma.
- Embolization
-
Blockade of blood vessels by an agglomeration of cancer cells or other substance.
- Periostin
-
An extracellular matrix component encoded by the POSTN gene. It is a ligand of integrins and has important roles in the niche supporting normal and cancer stem cells.
- Endothelial tip cells
-
Endothelial cells that sprout branches of blood vessels.
Rights and permissions
About this article
Cite this article
Satcher, R.L., Zhang, X.HF. Evolving cancer–niche interactions and therapeutic targets during bone metastasis. Nat Rev Cancer 22, 85–101 (2022). https://doi.org/10.1038/s41568-021-00406-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00406-5
This article is cited by
-
Cell-cell communication characteristics in breast cancer metastasis
Cell Communication and Signaling (2024)
-
MiR26a reverses enzalutamide resistance in a bone-tumor targeted system with an enhanced effect on bone metastatic CRPC
Journal of Nanobiotechnology (2024)
-
Targeting initial tumour–osteoclast spatiotemporal interaction to prevent bone metastasis
Nature Nanotechnology (2024)
-
Predictive and prognostic biomarkers of bone metastasis in breast cancer: current status and future directions
Cell & Bioscience (2023)
-
Bone serves as a transfer station for secondary dissemination of breast cancer
Bone Research (2023)