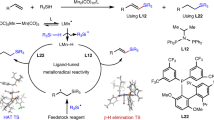
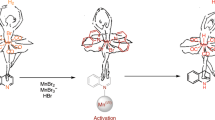
Abstract
The addition of X3Si–H or X2B–H (X = H, OR or R) across a C–C multiple bond is a well-established method for incorporating silane or borane groups, respectively, into hydrocarbon feedstocks. These hydrofunctionalization reactions are often mediated by transition metal catalysts, with precious metals being the most commonly used owing to the ability to optimize reaction scope, rates and selectivities. For example, platinum catalysts effect the hydrosilylation of alkenes with anti-Markovnikov selectivity and constitute an enabling technology in the multibillion dollar silicones industry. Increased emphasis on sustainable catalytic methods and on more economic processes has shifted the focus to catalysis with more earth-abundant transition metals, such as iron, cobalt and nickel. This Review describes the use of first-row transition metal complexes in catalytic alkene hydrosilylation and hydroboration. Defining advances in the field are covered, noting the chemistry that is unique to first-row transition metals and the design features that enable them to exhibit precious-metal-like reactivity. Other important features, such as catalyst activity and stability, are covered, as are practical considerations, such as cost and safety.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Principe, L. M. The Secrets of Alchemy (Univ. of Chicago Press, 2013).
U.S. Energy Information Administration. International Energy Outlook 2017. U.S. Energy Information Administration https://www.eia.gov/outlooks/ieo/pdf/0484(2017).pdf (2017).
Johnson, J. Global energy markets in turmoil, International Energy Agency says. Chem. Eng. News 95, 15 (2017).
Marciniec, B. Catalysis by transition metal complexes of alkene silylation — recent progress and mechanistic implications. Coord. Chem. Rev. 249, 2374–2390 (2005).
Marciniec, B., Maciejewski, H., Pietraszok, C. & Pawluc, P. Advances in Silicone Science Vol. 1 (Springer, 2009).
Nakajima, Y. & Shimada, S. Hydrosilylation reactions of olefins: recent advances and perspectives. RSC Adv. 5, 20603–20616 (2015).
Vogels, C. M. & Westcott, S. A. Recent advances in organic synthesis using transition metal-catalyzed hydroborations. Curr. Org. Chem. 9, 687–699 (2005).
Burgess, K. & Ohlmeyer, M. J. Transition-metal promoted hydroboration of alkenes, emerging methodology for organic transformations. Chem. Rev. 91, 1179–1191 (1991).
Komiyama, T., Minami, Y. & Hiyama, T. Recent advances in transition-metal-catalyzed synthetic transformations of organosilicon reagents. ACS Catal. 7, 631–651 (2017).
Pukhnarevitch, V. B., Lukevics, E., Kopylova, L. I. & Voronkov, M. Perspectives of Hydrosilylation (Institute for Organic Synthesis, Riga, Latvia, 1992).
Herzig, C. Siloxane copolymers containing alkenyl groups. US Patent 6265497 B1 (2001).
Friedman, G., Sperry, P. & Brossas, J. Oxygen-permeable transparent polymer compositions for contact lenses of the rigid type. US Patent 5166298 A (1992).
Lewis, L. N., Stein, J., Gao, Y., Colborn, R. E. & Hutchins, G. Platinum catalysts used in the silicones industry. Platin. Met. Rev. 41, 66–75 (1997).
Momentive Performance Materials. Silquest* A-137 Technical Data Sheet. HCD-10164. Momentive https://www.momentive.com/products/show-technical-datasheet.aspx?id=10164 (2011).
Troegel, D. & Stohrer, J. Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view. Coord. Chem. Rev. 255, 1440–1459 (2011). This review describes how the attributes desired in a hydrosilylation catalyst depend on the type of commercial hydrosilylation product being produced.
Speier, J. L., Webster, J. A. & Barnes, G. H. The addition of silicon hydrides to olefinic double bonds. Part II. The use of group viii metal catalysts. J. Am. Chem. Soc. 79, 974–979 (1957).
Lewis, L. N. & Lewis, N. Platinum-catalyzed hydrosilylation — colloid formation as the essential step. J. Am. Chem. Soc. 108, 7228–7231 (1986).
Stein, J., Lewis, L. N., Gao, L. & Scott, R. A. In situ determination of the active catalyst in hydrosilylation reactions using highly reactive Pt(0) catalyst precursors. J. Am. Chem. Soc. 121, 3693–3703 (1999).
Markó, I. E. et al. Selective and efficient platinum(0)–carbene complexes as hydrosilylation catalysts. Science 298, 204–206 (2002). This study demonstrates that strongly coordinating carbene ligands on Pt suppress Pt nanoparticle formation, which typically results in the formation of unwanted by-products in alkene hydrosilylation.
Berthon-Gelloz, G. et al. Expedient, direct synthesis of (L)Pt(0)(1,6-diene) complexes from H2PtCl6. Organometallics 26, 5731–5734 (2007).
Bai, H. In situ platinum recovery and color removal from organosilicon streams. Ind. Eng. Chem. Res. 51, 16457–16466 (2012).
Holwell, A. J. Optimised technologies are emerging which reduce platinum usage in silicone curing. Platin. Met. Rev. 52, 243–246 (2008).
Chirik, P. J. & Weighardt, K. Radical ligands confer nobility on base-metal catalysts. Science 327, 794–795 (2010).
Chirik, P. J. Iron- and cobalt-catalyzed alkene hydrogenation: catalysis with both redox-active and strong field ligands. Acc. Chem. Res. 48, 1687–1695 (2015).
Fürstner, A. Iron catalysis in organic synthesis: a critical assessment of what it takes to make this base metal a multitasking champion. ACS Cent. Sci. 2, 778–789 (2016).
Sun, J. & Deng, L. Cobalt complex-catalyzed hydrosilylation of alkenes and alkynes. ACS Catal. 6, 290–300 (2016).
Du, X. & Huang, Z. Advances in base-metal-catalyzed alkene hydrosilylation. ACS Catal. 7, 1227–1243 (2017).
Nesmeyanov, A. N., Freidlina, Kh,R., Chukovskaya, E. C., Petrova, R. G. & Belyavsky, A. B. Addition, substitution, and telomerization reactions of olefins in the presence of metal carbonyls or colloidal iron. Tetrahedron 17, 61–68 (1962).
Schroeder, M. A. & Wrighton, M. S. Pentacarbonyliron(0) photocatalyzed reactions of trialkylsilanes with alkenes. J. Organomet. Chem. 128, 345–358 (1977).
Mitchener, J. C. & Wrighton, M. S. Photogeneration of very active homogeneous catalysts using laser light excitation of iron carbonyl precursors. J. Am. Chem. Soc. 103, 975–977 (1981).
Whetten, R. L., Fu, K. J. & Grant, E. R. Pulsed-laser photocatalytic isomerization and hydrogenation of olefins. J. Am. Chem. Soc. 104, 4270–4272 (1982).
Small, B. L., Brookhart, M. & Bennett, A. M. A. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 120, 4049–4050 (1998).
Chirik, P. J. Preface: forum on redox-active ligands. Inorg. Chem. 50, 9737–9740 (2011).
Gibson, V. C., Redshaw, C. & Solan, G. A. Bis(imino)pyridines: surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 107, 1745–1776 (2007).
Darmon, J. M., Turner, Z. R., Lobkovsky, E. & Chirik, P. J. Electronic effects in 4-substituted bis(iminopyridines) and the corresponding reduced iron compounds. Organometallics 31, 2275–2285 (2012).
Bart, S. C., Lobkovsky, E. & Chirik, P. J. Preparation and molecular and electronic structures of iron(0) dinitrogen and silane complexes and their application to catalytic hydrogenation and hydrosilylation. J. Am. Chem. Soc. 126, 13794–13807 (2004). This work reports the synthesis of a well-defined Fe dinitrogen complex and provides a proof-of-principle that Fe, when in the appropriate coordination geometry and spin state, can be highly active, similar to precious metals, in a variety of catalytic reactions.
Archer, A. M., Bouwkamp, M. W., Cortez, M., Lobkovsky, E. & Chirik, P. J. Arene coordination in bis(imino)pyridine iron complexes: identification of catalyst deactivation pathways in iron-catalyzed hydrogenation and hydrosilation. Organometallics 25, 4269–4278 (2006).
Meciejewski, H., Marciniec, B. & Kownacki, I. Catalysis of hydrosilylation part xxxiv. High catalytic efficiency of the nickel equivalent of Karstedt catalyst [{Ni(η-CH2 = CHSiMe2)2O}2 {μ-CH2 = CHSiMe2)2O}]. J. Organomet. Chem. 597, 175–181 (2000).
LaPointe, A. M., Rix, F. C. & Brookhart, M. Mechanistic studies of palladium(II)-catalyzed hydrosilation and dehydrogenative silation reactions. J. Am. Chem. Soc. 119, 906–917 (1997).
Russell, S. K., Darmon, J. M., Lobkovsky, E. & Chirik, P. J. Synthesis of aryl-substituted bis(imino)pyridine iron dinitrogen complexes. Inorg. Chem. 49, 2782–2792 (2010).
Tondreau, A. M. et al. Iron catalysts for selective anti-Markovnikov alkene hydrosilylation using tertiary silanes. Science 335, 567–570 (2012). This work reports that well-defined Fe precatalysts effect the anti-Markovnikov hydrosilylation of commercially relevant substrates, with selectivities that exceed those of state-of-the-art Pt catalysts.
Plueddemann, E. P. Silane Coupling Agents 2nd edn (Plenum Press, New York, 1991).
Petrea, R. D. & Schuette, R. L. Finish for textile fibers containing silahydrocarbon lubricants and nonionic emulsifiers having a plurality of hydrocarbon chains. US Patent 5288416A (1994).
Plonsker, L. Textile lubrication. US Patent 4932976 A (1990).
Sprengers, J. W., de Greef, M., Duin, M. A. & Elsevier, C. J. Stable platinum(0) catalysts for catalytic hydrosilylation of styrene and synthesis of [Pt(Ar-bian)(η 2-alkene)] complexes. Eur. J. Inorg. Chem. 2003, 3811–3819 (2003).
Momentive Performance Materials. SilForce SL6000 Technical Data Sheet. HCD-10896. Momentive https://www.momentive.com/products/show-technical-datasheet.aspx?id=10896 (2016).
Atienza, C. C. H. et al. High selectivity bis(imino)pyridine iron catalysts for the hydrosilylation of 1,2,4-trivinylcyclohexane. ACS Catal. 2, 2169–2172 (2012).
Tondreau, A. M. et al. Synthesis, electronic structure, and alkene hydrosilylation activity of terpyridine and bis(imino)pyridine iron dialkyl complexes. Organometallics 31, 4886–4893 (2012).
Momentive Performance Materials. CoatOSil* 1770 Technical Data Sheet. HCD-10012. Momentive https://www.momentive.com/en-us/products/tds/coatosil-1770-silane/ (2016).
Toya, Y., Hayasaka, K. & Nakazawa, H. Hydrosilylation of olefins catalyzed by iron complexes bearing ketimine-type iminobipyridine ligands. Organometallics 36, 1727–1735 (2017).
Bouwkamp, M. W., Bowman, A. C., Lobkovsky, E. & Chirik, P. J. Iron-catalyzed [2π + 2π] cycloaddition of α, ω-dienes: the importance of redox-active supporting ligands. J. Am. Chem. Soc. 128, 13340–13341 (2006).
Chirik, P. J. et al. In-situ activation of metal complexes containing terdentate nitrogen ligands used as hydrosilylation catalysts. US Patent 8765987 B2 (2010).
Ryan, J. W. Redistribution and reduction reactions of alkoxysilanes. J. Am. Chem. Soc. 84, 4730–4734 (1962).
Buchwald, S. L. Silane disproportionation results in spontaneous ignition. Chem. Eng. News 71, 2 (1993).
Berk, S. C. & Buchwald, S. L. An air-stable catalyst system for the conversion of esters to alcohols. J. Org. Chem. 57, 3751–3753 (1992).
Buslov, I., Keller, S. C. & Hu, X. Alkoxy hydrosilanes as surrogates of gaseous silanes for hydrosilation of alkenes. Org. Lett. 18, 1928–1931 (2016).
Greenhalgh, M. D., Frank, D. J. & Thomas, S. P. Iron-catalyzed chemo-, regio-, and stereoselective hydrosilylation of alkenes and alkynes using a bench-stable iron(II) pre-catalyst. Adv. Synth. Catal. 356, 584–590 (2014).
Brandstadt, K. et al. Nickel containing hydrosilylation catalysts and compositions containing the catalysts. US Patent 9545624 B2 (2011).
Docherty, J. H., Peng, J., Dominey, A. P. & Thomas, S. P. Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide. Nat. Chem. 9, 595–600 (2017).
Gibson, V. C., Tellman, K. P., Humphries, M. J. & Wass, D. F. Bis(imino)pyridine cobalt alkyl complexes and their reactivity towards ethylene: a model system for β-hydrogen chain transfer. Chem. Commun. 2316–2317 (2002).
Friedfeld, M. R. et al. Cobalt precursors for high-throughput discovery of base metal asymmetric alkene hydrogenation catalysts. Science 342, 1076–1080 (2013).
Atienza, C. C. H. et al. Bis(imino)pyridine cobalt-catalyzed dehydrogenative silylation of alkenes: scope, mechanism, and origins of selective allylsilane formation. J. Am. Chem. Soc. 136, 12108–12118 (2014).
McAtee, J. R., Martin, S. E. S., Ahneman, D. T., Johnson, K. A. & Watson, D. A. Preparation of allyl and vinyl silanes by the palladium-catalyzed silylation of terminal olefins: a silyl-Heck reaction. Angew. Chem. Int. Ed. 51, 3663–3667 (2012).
Brookhart, M. & Grant, B. E. Mechanism of a cobalt(III)-catalyzed olefin hydrosilation reaction: direct evidence for a silyl migration pathway. J. Am. Chem. Soc. 115, 2151–2156 (1993).
Schuster, C. H., Diao, T., Pappas, I. & Chirik, P. J. Bench-stable, substrate-activated cobalt carboxylate pre-catalysts for alkene hydrosilylation with tertiary silanes. ACS Catal. 6, 2632–2636 (2016). This paper describes air-stable Co carboxylates that enable the efficient hydrosilylation of commercially relevant substrates without the need for external activators and also describes the catalyst design features to enable hydrosilylation over dehydrogenative silylation with Co.
Chen, C. et al. Rapid, regioconvergent, solvent-free alkene hydrosilylation with a cobalt catalyst. J. Am. Chem. Soc. 137, 13244–13247 (2015).
Ibrahim, A. D., Entsminger, S. W., Zhu, L. & Fout, A. R. A highly chemoselective cobalt catalyst for the hydrosilylation of alkenes using tertiary silanes and hydrosiloxanes. ACS Catal. 6, 3589–3593 (2016).
Scheuermann, M. L., Johnson, E. J. & Chirik, P. J. Alkene isomerization–hydroboration promoted by phosphine–ligated cobalt catalysts. Org. Lett. 17, 2716–2719 (2015).
Noda, D., Tahara, A., Sunada, Y. & Nagashima, H. Non-precious-metal catalytic systems involving iron or cobalt carboxylates and alkyl isocyanides for hydrosilylation of alkenes with hydrosiloxanes. J. Am. Chem. Soc. 138, 2480–2483 (2016).
Momentive Performance Materials. Silquest* A-1871 Technical Data Sheet. HCD-10053. Momentive https://www.momentive.com/en-us/products/tds/silquest-a-1871/ (2011).
Marciniec, B., Kownacka, A., Kownacki, I., Hoffmann, M. & Taylor, R. Hydrosilylation versus dehydrogenative silylation of styrene catalyzed by iron(0) carbonyl complexes with multivinylsilicon ligands — mechanistic implications. J. Organomet. Chem. 791, 58–65 (2015).
Sunada, Y., Soejima, H. & Nagashima, H. Disilaferracycle dicarbonyl complex containing weakly coordinated η2-(H–Si) ligands: application to C–H functionalization of indoles and arenes. Organometallics 33, 5936–5939 (2014).
Sunada, Y. et al. Catalyst design for iron-promoted reductions: an iron–disilyl–dicarbonyl complex bearing weakly coordinating η2-(H–Si) moieties. Dalton Trans. 42, 16687–16692 (2013).
Raya, B., Jing, S., Balasanthiran, V. & RajanBabu, T. V. Control of selectivity through synergy between catalysts, silanes, and reaction conditions in cobalt-catalyzed hydrosilylation of dienes and terminal alkenes. ACS Catal. 7, 2275–2283 (2017).
Liu, Y. & Deng, L. Mode of activation of cobalt(II) amides for catalytic hydrosilylation of alkenes with tertiary silanes. J. Am. Chem. Soc. 139, 1798–1801 (2017).
Buslov, I., Becouse, J., Mazza, S., Montandon-Clerc, M. & Hu, X. Chemoselective alkene hydrosilylation catalyzed by nickel pincer complexes. Angew. Chem. Int. Ed. 54, 14523–14526 (2015).
Pappas, I., Treacy, S. & Chirik, P. J. Alkene hydrosilylation using tertiary silanes with α-diimine nickel catalysts. Redox-active ligands promote a distinct mechanistic pathway from platinum catalysts. ACS Catal. 6, 4105–4109 (2016).
Dong, Q. et al. Synthesis and reactivity of nickel hydride complexes of an α-diimine ligand. Inorg. Chem. 51, 13162–13170 (2012).
Roy, A. K. & Taylor, R. B. The first alkene–platinum–silyl complexes: lifting the hydrosilylation mechanism shroud with long-lived precatalytic intermediates and true Pt catalysts. J. Am. Chem. Soc. 124, 9510–9524 (2002).
Meister, T. K. et al. Platinum catalysis revisited — unraveling principles of catalytic olefin hydrosilylation. ACS Catal. 6, 1274–1284 (2016).
Roy, A. et al. Platinum catalyzed hydrosilylation reactions utilizing cyclodiene additives. US Patent Application WO2015192029 A (2014).
Kiso, Y., Kumada, M., Tamao, K. & Umeno, M. Silicon hydrides and nickel complexes: I. Phosphine–nickel(II) complexes as hydrosilylation catalysts. J. Organomet. Chem. 50, 297–310 (1973).
Lappert, M. F., Nile, T. A. & Takahashi, S. Homogeneous catalysis: II. Ziegler systems as catalysts for hydrosilylation. J. Organomet. Chem. 72, 425–439 (1974).
Srinivas, V., Nakajima, Y., Ando, W., Sato, K. & Shimada, S. Bis(acetylacetonato)Ni(II)/NaHBEt3-catalyzed hydrosilylation of 1,3-dienes, alkenes and alkynes. J. Organomet. Chem. 809, 57–62 (2016).
Buslov, I., Song, F. & Hu, X. An easily accessed nickel nanoparticle catalyst for alkene hydrosilylation with tertiary silanes. Angew. Chem. Int. Ed. 55, 12295–12299 (2016).
Yu, R. P., Hesk, D., Rivera, N., Pelczer, I. & Chirik, P. J. Iron-catalyzed tritiation of pharmaceuticals. Nature 529, 195–199 (2016).
Obligacion, J. V., Semproni, S. P. & Chirik, P. J. Cobalt-catalyzed C–H borylation. J. Am. Chem. Soc. 136, 4133–4136 (2014).
Obligacion, J. V., Bezdek, M. J. & Chirik, P. J. C(sp 2)–H borylation of fluorinated arenes using an air-stable cobalt precatalyst: electronically enhanced site selectivity enables synthetic opportunities. J. Am. Chem. Soc. 139, 2825–2832 (2017).
Darmon, J. M. et al. Oxidative addition of carbon–carbon bonds with a redox-active bis(imino)pyridine iron complex. J. Am. Chem. Soc. 134, 17125–17137 (2012).
Brown, H. C. & Subba Rao, B. C. A new powerful reducing agent — sodium borohydride in the presence of aluminum chloride and other polyvalent metal halides. J. Am. Chem. Soc. 78, 2582–2588 (1956).
Carroll, A.-M., O’Sullivan, T. P. & Guiry, P. J. The development of enantioselective rhodium-catalyzed hydroboration of olefins. Adv. Synth. Catal. 347, 609–631 (2005).
Evans, D. A., Ratz, A. M., Huff, B. E. & Sheppard, G. S. Total synthesis of the polyether antibiotic lonomycin A (emericid). J. Am. Chem. Soc. 117, 3448–3467 (1995).
Volpicelli, R., Maragni, P., Cotarca, L., Foletto, J. & Massaccesi, F. Process for preparing nebivolol. US Patent 8258323 B2 (2012).
Beletskaya, I. & Pelter, A. Hydroborations catalyzed by transition metal complexes. Tetrahedron 53, 4957–5026 (1997).
Evans, D. A., Fu, G. C. & Hoveyda, A. H. Rhodium(I)- and iridium(I)-catalyzed hydroboration reactions: scope and synthetic applications. J. Am. Chem. Soc. 114, 6671–6679 (1992).
Männig, D. & Nöth, H. Catalytic hydroboration with rhodium complexes. Angew. Chem. Int. Ed. 24, 878–879 (1985).
Yamamoto, Y., Fujikawa, R., Umemoto, T. & Miyaura, N. Iridium-catalyzed hydroboration of alkenes with pinacolborane. Tetrahedron 60, 10695–10700 (2004).
Burgess, K. et al. Reactions of catecholborane with Wilkinson’s catalyst: implications for transition metal-catalyzed hydroborations of alkenes. J. Am. Chem. Soc. 114, 9350–9359 (1992).
Hayashi, T., Matsumoto, Y. & Ito, Y. Catalytic asymmetric hydroboration of styrenes. J. Am. Chem. Soc. 111, 3426–3428 (1989).
Evans, D. A., Fu, G. C. & Anderson, B. A. Mechanistic study of the rhodium(I)-catalyzed hydroboration reaction. J. Am. Chem. Soc. 114, 6679–6685 (1992).
Pereira, S. & Srebnik, M. Transition metal-catalyzed hydroboration of and CCl4 addition to alkenes. J. Am. Chem. Soc. 118, 909–910 (1996).
Lata, C. J. & Crudden, C. M. Dramatic effect of Lewis acids on the rhodium-catalyzed hydroboration of olefins. J. Am. Chem. Soc. 132, 131–137 (2010).
Pereira, S. & Srebnik, M. A study of hydroboration of alkenes and alkynes with pinacolborane catalyzed by transition metals. Tetrahedron Lett. 37, 3283–3286 (1996).
Hadebe, S. W. & Robinson, R. S. Microwave mediated rhodium-catalysed hydroboration of trans-4-octene with pinacolborane. Tetrahedron Lett. 47, 1299–1302 (2006).
Wu, J. Y., Moreau, B. & Ritter, T. Iron-catalyzed 1,4-hydroboration of 1,3-dienes. J. Am. Chem. Soc. 131, 12915–12917 (2009). This is a pioneering report demonstrating that C–B bond formation reactions are possible with reduced Fe complexes.
Monfette, S., Turner, Z. R., Semproni, S. P. & Chirik, P. J. Enantiopure C 1-symmetric bis(imino)pyridine cobalt complexes for asymmetric alkene hydrogenation. J. Am. Chem. Soc. 134, 4561–4564 (2012).
Zhang, L., Peng, D., Leng, X. & Huang, Z. Iron-catalyzed, atom-economical, chemo- and regioselective alkene hydroboration with pinacolborane. Angew. Chem. Int. Ed. 52, 3676–3680 (2013). This study includes the first example of a catalytic hydroboration of terminal alkenes with an Fe catalyst.
Balaraman, E., Gnanaprakasam, B., Shimon, L. J. W. & Milstein, D. Direct hydrogenation of amides to alcohols and amines under mild conditions. J. Am. Chem. Soc. 132, 16756–16758 (2010).
Zhang, L., Zuo, Z., Leng, X. & Huang, Z. A cobalt-catalyzed alkene hydroboration with pinacolborane. Angew. Chem. Int. Ed. 53, 2696–2700 (2014).
Obligacion, J. V. & Chirik, P. J. Highly selective bis(imino)pyridine iron-catalyzed alkene hydroboration. Org. Lett. 15, 2680–2683 (2013).
Ruddy, A. J., Sydora, O. L., Small, B. L., Stradiotto, M. & Turculet, L. (N-phosphinoamidate)cobalt-catalyzed hydroboration: alkene isomerization affords terminal selectivity. Chem. Eur. J. 20, 13918–13922 (2014).
Tseng, K.-N. T., Kampf, J. W. & Szymczak, N. K. Regulation of iron-catalyzed olefin hydroboration by ligand modifications at a remote site. ACS Catal. 5, 411–415 (2015).
Zheng, J., Sortais, J.-B. & Darcel, C. [(NHC)Fe(CO)4] efficient pre-catalyst for selective hydroboration of alkenes. ChemCatChem 6, 763–766 (2014).
Obligacion, J. V. & Chirik, P. J. Bis(imino)pyridine cobalt-catalyzed alkene isomerization–hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 135, 19107–19110 (2013). This report describes Co precatalysts that offer unprecedented activity and selectivity in catalytic alkene hydroboration through an isomerization–hydroboration sequence.
Weliange, N. M., McGuinness, D. S., Gardiner, M. G. & Patel, J. Cobalt-bis(imino)pyridine complexes as catalysts for hydroalumination–isomerization of internal olefins. Dalton Trans. 45, 10842–10849 (2016).
de Klerk, A. et al. Linear α-olefins from linear internal olefins by a boron-based continuous double-bond isomerization process. Ind. Eng. Chem. Res. 46, 400–410 (2007).
Palmer, W. N., Diao, T., Pappas, I. & Chirik, P. J. High-activity cobalt catalysts for alkene hydroboration with electronically responsive terpyridine and α-diimine ligands. ACS Catal. 5, 622–626 (2015).
Sacco, A. & Rossi, M. Hydride and nitrogen complexes of cobalt. Chem. Commun., 316 (1967).
Lee et al. Stereoselective hydroboration of diynes and triyne to give products containing multiple vinylene bridges: a versatile application to fluorescent dyes and light-emitting copolymers. Organometallics 23, 4659–4575 (2004).
Nielson, B. M. & Bielawski, C. W. Photoswitchable metal-mediated catalysis: remotely tuned alkene and alkyne hydroborations. Organometallics 32, 3121–3128 (2013).
Pereira, S. & Srebnik, M. Hydroboration of alkynes with pinacolborane catalyzed by HZrCp2Cl. Organometallics 14, 3127–3128 (1995).
He, X. & Hartwig, J. F. True metal-catalyzed hydroboration with titanium. J. Am. Chem. Soc. 118, 1696–1702 (1996).
Otsuka, S. & Nakamura, A. Acetylene and allene complexes: their implication in homogeneous catalysis. Adv. Organomet. Chem. 14, 245–283 (1976).
Hermann, W. A. & Prinz, M. in Applied Homogeneous Catalysis with Organometallic Compounds 2nd edn (eds Cornils, B. & Herrmann, W. A.) 1119–1124 (Wiley, 2002).
Ohmura, T., Yamamoto, Y. & Miyaura, N. Rhodium- or iridium-catalyzed trans-hydroboration of terminal alkynes giving (Z)-1-alkenylboron compounds. J. Am. Chem. Soc. 122, 4990–4991 (2000).
Gunanathan, C., Hölscher, M., Pan, F. & Leitner, W. Ruthenium catalyzed hydroboration of terminal alkynes to Z-vinylboronates. J. Am. Chem. Soc. 134, 14349–14352 (2012).
Bruneau, C. & Dixneuf, P. H. Metal vinylidenes and allenylidenes in catalysis: applications in anti-Markovnikov additions to terminal alkynes and alkene metathesis. Angew. Chem. Int. Ed. 45, 2176–2203 (2006).
Obligacion, J. V., Neely, J. M., Yazdani, A. N., Pappas, I. & Chirik, P. J. Cobalt catalyzed Z-selective hydroboration of terminal alkynes and elucidation of the origin of selectivity. J. Am. Chem. Soc. 137, 5855–5858 (2015). This work highlights that the steric and electronic modularity of [pyridine(diimine)]Co catalysts can switch the mode of precatalyst activation, which eventually leads to a switch in stereoselectivity in terminal alkyne hydroboration with pinacolborane.
Gorgas, N. et al. Stable, yet highly reactive nonclassical iron(II) polyhydride pincer complexes: Z-selective dimerization and hydroboration of terminal alkynes. J. Am. Chem. Soc. 139, 8130–8133 (2017).
Krautwald, S., Bezdek, M. J. & Chirik, P. J. Cobalt-catalyzed 1,1-diboration of terminal alkynes: scope, mechanism, and synthetic applications. J. Am. Chem. Soc. 139, 3868–3875 (2017).
Gilbert-Wilson, R., Chu, W.-Y. & Rauchfuss, T. B. Phosphine-iminopyridines as platforms for hydrofunctionalization of alkenes. Inorg. Chem. 54, 5596–5603 (2015).
Espinal-Viguri, M., Woof, C. R. & Webster, R. L. Iron-catalyzed hydroboration: unlocking reactivity through ligand modulation. Chem. Eur. J. 22, 11605–11608 (2016).
MacNair, A. J., Millet, C. R. P., Nichol, G. S., Ironmonger, A. & Thomas, S. P. Markovnikov-selective, activator-free iron-catalyzed vinylarene hydroboration. ACS Catal. 6, 7217–7221 (2016).
Reilly, S. W., Webster, C. E., Hollis, T. K. & Valle, H. U. Transmetallation from CCC-NHC pincer Zr complexes in the synthesis of air-stable CCC-NHC pincer Co(III) complexes and initial hydroboration trials. Dalton Trans. 45, 2823–2828 (2016).
Ibrahim, A. D., Entsminger, S. W. & Fout, A. R. Insights into a chemoselective cobalt catalyst for the hydroboration of alkenes and nitriles. ACS Catal. 7, 3730–3734 (2017).
Zhang, T., Manna, K. & Lin, W. Metal–organic frameworks stabilize solution-inaccessible cobalt catalysts for highly efficient broad-scope organic transformations. J. Am. Chem. Soc. 138, 3241–3249 (2016).
Touney, E. E. et al. Heteroleptic nickel complexes for the Markovnikov-selective hydroboration of styrenes. Organometallics 35, 3436–3439 (2016).
Zhang, G. et al. Highly selective hydroboration of alkenes, ketones and aldehydes catalyzed by a well-defined manganese complex. Angew. Chem. Int. Ed. 55, 14369–14372 (2016).
Rauch, M., Ruccolo, S. & Parkin, G. Synthesis, structure, and reactivity of a terminal magnesium hydride compound with a carbatrane motif, [TismPriBenz]MgH: a multifunctional catalyst for hydrosilylation and hydroboration. J. Am. Chem. Soc. 139, 13264–13267 (2017).
Zhang, L. & Huang, Z. Synthesis of 1,1,1-tris(boronates) from vinylarenes by Co-catalyzed dehydrogenative borylations–hydroboration. J. Am. Chem. Soc. 137, 15600–15603 (2015).
Zhang, L., Zuo, Z., Wan, X. & Huang, Z. Cobalt-catalyzed enantioselective hydroboration of 1,1-disubstituted aryl alkenes. J. Am. Chem. Soc. 136, 15501–15504 (2014).
Chen, J., Xi, T. & Lu, Z. Iminopyridine oxazoline iron catalyst for asymmetric hydroboration of 1,1-disubstituted aryl alkenes. Org. Lett. 16, 6452–6455 (2014).
Masamune, S. et al. Organoboron compounds in organic synthesis. 1. Asymmetric hydroboration. J. Am. Chem. Soc. 107, 4549–4551 (1985).
Mazet, C. & Gérard, D. Highly regio- and enantioselective catalytic asymmetric hydroboration of α-substituted styrenyl derivatives. Chem. Commun. 47, 298–300 (2011).
Bianchini, C. et al. Oligomerisation of ethylene to linear α-olefins by C S and C 1-symmetric [2,6-bis(imino)pyridyl]iron and -cobalt dichloride complexes. Eur. J. Inorg. Chem. 2003, 1620–1631 (2003).
Tondreau, A. M. et al. Enantiopure pyridine bis(oxazoline) “pybox” and bis(oxazoline) “box” iron dialkyl complexes: comparison to bis(imino)pyridine compounds and application to catalytic hydrosilylation of ketones. Organometallics 28, 3928–3940 (2009).
Wile, B. M. et al. Reduction chemistry of aryl- and alkyl-substituted bis(imino)pyridine iron dihalide compounds: molecular and electronic structures of [(PDI)2Fe] derivatives. Inorg. Chem. 48, 4190–4200 (2009).
Guo, J., Cheng, B., Shen, X. & Lu, Z. Cobalt-catalyzed asymmetric sequential hydroboration/ hydrogenation of internal alkynes. J. Am. Chem. Soc. 139, 15316–15319 (2017).
Yu, S., Wu, C. & Ge, S. Cobalt-catalyzed asymmetric hydroboration/cyclization of 1,6-enynes with pinacolborane. J. Am. Chem. Soc. 139, 6526–6529 (2017).
Jang, W. J., Song, S. M., Moon, J. H., Lee, J. Y. & Yun, J. Copper-catalyzed enantioselective hydroboration of unactivated 1,1-disubstituted alkenes. J. Am. Chem. Soc. 139, 13660–13663 (2017).
Kerchner, H. A. & Montgomery, J. Synthesis of secondary and tertiary alkylboranes via formal hydroboration of terminal and 1,1-disubstituted alkenes. Org. Lett. 18, 5760–5763 (2016).
Jang, H., Zhugralin, A. R., Lee, Y. & Hoveyda, A. H. Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC–Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. J. Am. Chem. Soc. 133, 7859–7871 (2011).
Corberán, R., Mszar, N. W. & Hoveyda, A. H. NHC-Cu-catalyzed enantioselective hydroboration of acyclic and exocyclic 1,1-disubstituted aryl alkenes. Angew. Chem. Int. Ed. 50, 7079–7082 (2011).
Smith, J. R. et al. Enantioselective rhodium(III)-catalyzed Markovnikov hydroboration of unactivated terminal alkenes. J. Am. Chem. Soc. 139, 9148–9151 (2017).
Acknowledgements
The authors thank Princeton University for financial support. J.V.O. acknowledges the Howard Hughes Medical Institute International Student Research Fellowship and the 2016 Harold W. Dodds Honourific Fellowship (awarded by the Graduate School at Princeton University).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obligacion, J.V., Chirik, P.J. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat Rev Chem 2, 15–34 (2018). https://doi.org/10.1038/s41570-018-0001-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0001-2
This article is cited by
-
Cobalt catalyzed practical hydroboration of terminal alkynes with time-dependent stereoselectivity
Nature Communications (2024)
-
Precise immobilization of metal single atoms into a porphyrinic metal-organic framework for an efficient alkene hydrosilylation
Nano Research (2024)
-
Regio- and enantioselective CuH-catalyzed 1,2- and 1,4-hydrosilylation of 1,3-enynes
Nature Communications (2023)
-
Dearomative triple elementalization of quinolines driven by visible light
Nature Communications (2023)
-
Catalytic asymmetric silicon-carbon bond-forming transformations based on Si-H functionalization
Science China Chemistry (2023)