Abstract
Remarkable progress has been made in the development of biomarker-driven targeted therapies for patients with multiple cancer types, including melanoma, breast and lung tumours, although precision oncology for patients with colorectal cancer (CRC) continues to lag behind. Nonetheless, the availability of patient-derived CRC models coupled with in vitro and in vivo pharmacological and functional analyses over the past decade has finally led to advances in the field. Gene-specific alterations are not the only determinants that can successfully direct the use of targeted therapy. Indeed, successful inhibition of BRAF or KRAS in metastatic CRCs driven by activating mutations in these genes requires combinations of drugs that inhibit the mutant protein while at the same time restraining adaptive resistance via CRC-specific EGFR-mediated feedback loops. The emerging paradigm is, therefore, that the intrinsic biology of CRC cells must be considered alongside the molecular profiles of individual tumours in order to successfully personalize treatment. In this Review, we outline how preclinical studies based on patient-derived models have informed the design of practice-changing clinical trials. The integration of these experiences into a common framework will reshape the future design of biology-informed clinical trials in this field.
Key points
-
The efficacy of targeted therapies in patients with solid tumours is largely unpredictable owing to intrinsic genetic complexity and a high level of tissue context specificity.
-
The development of patient-derived models that reflect the genetic heterogeneity of colorectal cancer (CRC) constitutes a successful platform for the development of targeted therapies.
-
These models have enabled the validation of retrospectively identified biomarkers in clinical trials and the optimization of prospective biomarkers to guide the selection of novel targeted therapies, such as those targeting HER2.
-
Longitudinal evaluations of the genomic evolution of CRC enabled by analysis of liquid biopsy samples have further increased the understanding of the mechanisms of resistance to targeted agents.
-
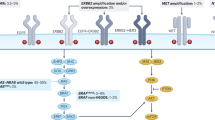
Investigations of resistance to targeted therapies have revealed convergence on CRC-specific feedback loops within the MAPK signalling pathway as a core mechanism of survival.
-
Co-inhibition with agents targeting EGFR and the specific oncogenic mutation has proved crucial in the clinical development of effective regimens for BRAF-mutant CRCs, and has also been demonstrated to be beneficial in the context of KRASG12C-mutant CRC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Remon, J. & Dienstmann, R. Precision oncology: separating the wheat from the chaff. ESMO Open 3, e000446 (2018).
Banerji, U. & Workman, P. Critical parameters in targeted drug development: the pharmacological audit trail. Semin. Oncol. 43, 436–445 (2016).
Yap, T. A., Sandhu, S. K., Workman, P. & de Bono, J. S. Envisioning the future of early anticancer drug development. Nat. Rev. Cancer 10, 514–523 (2010). A framework for evidence-based decision-making during drug discovery and development.
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Corcoran, R. B. et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2, 227–235 (2012). The first evidence of the mechanism of resistance to BRAF inhibitor in BRAF-mutant CRC through feedback reactivation of the EGFR-MAPK axis.
Amodio, V. et al. EGFR blockade reverts resistance to KRAS G12C inhibition in colorectal cancer. Cancer Discov. 10, 1129–1139 (2020). A recent paper highlighting the mechanism of resistance to selective KRAS-G12C inhibitors in CRC through feedback reactivation of EGFR.
Iorio, F. et al. A landscape of pharmacogenomic interactions in cancer. Cell 166, 740–754 (2016).
Najgebauer, H. et al. CELLector: genomics-guided selection of cancer in vitro models. Cell Syst. 10, 424–4326 (2020).
Drost, J. & Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 18, 407–418 (2018).
Ballard, D. H., Boyer, C. J. & Alexander, J. S. Organoids – preclinical models of human disease. N. Engl. J. Med. 380, 1981–1982 (2019).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Hidalgo, M. et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 4, 998–1013 (2014).
Byrne, A. T. et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 17, 254–268 (2017).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Rad, R. et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell 24, 15–29 (2013).
Bürtin, F., Mullins, C. S. & Linnebacher, M. Mouse models of colorectal cancer: past, present and future perspectives. World J. Gastroenterol. 26, 1394–1426 (2020).
Diaz, L. A. & Bardelli, A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 32, 579–586 (2014).
Normanno, N., Cervantes, A., Ciardiello, F., De Luca, A. & Pinto, C. The liquid biopsy in the management of colorectal cancer patients: current applications and future scenarios. Cancer Treat. Rev. 70, 1–8 (2018).
Siravegna, G., Marsoni, S., Siena, S. & Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Siravegna, G. et al. Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin. Cancer Res. 25, 3046–3053 (2019).
Parikh, A. R. et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 25, 1415–1421 (2019).
Khan, K. H. et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov. 8, 1270–1285 (2018).
Parikh, A. R. et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin. Cancer Res. 26, 1877–1885 (2020).
Nakamura, Y. et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 26, 1859–1864 (2020).
Siravegna, G. et al. How liquid biopsies can change clinical practice in oncology. Ann. Oncol. 30, 1580–1590 (2019).
Ganesh, K. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 16, 361–375 (2019).
Ciardiello, D. et al. Immunotherapy of colorectal cancer: challenges for therapeutic efficacy. Cancer Treat. Rev. 76, 22–32 (2019).
Germano, G. et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 552, 116–120 (2017).
Rospo, G. et al. Evolving neoantigen profiles in colorectal cancers with DNA repair defects. Genome Med. 11, 42 (2019).
Germano, G., Amirouchene-Angelozzi, N., Rospo, G. & Bardelli, A. The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov. 8, 1518–1528 (2018).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Olson, B., Li, Y., Lin, Y., Liu, E. T. & Patnaik, A. Mouse models for cancer immunotherapy research. Cancer Discov. 8, 1358–1365 (2018).
Segal, N. H. & Saltz, L. B. Translational considerations on the outlook of immunotherapy for colorectal cancer. Curr. Colorectal Cancer Rep. 11, 92–97 (2015).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Wieduwilt, M. J. & Moasser, M. M. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell. Mol. Life Sci. 65, 1566 (2008).
Mendelsohn J, B. J. The EGF receptor family as targets for cancer therapy. Oncogene 19, 6550–6565 (2000).
Salomon, D. S., Brandt, R., Ciardiello, F. & Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 19, 183–232 (1995).
Wu, X., Fan, Z., Masui, H., Rosen, N. & Mendelsohn, J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J. Clin. Invest. 95, 1897–1905 (1995).
Ciardiello, F. & Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 358, 1160–1174 (2008).
Van Cutsem, E. et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 25, 1658–1664 (2007).
Saltz, L. B. et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J. Clin. Oncol. 22, 1201–1208 (2004). The first trial demonstrating the clinical efficacy of an anti-EGFR agent in mCRC.
Mendelsohn, J. & Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 21, 2787–2799 (2003).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Jonker, D. J. et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 357, 2040–2048 (2007).
Chung, K. Y. et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J. Clin. Oncol. 23, 1803–1810 (2005).
Lièvre, A. et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 66, 3992–3995 (2006).
Benvenuti, S. et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 67, 2643–2648 (2007). The first two works, with Lièvre et al., to show how the presence of activating RAS or RAF mutations impair the activity of anti-EGFR antibodies in a preclinical model.
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008). The first clinical report showing the lack of clinical activity of anti-EGFR antibodies in KRAS-mutated cancers.
Karapetis, C. S. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 (2008).
Peeters, M. et al. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J. Clin. Oncol. 31, 759–765 (2013).
Segelov, E. et al. Response to cetuximab with or without irinotecan in patients with refractory metastatic colorectal cancer harboring the KRAS G13D mutation: Australasian Gastro-Intestinal Trials Group ICECREAM study. J. Clin. Oncol. 34, 2258–2264 (2016).
Siena, S. et al. Phase II open-label study to assess efficacy and safety of lenalidomide in combination with cetuximab in KRAS-mutant metastatic colorectal cancer. PLoS ONE 8, e62264 (2013).
Douillard, J.-Y. et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 28, 4697–4705 (2010).
Bardelli, A. & Siena, S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J. Clin. Oncol. 28, 1254–1261 (2010).
De Roock, W. C. B., Bernasconi, D., De Schutter, J., Biesmans, B., Fountzilas, G. et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 11, 753–762 (2010).
Douillard, J.-Y. et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 369, 1023–1034 (2013).
Di Nicolantonio, F. et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 26, 5705–5712 (2008).
Jhawer, M. et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 68, 1953–1961 (2008).
Sartore-Bianchi, A. et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 69, 1851–1857 (2009).
van Brummelen, E. M. J., de Boer, A., Beijnen, J. H. & Schellens, J. H. M. BRAF mutations as predictive biomarker for response to anti-EGFR monoclonal antibodies. Oncologist 22, 864–872 (2017).
Rowland, A. et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer 112, 1888–1894 (2015).
Pietrantonio, F. et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur. J. Cancer 51, 587–594 (2015).
Smith, C. G. et al. Somatic profiling of the epidermal growth factor receptor pathway in tumors from patients with advanced colorectal cancer treated with chemotherapy ± cetuximab. Clin. Cancer Res. 19, 4104–4113 (2013).
Loupakis, F. et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br. J. Cancer 101, 715–721 (2009).
Peeters, M. et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin. Cancer Res. 19, 1902–1912 (2013).
Karapetis, C. S. et al. PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer–results from NCIC CTG/AGITG CO.17. Clin. Cancer Res. 20, 744–753 (2014).
Orlandi, A. et al. BRAF in metastatic colorectal cancer: the future starts now. Pharmacogenomics 16, 2069–2081 (2015).
Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
Zhao, L. & Vogt, P. K. Helical domain and kinase domain mutations in p110α of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc. Natl Acad. Sci. USA 105, 2652–2657 (2008).
Day, F. L. et al. PIK3CA and PTEN gene and exon mutation-specific clinicopathologic and molecular associations in colorectal cancer. Clin. Cancer Res. 19, 3285–3296 (2013).
Prenen, H. et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin. Cancer Res. 15, 3184–3188 (2009).
Perrone, F. et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann. Oncol. 20, 84–90 (2009).
Huang, L. et al. Anti-epidermal growth factor receptor monoclonal antibody-based therapy for metastatic colorectal cancer: a meta-analysis of the effect of PIK3CA mutations in KRAS wild-type patients. Arch. Med. Sci. 10, 1–9 (2014).
Sepulveda, A. R. et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 35, 1453–1486 (2017).
Ciardiello, F. et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann. Oncol. 27, 1055–1061 (2016).
Yonesaka, K. et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci. Transl. Med. 3, 99ra86 (2011).
Bardelli, A. et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 3, 658–673 (2013).
Scartozzi, M. et al. Analysis of HER-3, insulin growth factor-1, nuclear factor-kB and epidermal growth factor receptor gene copy number in the prediction of clinical outcome for K-RAS wild-type colorectal cancer patients receiving irinotecan-cetuximab. Ann. Oncol. 23, 1706–1712 (2012).
Martinelli, E. et al. AXL is an oncotarget in human colorectal cancer. Oncotarget 6, 23281–23296 (2015).
Cardone, C. et al. AXL is a predictor of poor survival and of resistance to anti-EGFR therapy in RAS wild-type metastatic colorectal cancer. Eur. J. Cancer 138, 1–10 (2020).
De Robertis, M. et al. Dysregulation of EGFR pathway in EphA2 cell subpopulation significantly associates with poor prognosis in colorectal cancer. Clin. Cancer Res. 23, 159–170 (2017).
Martini, G. et al. EPHA2 is a predictive biomarker of resistance and a potential therapeutic target for improving antiepidermal growth factor receptor therapy in colorectal cancer. Mol. Cancer Ther. 18, 845–855 (2019).
Pietrantonio, F. et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J. Natl Cancer Inst. 109, djx089 (2017).
Cremolini, C. et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann. Oncol. 28, 3009–3014 (2017).
Morano, F. et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J. Clin. Oncol. 37, 3099–3110 (2019).
Misale, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536 (2012). The first report of the causative role of KRAS mutations in acquired resistance to anti-EGFR agents in CRC.
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra24 (2014).
Martini, G. et al. Resistance to anti-epidermal growth factor receptor in metastatic colorectal cancer: what does still need to be addressed? Cancer Treat. Rev. 86, 102023 (2020).
Misale S, A. S., Lamba, S., Siravegna, G., Lallo, A., Hobor, S. et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci. Transl. Med. 6, 224ra26 (2014).
Russo, M. et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 6, 147–153 (2016).
Troiani, T. N. S., Vitagliano, D., Morgillo, F., Capasso, A., Sforza, V. et al. Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition. Clin. Cancer Res. 20, 3775–3786 (2014).
Siena, S. et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann. Oncol. 29, 119–126 (2018).
Siravegna, G. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 21, 795–801 (2015).
Siravegna, G. et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell 34, 148–1627 (2018).
Van Emburgh, B. O. et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat. Commun. 7, 13665 (2016).
Parseghian, C. M. et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann. Oncol. 30, 243–249 (2019).
Cremolini, C. et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 5, 343–350 (2019).
Martinelli, E. et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: challenges and future perspectives. Ann. Oncol. 31, 30–40 (2020).
Dienstmann, R. et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 17, 79–92 (2017).
Garrett, T. P. J. et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 11, 495–505 (2003).
Siena, S. et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann. Oncol. 29, 1108–1119 (2018).
Valtorta, E. et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod. Pathol. 28, 1481–1491 (2015).
Seo, A. N. et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS ONE 9, e98528 (2014).
Bertotti, A. et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 1, 508–523 (2011). This proof-of-concept work establishing the translational potential of PDXs and the potential targeted approach in HER2-amplified CRC.
Richman, S. D. et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J. Pathol. 238, 562–570 (2016).
Nam, S. K. et al. BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS ONE 11, e0151865 (2016).
Ingold Heppner, B. et al. HER2/neu testing in primary colorectal carcinoma. Br. J. Cancer 111, 1977–1984 (2014).
Missiaglia, E. et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 25, 1995–2001 (2014).
Laurent-Puig, P. et al. ERBB2 alterations a new prognostic biomarker in stage III colon cancer from a FOLFOX based adjuvant trial (PETACC8). Ann. Oncol. 27, vi151 (2016).
Raghav, K. P. S. et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. J. Clin. Oncol. 34, 3517 (2016).
Schuell, B., Gruenberger, T., Scheithauer, W., Zielinski, C. & Wrba, F. HER 2/neu protein expression in colorectal cancer. BMC Cancer 6, 123 (2006).
Sun, S.-J. et al. High HER-2 protein levels correlate with clinicopathological features in colorectal cancer. J. Cancer Res. Ther. 12, 323–333 (2020).
Sartore-Bianchi, A. et al. HER2 positivity predicts unresponsiveness to EGFR-targeted treatment in metastatic colorectal cancer. Oncologist 24, 1395–1402 (2019).
Brannon, A. R. et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 15, 454 (2014).
Kavuri, S. M. et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 5, 832–841 (2015).
Loree, J. M. et al. Molecular landscape of ERBB2/ERBB3 mutated colorectal cancer. J. Natl Cancer Inst. 110, 1409–1417 (2018).
Leto, S. M. et al. Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2-amplified gastrointestinal carcinomas. Clin. Cancer Res. 21, 5519–5531 (2015).
Martin, V. et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br. J. Cancer 108, 668–675 (2013).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Clark, J., Niedzwiecki, D., Hollis, D. & Mayer, R. Phase II trial of 5-fluororuacil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzumab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy [abstract]. Proc. Am. Soc. Clin. Oncol. 22, 3584 (2003).
Ramanathan, R. K. et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 22, 858–865 (2004).
Sartore-Bianchi, A. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 738–746 (2016).
Sartore-Bianchi, A. et al. Central nervous system as possible site of relapse in ERBB2-positive metastatic colorectal cancer: long-term results of treatment with trastuzumab and lapatinib. JAMA Oncol. 6, 927–929 (2020).
Tosi, F. et al. Long-term clinical outcome of trastuzumab and lapatinib for HER2-positive metastatic colorectal cancer. Clin. Colorectal Cancer 19, 256–262.e2 (2020). Three papers reporting the results of the HERACLES-A trial, investigating the combination of trastuzumab and lapatinib in HER2+ mCRC.
Sartore-Bianchi, A. et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open 5, e000911 (2020).
Sakai, K. et al. Pertuzumab, a novel HER dimerization inhibitor, inhibits the growth of human lung cancer cells mediated by the HER3 signaling pathway. Cancer Sci. 98, 1498–1503 (2007).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Nakada, T. et al. Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg. Med. Chem. Lett. 26, 1542–1545 (2016).
Siena, S. et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. (in the press).
Meric-Bernstam, F. et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 20, 518–530 (2019).
Nakamura, Y. et al. TRIUMPH: Primary efficacy of a phase II trial of trastuzumab (T) and pertuzumab (P) in patients (pts) with metastatic colorectal cancer (mCRC) with HER2 (ERBB2) amplification (amp) in tumour tissue or circulating tumour DNA (ctDNA): A GOZILA sub-study [abstract 526PD]. Ann. Oncol. 30 (Suppl. 5), v199–v200 (2019).
Kulukian, A. et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 19, 976–987 (2020).
Strickler, J. H. et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial [abstract 527PD]. Ann. Oncol. 30 (Suppl. 5), v200 (2019).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Grothey, A. et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 303–312 (2013).
Li, J. et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 16, 619–629 (2015).
Mayer, R. J. et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 372, 1909–1919 (2015).
Xu, J. et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J. Clin. Oncol. 36, 350–358 (2018).
Tol, J., Nagtegaal, I. D. & Punt, C. J. BRAF mutation in metastatic colorectal cancer. N. Engl. J. Med. 361, 98–99 (2009).
Roth, A. D. et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 28, 466–474 (2010).
Giannakis, M. et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 15, 857–865 (2016).
Clarke, C. N. & Kopetz, E. S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest. Oncol. 6, 660–667 (2015).
Michaloglou, C., Vredeveld, L. C., Mooi, W. J. & Peeper, D. S. BRAF(E600) in benign and malignant human tumours. Oncogene 27, 877–895 (2008).
Sebolt-Leopold, J. S. & Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer 4, 937–947 (2004).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Maughan, T. S. et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377, 2103–2114 (2011).
Samowitz, W. S. et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 65, 6063–6069 (2005).
Tran, B. et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 117, 4623–4632 (2011).
Richman, S. D. et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J. Clin. Oncol. 27, 5931–5937 (2009).
Ogino, S. et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin. Cancer Res. 18, 890–900 (2012).
Taieb, J. et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J. Natl Cancer Inst. 109, djw272 (2017).
Matos, I., Elez, E., Capdevila, J. & Tabernero, J. Emerging tyrosine kinase inhibitors for the treatment of metastatic colorectal cancer. Expert Opin. Emerg. Drugs 21, 267–282 (2016).
Sinicrope, F. A. et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 148, 88–99 (2015).
French, A. J. et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin. Cancer Res. 14, 3408–3415 (2008).
Lochhead, P. et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J. Natl Cancer Inst. 105, 1151–1156 (2013).
Morris, V. et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin. Colorectal Cancer 13, 164–171 (2014).
Jones, J. C. et al. Non-V600 BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J. Clin. Oncol. 35, 2624–2630 (2017).
Cremolini, C. et al. BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann. Oncol. 26, 2092–2097 (2015).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Long, G. V. et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371, 1877–1888 (2014).
Kopetz, S. et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J. Clin. Oncol. 33, 4032–4038 (2015). The first study investigating BRAF inhibition in BRAF-mutant CRC.
Gomez-Roca, C. A. et al. Encorafenib (Lgx818), an oral Braf inhibitor, in patients (pts) with Braf V600e metastatic colorectal cancer (Mcrc): results of dose expansion in an open-label, phase 1 study [abstract 535P]. Ann. Oncol. 25 (Suppl. 4), iv182 (2014).
Bollag, G. et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010).
Hyman, D. M. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736 (2015).
Yaeger, R. et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin. Cancer Res. 21, 1313–1320 (2015).
Corcoran, R. B. et al. Efficacy and circulating tumor DNA (ctDNA) analysis of the BRAF inhibitor dabrafenib (D), MEK inhibitor trametinib (T), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E-mutated (BRAFm) metastatic colorectal cancer (mCRC) [abstract 455O]. Ann. Oncol. 27 (Suppl. 6), vi150 (2016).
Corcoran, R. B. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov. 8, 428–443 (2018).
Bendell, J. C. et al. Efficacy and tolerability in an open-label phase I/II study of MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in combination in patients (pts) with BRAF V600E mutated colorectal cancer (CRC) [abstract]. J. Clin. Oncol. 32 (Suppl. 15), 3515 (2014).
Corcoran, R. B. et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J. Clin. Oncol. 33, 4023–4031 (2015).
Corcoran, R. B. et al. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci. Signal. 3, ra84 (2010).
Kopetz, S. et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N. Engl. J. Med. 381, 1632–1643 (2019). Results from the BEACON-CRC phase III trial that led to the registration of cetuximab + encorafenib ± binimetinib in BRAF-mutant mCRC.
Grothey, A. et al. ANCHOR CRC: a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer [abstract LBA-5]. Ann. Oncol. 31 (Suppl. 3), S242–S243 (2020).
Mao, M. et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin. Cancer Res. 19, 657–667 (2013).
van Geel, R. et al. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov. 7, 610–619 (2017).
Juric, D. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518, 240–244 (2015).
Hong, D. S. et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 6, 1352–1365 (2016).
Ahronian, L. G. et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 5, 358–367 (2015).
Yaeger, R. et al. Mechanisms of acquired resistance to BRAF V600E inhibition in colon cancers converge on RAF dimerization and are sensitive to its inhibition. Cancer Res. 77, 6513–6523 (2017).
Oddo, D. et al. Emergence of MET hyper-amplification at progression to MET and BRAF inhibition in colorectal cancer. Br. J. Cancer 117, 347–352 (2017).
Oddo, D. et al. Molecular landscape of acquired resistance to targeted therapy combinations in BRAF-mutant colorectal cancer. Cancer Res. 76, 4504–4515 (2016).
Pietrantonio, F. et al. MET-driven resistance to dual EGFR and BRAF blockade may be overcome by switching from EGFR to MET inhibition in BRAF-mutated colorectal cancer. Cancer Discov. 6, 963–971 (2016).
Rajagopalan, H. et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 418, 934 (2002).
Tie, J. et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int. J. Cancer 128, 2075–2084 (2011).
Fransen, K. et al. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis 25, 527–533 (2004).
Cisowski, J., Sayin, V. I., Liu, M., Karlsson, C. & Bergo, M. O. Oncogene-induced senescence underlies the mutual exclusive nature of oncogenic KRAS and BRAF. Oncogene 35, 1328–1333 (2016).
Heidorn, S. J. et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 (2010).
Lavoie, H. et al. Inhibitors that stabilize a closed RAF kinase domain conformation induce dimerization. Nat. Chem. Biol. 9, 428–436 (2013).
Schram, A. M., Chang, M. T., Jonsson, P. & Drilon, A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 14, 735–748 (2017).
Sveen, A., Kopetz, S. & Lothe, R. A. Biomarker-guided therapy for colorectal cancer: strength in complexity. Nat. Rev. Clin. Oncol. 17, 11–32 (2020). A recent review focused on the role of biomarkers in the therapeutic management of mCRC.
Pulciani, S. et al. Oncogenes in solid human tumours. Nature 300, 539–542 (1982).
Créancier, L. et al. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett. 365, 107–111 (2015).
Hechtman, J. F. et al. Identification of targetable kinase alterations in patients with colorectal carcinoma that are preferentially associated with wild-type RAS/RAF. Mol. Cancer Res. 14, 296–301 (2016).
Ardini, E. et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol. Oncol. 8, 1495–1507 (2014). The first report of the sensitivity of NTRK fusion-positive CRC to TRKA inhibition.
Vaishnavi, A., Le, A. T. & Doebele, R. C. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 5, 25–34 (2015).
Drilon, A. et al. Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 7, 400–409 (2017).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 21, 531–540 (2020).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 21, 271–282 (2020).
FDA. FDA approves larotrectinib for solid tumors with NTRK gene fusions. https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0 (2018).
EMA. First ‘histology-independent’ treatment for solid tumours with a specific gene mutation. https://www.ema.europa.eu/en/news/first-histology-independent-treatment-solid-tumours-specific-gene-mutation (2019).
Nathenson, M. et al. Activity of larotrectinib in patients with TRK fusion GI malignancies [abstract O-020]. Ann. Oncol. 29 (Suppl. 5), v107 (2018).
Doebele, R.C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 21, 271–282 (2020).
Sgambato, A., Casaluce, F., Maione, P. & Gridelli, C. Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors. Expert. Rev. Anticancer. Ther. 18, 71–80 (2018).
Amatu, A. et al. Novel CAD-ALK gene rearrangement is drugable by entrectinib in colorectal cancer. Br. J. Cancer 113, 1730–1734 (2015).
Pietrantonio, F. et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann. Oncol. 29, 1394–1401 (2018).
Weaver, A. & Bossaer, J. B. Fibroblast growth factor receptor (FGFR) inhibitors: a review of a novel therapeutic class. J. Oncol. Pharm. Pract. https://doi.org/10.1177/1078155220983425 (2020).
Pagani, F. et al. The landscape of actionable gene fusions in colorectal cancer. Int. J. Mol. Sci. 20, 5319 (2019).
Drilon, A. et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann. Oncol. 27, 920–926 (2016).
Drilon, A. et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 7, 963–972 (2017).
Russo, M. et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov. 6, 36–44 (2016).
Cocco, E., Scaltriti, M. & Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 15, 731–747 (2018). A thorough review on the key discoveries in NTRK fusion-positive cancers and their treatment.
Drilon, A. et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov. 8, 1227–1236 (2018).
Cocco, E. et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat. Med. 25, 1422–1427 (2019). The first report that MAPK reactivation through parallel signalling participates to the onset of acquired resistance to NTRK inhibitors in gastrointestinal cancers.
Pai, E. F. et al. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature 341, 209–214 (1989).
Papke, B. & Der, C. J. Drugging RAS: know the enemy. Science 355, 1158–1163 (2017).
Schubbert, S., Shannon, K. & Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 7, 295–308 (2007).
Yuan, T. L. et al. Differential effector engagement by oncogenic KRAS. Cell Rep. 22, 1889–1902 (2018).
Hunter, J. C. et al. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol. Cancer Res. 13, 1325–1335 (2015).
Ihle, N. T. et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J. Natl. Cancer Inst. 104, 228–239 (2012).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Cox, A. D., Fesik, S. W., Kimmelman, A. C., Luo, J. & Der, C. J. Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 13, 828–851 (2014).
Patricelli, M. P. et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 6, 316–329 (2016).
Janes, M. R. et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell 172, 578–589.e17 (2018).
Canon, J. et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Hallin, J. et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 10, 54–71 (2020).
Govindan, R. et al. Phase I study of AMG 510, a novel molecule targeting KRAS G12C mutant solid tumours [abstract 446PD]. Ann. Oncol. 30 (Suppl. 5), v163–v164 (2019).
Strickler, J. et al. AMG 510, a novel small molecule inhibitor of KRAS G12C, for patients with advanced gastrointestinal cancers: results from the CodeBreak 100 phase 1 trial [abstract SO-24]. Ann. Oncol. 31 (Suppl. 3), S226 (2020).
Hong, D. S. et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 393, 1207–1217 (2020). Summarizes, with Canon et al., Hallin et al., Govindan et al. and Strickler et al. (2020), the preclinical and clinical development of selective KRAS-G12C inhibitors.
Nagasaka, M. et al. KRAS G12C game of thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat. Rev. 84, 101974 (2020).
Lou, K. et al. KRAS G12C inhibition produces a driver-limited state revealing collateral dependencies. Sci. Signal. 12, 583 (2019).
Misale, S. et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin. Cancer Res. 25, 796–807 (2019).
Molina-Arcas, M. et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci. Transl. Med. 11, eaaw7999 (2019).
Lito P, R. N. & Solit, D. B. Tumor adaptation and resistance to RAF inhibitors. Nat. Med. 19, 1401–1409 (2013).
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
Ryan, M. B. et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRASG12C inhibition. Clin. Cancer Res. 26, 1633–1643 (2020).
Schneider, G., Schmidt-Supprian, M., Rad, R. & Saur, D. Tissue-specific tumorigenesis: context matters. Nat. Rev. Cancer 17, 239–253 (2017).
Li, M. & Belmonte, J. C. I. Organoids — preclinical models of human disease. N. Engl. J. Med. 380, 569–579 (2019).
Yoshida, G. J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 13, 1–16 (2020).
Mainardi, S. et al. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat. Med. 24, 961–967 (2018).
Deming, D. A. et al. A phase I study of selumetinib (AZD6244/ARRY-142866), a MEK1/2 inhibitor, in combination with cetuximab in refractory solid tumors and KRAS mutant colorectal cancer. Invest. New Drugs 34, 168–175 (2016).
Neto, J. M. F. et al. Multiple low dose therapy as an effective strategy to treat EGFR inhibitor-resistant NSCLC tumours. Nat. Commun. 11, 3157 (2020).
Ozkan-Dagliyan, I. et al. Low-dose vertical inhibition of the RAF-MEK-ERK cascade causes apoptotic death of KRAS mutant cancers. Cell Rep. 31, 107764 (2020).
Dienstmann, R. et al. Evolving landscape of molecular prescreening strategies for oncology early clinical trials. JCO Precis. Oncol. 4, 505–513 (2020).
Colomer, R. et al. When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine 25, 100487 (2020).
Mosele, F. et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 31, 1491–1505 (2020).
Mateo, J. et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 29, 1895–1902 (2018). The ESCAT framework to rank genomic alterations on the basis of their actionability in cancer.
Dickson, D. et al. The master observational trial: a new class of master protocol to advance precision medicine. Cell 180, 9–14 (2020).
Siena, S. et al. Pembrolizumab in MMR-proficient metastatic colorectal cancer pharmacologically primed to trigger dynamic hypermutation status: the ARETHUSA trial [abstract]. J. Clin. Oncol. 37 (Suppl. 15), TPS2659 (2019).
Lonardi, S. et al. The PEGASUS trial: post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients [abstract]. J. Clin. Oncol. 38 (Suppl. 15), TPS4124 (2020).
Andre, T. et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Overman, M. J. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36, 773–779 (2018).
Arena, S. et al. A subset of colorectal cancers with cross-sensitivity to olaparib and oxaliplatin. Clin. Cancer Res. 26, 1372–1384 (2020).
Reilly, N. M., Novara, L., Di Nicolantonio, F. & Bardelli, A. Exploiting DNA repair defects in colorectal cancer. Mol. Oncol. 13, 681–700 (2019).
Mauri, G., Arena, S., Siena, S., Bardelli, A. & Sartore-Bianchi, A. The DNA damage response pathway as a land of therapeutic opportunities for colorectal cancer. Ann. Oncol. 31, 1135–1147 (2020).
Van Cutsem E, L. H. et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and ras mutations in colorectal cancer. J. Clin. Oncol. 33, 692–700 (2015).
Van Cutsem, E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, 1408–1417 (2009).
Bokemeyer, C. et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 27, 663–671 (2009).
Bokemeyer, C. et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur. J. Cancer 51, 1243–1252 (2015).
Tveit, K. M. et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J. Clin. Oncol. 30, 1755–1762 (2012).
Patterson, S. D. et al. Comprehensive analysis of KRAS and NRAS mutations as predictive biomarkers for single agent panitumumab (pmab) response in a randomized, phase III metastatic colorectal cancer (mCRC) study (20020408) [abstract]. J. Clin. Oncol. 31 (Suppl. 15), 3617 (2013).
Seymour, M. T. et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 14, 749–759 (2013).
Peeters, M. et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. 28, 4706–4713 (2010).
Peeters, M. et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin. Cancer Res. 21, 5469–5479 (2015).
Venook, A. P. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 317, 2392–2401 (2017).
Innocenti, F. et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J. Clin. Oncol. 37, 1217–1227 (2019).
Heinemann, V. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1065–1075 (2014).
Stintzing, S. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 17, 1426–1434 (2016).
Schwartzberg, L. S. et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 32, 2240–2247 (2014).
Rivera, F. et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int. J. Colorectal Dis. 32, 1179–1190 (2017).
Ciardiello, F. et al. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann. Oncol. 25, 1756–1761 (2014).
Kopetz, S. et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J. Clin. Oncol. 39, 285–294 (2017).
Parikh, A. R. & Corcoran, R. B. Fast-TRKing drug development for rare molecular targets. Cancer Discov. 7, 934–936 (2017).
Park, J. J. H., Hsu, G., Siden, E. G., Thorlund, K. & Mills, E. J. An overview of precision oncology basket and umbrella trials for clinicians. CA Cancer J. Clin. 70, 125–137 (2020).
Sidaway, P. MSI-H: a truly agnostic biomarker? Nat. Rev. Clin. Oncol. 17, 68 (2020).
Hyman, D. et al. Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi) [abstract]. Cancer Res. 79 (Suppl. 13), CT127 (2019).
Jarow, J. P., Lurie, P., Ikenberry, S. C. & Lemery, S. Overview of FDA’s expanded access program for investigational drugs. Ther. Innov. Regul. Sci. 51, 177–179 (2017).
Acknowledgements
This work is supported by Fondazione AIRC, Associazione Italiana per la Ricerca sul Cancro, Investigator Grants 20685 (S.S.), 21923 (A.B.), 21407 (F.D.N.) and 22802 (L.T.); Fondazione AIRC under 5 per Mille 2018-ID 21091 program (to A.B., F.D.N., S.M., S.S. and L.T); Instituto de Salud Carlos III through the project “AC15/00018” (co-funded by the European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future”) (J.T.). AIRC-CRUK-FC AECC Accelerator Award contract 22795 (A.B., L.T. and J.T); Fondazione Piemontese per la Ricerca sul Cancro-ONLUS, 5x1000 Ministero della Salute 2015 Project “STRATEGY” (F.D.N.); Fondazione Piemontese per la Ricerca sul Cancro-ONLUS, 5x1000 Ministero della Salute 2015 Project “IMMUNOGENOMICA” (A.B. and L.T.); BiLiGeCT - Progetto PON ARS01_00492 (A.B.); Fondazione Piemontese per la Ricerca sul Cancro-ONLUS, 5x1000 Ministero della Salute 2014 and 2016 (L.T.); H2020 grant agreement no. 754923 COLOSSUS (L.T. and J.T.); CORDIS Community Research and Development Information Service, Horizon 2020 (project ID 635342) grant, Molecularly Guided Trials with Specific Treatment Strategies in Patients with Advanced Newly Molecular Defined Subtypes of Colorectal Cancer (MoTriColor) (J.T.); and Fondazione Oncologia Niguarda Onlus, grant Terapia Molecolare dei Tumori (S.S.).
Author information
Authors and Affiliations
Contributions
F.D.N., P.P.V., S.M., L.T. and A.B. researched data for this article, all authors made a substantial contribution to discussions of content, F.D.N., P.P.V., S.M., J.T., L.T., S.S. and A.B. wrote the manuscript, and all authors reviewed and/or edited the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
P.P.V. has acted as a consultant of Biocartis and speaker for Merck. S.S. has acted as an advisor to Amgen, Bayer, BMS, Celgene, CheckmAb, Daiichi-Sankyo, Incyte, Merck, Novartis, Roche and Seattle Genetics. J.T. has acted as an advisor to Array BioPharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai Pharma, Eli Lilly, Foundation Medicine, Genentech, HalioDX SAS, Menarini, Merck Serono, Merus, MSD, Novartis, Peptomyc, Pfizer, Roche, Roche Diagnostics, Sanofi, Seattle Genetics, Servier, and Taiho Pharmaceutical. L.T. has acted as a speaker for AstraZeneca, Eli Lilly and Merck KGaA, and has received research grants from Menarini, Merus, Pfizer, Servier and Symphogen. R.B. is an employee of and holds shares in Agendia, holds shares in Oncosence, and has received research funding from Astex and Eli Lilly. A.B. has acted as an advisor to Biocartis, Guardant, Horizon Discovery, Illumina, Inivata, Neophore, Roche and Third Rock, declares ownership interests (including patents) in Phoremost and Neophore, and has received commercial research grants from Neophore. The remaining authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks T. Yoshino, B. Ma, T. Maughan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Nicolantonio, F., Vitiello, P.P., Marsoni, S. et al. Precision oncology in metastatic colorectal cancer — from biology to medicine. Nat Rev Clin Oncol 18, 506–525 (2021). https://doi.org/10.1038/s41571-021-00495-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00495-z
This article is cited by
-
Regulation of tumor metastasis and CD8+ T cells infiltration by circRNF216/miR-576-5p/ZC3H12C axis in colorectal cancer
Cellular & Molecular Biology Letters (2024)
-
New clinical trial design in precision medicine: discovery, development and direction
Signal Transduction and Targeted Therapy (2024)
-
Feedback activation of EGFR/wild-type RAS signaling axis limits KRASG12D inhibitor efficacy in KRASG12D-mutated colorectal cancer
Oncogene (2023)
-
The emergence of RAS mutations in patients with RAS wild-type mCRC receiving cetuximab as first-line treatment: a noninterventional, uncontrolled multicenter study
British Journal of Cancer (2023)
-
Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer
Nature Communications (2023)