Abstract
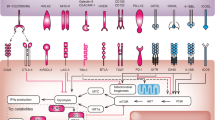
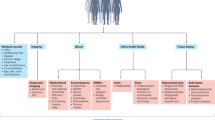
Accumulating evidence suggests that a high tumour burden has a negative effect on anticancer immunity. The concept of tumour burden, simply defined as the total amount of cancer in the body, in contrast to molecular tumour burden, is often poorly understood by the wider medical community; nonetheless, a possible role exists in defining the optimal treatment strategy for many patients. Historically, tumour burden has been assessed using imaging. In particular, CT scans have been used to evaluate both the number and size of metastases as well as the number of organs involved. These methods are now often complemented by metabolic tumour burden, measured using the more recently developed 2-deoxy-2-[18F]-fluoro-d-glucose (FDG)-PET/CT. Serum-based biomarkers, such as lactate dehydrogenase, can also reflect tumour burden and are often also correlated with a poor response to immune-checkpoint inhibitors. Other circulating markers (such as circulating free tumour DNA and/or circulating tumour cells) are also attracting research interest as surrogate markers of tumour burden. In this Review, we summarize evidence supporting the utility of tumour burden as a biomarker to guide the use of immune-checkpoint inhibitors. We also describe data and provide perspective on the various tools used for tumour burden assessment, with a particular emphasis on future therapeutic strategies that might address the issue of inferior outcomes among patients with cancer with a high tumour burden.
Key points
-
The search for predictive biomarkers of responsiveness to immune-checkpoint inhibitors (ICIs) remains an active area of research.
-
Tumour burden can be assessed using imaging, liquid biopsy methods or through the quantification of biological tumour derivatives such as lactate dehydrogenase or serum-based biomarkers.
-
Accumulating evidence supports a prognostic role for tumour burden in patients receiving ICIs.
-
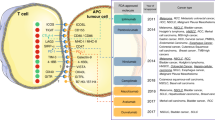
The detrimental effects of tumour burden on the efficacy of ICIs probably reflect differences in tumour biology relative to lower-burden disease.
-
Further investigations are warranted to distinguish between the prognostic and potentially predictive validity of tumour burden in patients receiving ICIs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ferrara, R. et al. Hyperprogressive disease in patients with advanced non–small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 4, 1543–1552 (2018).
Kim, C. G. et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann. Oncol. 30, 1104–1113 (2019).
Ott, P. A. et al. T-cell–inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 37, 318–327 (2019).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Fehrenbacher, L. et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non–small cell lung cancer. J. Thorac. Oncol. 13, 1156–1170 (2018).
Addeo, A., Banna, G. L. & Weiss, G. J. Tumor mutation burden - from hopes to doubts. JAMA Oncol. 5, 934–935 (2019).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N. Engl. J. Med. 381, 2020–2031 (2019).
Iams, W. T., Porter, J. & Horn, L. Immunotherapeutic approaches for small-cell lung cancer. Nat. Rev. Clin. Oncol. 17, 300–312 (2020).
Prasad, V. & Addeo, A. The FDA approval of pembrolizumab for patients with TMB>10 mut/Mb: was it a wise decision? No. Ann. Oncol. 31, 1112–1114 (2020).
Joseph, R. W. et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin. Cancer Res. 24, 4960–4967 (2018).
Davis, E. J. et al. Clinical correlates of response to anti-PD-1-based therapy in patients with metastatic melanoma. J. Immunother. 42, 221–227 (2019).
Hopkins, A. M., Kichenadasse, G., McKinnon, R. A., Rowland, A. & Sorich, M. J. Baseline tumor size and survival outcomes in lung cancer patients treated with immune checkpoint inhibitors. Semin. Oncol. 46, 380–384 (2019).
Faehling, M. et al. Immuno-oncological treatment and tumor mass in non-small cell lung cancer: case-control analysis of overall survival in routine clinical practice. Oncology 97, 228–235 (2019).
Katsurada, M. et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer. Res. 39, 815–825 (2019).
Hakozaki, T., Hosomi, Y., Kitadai, R., Kitagawa, S. & Okuma, Y. Efficacy of immune checkpoint inhibitor monotherapy for patients with massive non-small-cell lung cancer. J. Cancer Res. Clin. Oncol. 146, 2957–2966 (2020).
Inoue, H. et al. Pre-treatment tumor size impacts on response to nivolumab in head and neck squamous cell carcinoma. Auris Nasus Larynx 47, 650–657 (2020).
Friedlander, P. The use of baseline tumor size to prognosticate overall survival in stage IV melanoma patients treated with the PD-1 inhibitor pembrolizumab. Ann. Transl. Med. 7 (Suppl. 1), S24 (2019).
Pires da Silva, I. et al. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti–PD-1 therapy. Cancer 126, 86–97 (2020).
Seban, R. D. et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur. J. Nucl. Med. Mol. Imaging 47, 1147–1157 (2019).
Hashimoto, K. et al. Potential of FDG-PET as prognostic significance after anti-PD-1 antibody against patients with previously treated non-small cell lung cancer. J. Clin. Med. 9, 725 (2020).
Seban, R. D. et al. FDG-PET biomarkers associated with long-term benefit from first-line immunotherapy in patients with advanced non-small cell lung cancer. Ann. Nucl. Med. 34, 968–974 (2020).
Chardin, D. et al. Baseline metabolic tumor volume as a strong predictive and prognostic biomarker in patients with non-small cell lung cancer treated with PD1 inhibitors: a prospective study. J. Immunother. Cancer 8, e000645 (2020).
Lo Russo, G. et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin. Cancer Res. 25, 989–999 (2019).
Castello, A., Rossi, S., Mazziotti, E., Toschi, L. & Lopci, E. Hyperprogressive disease in patients with non-small cell lung cancer treated with checkpoint inhibitors: the Role of 18F-FDG PET/CT. J. Nucl. Med. 61, 821–826 (2020).
Ito, K. et al. Prognostic value of baseline metabolic tumor volume measured on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur. J. Nucl. Med. Mol. Imaging 46, 930–939 (2019).
Seban, R. D. et al. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur. J. Nucl. Med. Mol. Imaging 46, 2298–2310 (2019).
Wong, A. et al. 18F-FDG PET/CT based spleen to liver ratio associates with clinical outcome to ipilimumab in patients with metastatic melanoma. Cancer Imaging 20, 36 (2020).
Seban, R. D. et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur. J. Nucl. Med. Mol. Imaging 47, 2301–2312 (2020).
Weppler, A. M. et al. Clinical, FDG-PET and molecular markers of immune checkpoint inhibitor response in patients with metastatic Merkel cell carcinoma. J. Immunother. Cancer 8, e000700 (2020).
Dall’Olio, F. G. et al. Baseline total metabolic tumour volume on 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography-computed tomography as a promising biomarker in patients with advanced non–small cell lung cancer treated with first-line pembrolizumab. Eur. J. Cancer 150, 99–107 (2021).
Chang, Y. S. et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc. Natl Acad. Sci. USA 97, 14608–14613 (2000).
Tanaka, F. et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin. Cancer Res. 15, 6980–6986 (2009).
Krebs, M. G. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 29, 1556–1563 (2011).
Kang, B. J. et al. Circulating tumor cell number is associated with primary tumor volume in patients with lung adenocarcinoma. Tuberc. Respir. Dis. 83, 61–70 (2020).
Dall’Olio, F. G. et al. PD-L1 expression in circulating tumor cells as promising prognostic biomarker in advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Clin. Lung Cancer https://doi.org/10.1016/j.cllc.2021.03.005 (2021).
Zhou, J., Dong, F., Cui, F., Xu, R. & Tang, X. The role of circulating tumor cells in evaluation of prognosis and treatment response in advanced non-small-cell lung cancer. Cancer Chemother. Pharmacol. 79, 825–833 (2017).
Chen, Q. et al. Lung cancer circulating tumor cells isolated by the EpCAM-independent enrichment strategy correlate with Cytokeratin 19-derived CYFRA21-1 and pathological staging. Clin. Chim. Acta 419, 57–61 (2013).
Guibert, N. et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 120, 108–112 (2018).
Tamminga, M. et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J. Immunother. Cancer 7, 173 (2019).
Alama, A. et al. Prognostic relevance of circulating tumor cells and circulating cell-free DNA association in metastatic non-small cell lung cancer treated with nivolumab. J. Clin. Med. 8, 1011 (2019).
Papadaki, M. A. et al. Optimization of the enrichment of circulating tumor cells for downstream phenotypic analysis in patients with non-small cell lung cancer treated with anti-PD-1 immunotherapy. Cancers 12, 1556 (2020).
Cheng, M. L. et al. Circulating tumor DNA in advanced solid tumors: clinical relevance and future directions. CA Cancer J. Clin. 71, 176–190.
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra24 (2014).
Parkinson, C. A. et al. Exploratory analysis of TP53 mutations in circulating tumour DNA as biomarkers of treatment response for patients with relapsed high-grade serous ovarian carcinoma: a retrospective study. PLoS Med. 13, e1002198 (2016).
Abbosh, C. et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545, 446–451 (2017).
Iijima, Y. et al. Very early response of circulating tumour–derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non–small cell lung cancer. Eur. J. Cancer 86, 349–357 (2017).
Valpione, S. et al. Plasma total cell-free DNA (cfDNA) is a surrogate biomarker for tumour burden and a prognostic biomarker for survival in metastatic melanoma patients. Eur. J. Cancer 88, 1–9 (2018).
Lee, J. H. et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann. Oncol. 28, 1130–1136 (2017).
Lee, J. H. et al. Longitudinal monitoring of ctDNA in patients with melanoma and brain metastases treated with immune checkpoint inhibitors. Clin. Cancer Res. 26, 4064–4071 (2020).
Seremet, T. et al. Undetectable circulating tumor DNA (ctDNA) levels correlate with favorable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J. Transl. Med. 17, 303 (2019).
Marsavela, G. et al. Circulating tumor DNA predicts outcome from first-, but not second-line treatment and identifies melanoma patients who may benefit from combination immunotherapy. Clin. Cancer Res. 26, 5926–5933 (2020).
Bratman, S. V. et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 1, 873–881 (2020).
Herbreteau, G. et al. Circulating tumor DNA as a prognostic determinant in small cell lung cancer patients receiving atezolizumab. J. Clin. Med. 9, 3861 (2020).
Nabet, B. Y. et al. Noninvasive early identification of therapeutic benefit from immune checkpoint inhibition. Cell 183, 363–376.e13 (2020).
Van Wilpe, S. et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology 9, 1731942 (2020).
Agarwala, S. S. et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur. J. Cancer 45, 1807–1814 (2009).
Koukourakis, M. I. et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (Vatalanib) antiangiogenic therapy. Clin. Cancer Res. 17, 4892–4900 (2011).
Dercle, L. et al. Rapid and objective CT scan prognostic scoring identifies metastatic patients with long-term clinical benefit on anti-PD-1/-L1 therapy. Eur. J. Cancer 65, 33–42 (2016).
Braune, J. et al. Circulating tumor DNA allows early treatment monitoring in BRAF- and NRAS-mutant malignant melanoma. JCO Precis. Oncol. 4, 20–31 (2020).
Gill, A. B. et al. Correlating radiomic features of heterogeneity on CT with circulating tumor DNA in metastatic melanoma. Cancers 12, 3493 (2020).
van Wilpe, S., Tolmeijer, S. H., de Vries, I. J. M., Koornstra, R. H. T. & Mehra, N. LDH isotyping for checkpoint inhibitor response prediction in patients with metastatic melanoma. Immuno 1, 67–77 (2021).
Zhang, Z. et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Cancer Med. 8, 1467–1473 (2019).
Petrelli, F. et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res. 29, 1–12 (2019).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Spigel, D. R. et al. Second-line Nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann. Oncol. 32, 631–641 (2021).
Knispel, S. et al. Outcome of melanoma patients with elevated LDH treated with first-line targeted therapy or PD-1-based immune checkpoint inhibition. Eur. J. Cancer 148, 61–75 (2021).
Gupta, G. S. LDH-C4: a target with therapeutic potential for cancer and contraception. Mol. Cell Biochem. 371, 115–127 (2012).
Skude, G., von Eyben, F. E. & Kristiansen, P. Additional lactate dehydrogenase (LDH) isoenzymes in normal testis and spermatozoa of adult man. Mol. Gen. Genet. 198, 172–174 (1984).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218 (2016).
Ding, J., Karp, J. E. & Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 19, 353–363 (2017).
Zhang, W. et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178, 176–189.e15 (2019).
Kim, J. Y. et al. Hyperprogressive disease during anti-PD-1 (PDCD1) / PD-L1 (CD274) therapy: a systematic review and meta-analysis. Cancers 11, 1699 (2019).
Duffy, M. J. Clinical uses of tumor markers: a critical review. Crit. Rev. Clin. Lab. Sci. 38, 225–262 (2001).
Harpio, R. & Einarsson, R. S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin. Biochem. 37, 512–518 (2004).
Shi, P. et al. Association between serum tumor markers and metabolic tumor volume or total lesion glycolysis in patients with recurrent small cell lung cancer. Oncol. Lett. 10, 3123–3128 (2015).
Dogan, I., Karyagar, S. S. S., Karyagar, S. S. S., Kahraman, C. & Alver, A. Relationship between pretreatment levels of serum Cyfra 21.1, CEA and PET metabolic parameters in NSCLC. Ann. Nucl. Med. 28, 829–835 (2014).
Deckers, E. A. et al. The association between active tumor volume, total lesion glycolysis and levels of S-100B and LDH in stage IV melanoma patients. Eur. J. Surg. Oncol. 46, 2147–2153 (2020).
Dal Bello, M. G. et al. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J. Transl. Med. 17, 74 (2019).
Dall’Olio, F. G. et al. CEA and CYFRA 21-1 as prognostic biomarker and as a tool for treatment monitoring in advanced NSCLC treated with immune checkpoint inhibitors. Ther. Adv. Med. Oncol. 12, 175883592095299 (2020).
Wagner, N. B., Forschner, A., Leiter, U., Garbe, C. & Eigentler, T. K. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br. J. Cancer 119, 339–346 (2018).
Ballman, K. V. Biomarker: predictive or prognostic? J. Clin. Oncol. 33, 3968–3971 (2015).
DeBerardinis, R. J. & Chandel, N. S. We need to talk about the Warburg effect. Nat. Metab. 2, 127–129 (2020).
Sugiura, A. & Rathmell, J. C. Metabolic barriers to T cell function in tumors. J. Immunol. 200, 400–407 (2018).
de Geus-Oei, L. F. et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer 55, 79–87 (2007).
Brown, R. S. et al. Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J. Nucl. Med. 40, 556–565 (1999).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
de la Cruz-López, K. G., Castro-Muñoz, L. J., Reyes-Hernández, D. O., García-Carrancá, A. & Manzo-Merino, J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol. 9, 1143 (2019).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Augustin, R. C., Delgoffe, G. M. & Najjar, Y. G. Characteristics of the tumor microenvironment that influence immune cell functions: hypoxia, oxidative stress, metabolic alterations. Cancers 12, 3802 (2020).
Suzuki, J. et al. The tumor suppressor menin prevents effector CD8 T-cell dysfunction by targeting mTORC1-dependent metabolic activation. Nat. Commun. 9, 3296 (2018).
Kuwahara, M. et al. The menin-bach2 axis is critical for regulating CD4 T-cell senescence and cytokine homeostasis. Nat. Commun. 5, 3555 (2014).
Petrova, V., Annicchiarico-Petruzzelli, M., Melino, G. & Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 7, 10 (2018).
Kiraga, Ł. et al. Changes in hypoxia level of CT26 tumors during various stages of development and comparing different methods of hypoxia determination. PLoS ONE 13, e0206706 (2018).
Milross, C. G. et al. The effect of tumor size on necrosis and polarographically measured pO2. Acta Oncol. 36, 183–189 (1997).
Zhang, W. J. et al. Hypoxia-inducible factor-1 alpha correlates with tumor-associated macrophages infiltration, influences survival of gastric cancer patients. J. Cancer 8, 1818–1825 (2017).
Scharping, N. E. & et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Xie, H. & Simon, M. C. Oxygen availability and metabolic reprogramming in cancer. J. Biol. Chem. 292, 16825–16832 (2017).
Ferrara, R., Mezquita, L., Auclin, E., Chaput, N. & Besse, B. Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: does age really matter? Cancer Treat. Rev. 60, 60–68 (2017).
Plunkett, F. J. et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech. Ageing Dev. 126, 855–865 (2005).
Lanna, A., Henson, S. M., Escors, D. & Akbar, A. N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 15, 965–972 (2014).
Henson, S. M. et al. P38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J. Clin. Invest. 124, 4004–4016 (2014).
Akbar, A. N., Henson, S. M. & Lanna, A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 37, 866–876 (2016).
van de Berg, P. J. E. J. et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J. Immunol. 184, 3417–3423 (2010).
Fulop, T. et al. Potential role of immunosenescence in cancer development. Ann. N. Y. Acad. Sci. 1197, 158–165 (2010).
Plunkett, F. J. et al. The loss of telomerase activity in highly differentiated CD8+CD28−CD27− T cells is associated with decreased Akt (Ser 473) phosphorylation. J. Immunol. 178, 7710–7719 (2007).
Strioga, M., Pasukoniene, V. & Characiejus, D. CD8+CD28- and CD8+CD57+ T cells and their role in health and disease. Immunology 134, 17–32 (2011).
Brenchley, J. M. et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 (2003).
Voehringer, D., Koschella, M. & Pircher, H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 100, 3698–3702 (2002).
Ferrara, R. et al. Circulating T-cell immunosenescence in patients with advanced non–small cell lung cancer treated with single-agent PD-1/PD-L1 inhibitors or platinum-based chemotherapy. Clin. Cancer Res. 27, 492–503 (2021).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Tu, W. & Rao, S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front. Microbiol. 7, 2111 (2016).
Onyema, O. O. et al. Shifts in subsets of CD8+ T-cells as evidence of immunosenescence in patients with cancers affecting the lungs: an observational case-control study. BMC Cancer 15, 1016 (2015).
Tsukishiro, T., Donnenberg, A. D. & Whiteside, T. L. Rapid turnover of the CD8+CD28- T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol. Immunother. 52, 599–607 (2003).
Shen, Y., Qu, Q. X., Zhu, Y. B. & Zhang, X. G. Analysis of CD8+CD28- T-suppressor cells in gastric cancer patients. J. Immunoass. Immunochem. 33, 149–155 (2012).
Meermeier, E. W. et al. Tumor burden limits bispecific antibody efficacy through T cell exhaustion averted by concurrent cytotoxic therapy. Blood Cancer Discov. 2, 354–369 (2021).
Swan, D., Gurney, M., Krawczyk, J., Ryan, A. E. & O’Dwyer, M. Beyond DNA damage: exploring the immunomodulatory effects of cyclophosphamide in multiple myeloma. HemaSphere 4, e350 (2020).
Wei, A. H. et al. Biomarkers associated with blinatumomab outcomes in acute lymphoblastic leukemia. Leukemia 35, 2220–2231 (2021).
Kaufmann, D. E. et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8, 1246–1254 (2007).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Han, S., Asoyan, A., Rabenstein, H., Nakano, N. & Obst, R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc. Natl Acad. Sci. USA 107, 20453–20458 (2010).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Philip, M. & Schietinger, A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunol. 58, 98–103 (2019).
Kallies, A., Zehn, D. & Utzschneider, D. T. Precursor exhausted T cells: key to successful immunotherapy? Nat. Rev. Immunol. 20, 128–136 (2020).
Ghorani, E. et al. The T cell differentiation landscape is shaped by tumour mutations in lung cancer. Nat. Cancer 1, 546–561 (2020).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Sylman, J. L. et al. The predictive value of inflammation-related peripheral blood measurements in cancer staging and prognosis. Front. Oncol. 8, 78 (2018).
Arda, E., Yuksel, I., Cakiroglu, B., Akdeniz, E. & Cilesiz, N. Valuation of neutrophil/lymphocyte ratio in renal cell carcinoma grading and progression. Cureus 10, e2051 (2018).
Sorich, M. J., Rowland, A., Karapetis, C. S. & Hopkins, A. M. Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for non-small cell lung cancer: pooled analysis of clinical trials. J. Thorac. Oncol. 14, 1440–1446 (2019).
Kinoshita, T., Ito, H. & Miki, C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer 85, 2526–2531 (1999).
Sanmamed, M. F. et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin. Cancer Res. 20, 5697–5707 (2014).
Shang, G.-S., Liu, L. & Qin, Y.-W. IL-6 and TNF-α promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncol. Lett. 13, 4657–4660 (2017).
Zhou, X. L., Fan, W., Yang, G. & Yu, M. X. The clinical significance of PR, ER, NF-B, and TNF-α in breast cancer. Dis. Markers 2014, 494581 (2014).
Zhang, Z. et al. Transmembrane TNF-alpha promotes chemoresistance in breast cancer cells. Oncogene 37, 3456–3470 (2018).
Al Obeed, O. A. et al. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J. Gastroenterol. 20, 18390–18396 (2014).
Fade, A., Mahmoud, N. I. & Rivera, A. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr. Oncol. Rep. 4, 250–255 (2002).
Allin, K. H., Bojesen, S. E. & Nordestgaard, B. G. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J. Clin. Oncol. 27, 2217–2224 (2009).
Cassidy, M. R. et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine 18, 56–61 (2017).
Bigot, F. et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy Immune Score (GRIm-Score). Eur. J. Cancer 84, 212–218 (2017).
Ferrucci, P. F. et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br. J. Cancer 112, 1904–1910 (2015).
Mezquita, L. et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 4, 351–357 (2018).
Laino, A. S. et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 8, 842 (2020).
Schalper, K. A. et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 26, 688–692 (2020).
Mercogliano, M. F., Bruni, S., Mauro, F., Elizalde, P. V. & Schillaci, R. Harnessing tumor necrosis factor alpha to achieve effective cancer immunotherapy. Cancers 13, 564 (2021).
Jeong, H. et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res. 79, 795–806 (2019).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051 (2018).
Dummer, R. et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: safety run-in and biomarker cohorts of COMBI-i. Nat. Med. 26, 1557–1563 (2020).
Ribas, A. et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF -mutant melanoma. Nat. Med. 25, 936–940 (2019).
Méjean, A. et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N. Engl. J. Med. 379, 417–427 (2018).
Bex, A. et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib. JAMA Oncol. 5, 164 (2019).
Guisier, F., Cousse, S., Jeanvoine, M., Thiberville, L. & Salaun, M. A rationale for surgical debulking to improve anti-PD1 therapy outcome in non small cell lung cancer. Sci. Rep. 9, 16902 (2019).
Kordbacheh, T., Honeychurch, J., Blackhall, F., Faivre-Finn, C. & Illidge, T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: building better translational research platforms. Ann. Oncol. 29, 301–310 (2018).
Hendriks, L. E. L., Menis, J., De Ruysscher, D. K. M. & Reck, M. Combination of immunotherapy and radiotherapy — the next magic step in the management of lung cancer? J. Thorac. Oncol. 15, 166–169 (2020).
Popat, V. et al. Lack of association between radiographic tumor burden and efficacy of immune checkpoint inhibitors in advanced lung cancer. Oncologist 25, 515–522 (2020).
Acknowledgements
We wish to acknowledge Dr Stephan De Botton of the Département d’Hématologie, Gustave Roussy Cancer Campus, Villejuif, France, for his help in revising this manuscript.
Author information
Authors and Affiliations
Contributions
F.G.D., C.C., N.C. and B.B. researched data for this article. All authors made a substantial contribution to discussions of content. F.G.D. and B.B. wrote the manuscript. All authors reviewed and/or edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
A.M. has acted as an adviser and/or consultant of Amgen, AstraZeneca/Medimmune, BMS, GSK, IMCheck, J&J, Merck (MSD), Merck Serono, OSE immunotherapeutics, Pfizer, Pierre Fabre, Roche/Genentech, Sanofi and Symphogen/Servier, has received travel support from AstraZeneca, BMS, Merck (MSD) and Roche, and has received research funding from BMS, Boehringer Ingelheim, Fondation MSD Avenir, Merus and Transgene. C.C. has acted as a consultant of AstraZeneca, BMS, MSD and Roche. N.C. has received research funding from AstraZeneca, BMS Foundation, Cytune pharma, GSK and Roche. C.R. has acted as a consultant of Amgen, BMS, MSD, Novartis, Pfizer, Pierre Fabre, Roche and Sanofi. B.B. has conducted research funded by Abbvie, Amgen, Aptitude Health, AstraZeneca, Beigene, Biogen, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Ignyta, Inivata, Ipsen, Merck, MSD, Nektar, Onxeo, OSE immunotherapeutics, Pfizer, Pharma Mar, Roche-Genentech, Sanofi, Spectrum Pharmaceuticals, Takeda,Tiziana Pharm 4D Pharma and Tolero Pharmaceuticals.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks Richard Joseph, Michael Sorich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dall’Olio, F.G., Marabelle, A., Caramella, C. et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol 19, 75–90 (2022). https://doi.org/10.1038/s41571-021-00564-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00564-3
This article is cited by
-
Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation
Journal of Experimental & Clinical Cancer Research (2024)
-
Prediction of hematologic toxicity in luminal type breast cancer patients receiving neoadjuvant chemotherapy using CT L1 level skeletal muscle index
Scientific Reports (2024)
-
Development of a whole spinal MRI-based tumor burden scoring method in participants with multiple myeloma: a pilot study of prognostic significance
Annals of Hematology (2024)
-
HDAC6 inhibitor ACY-1215 enhances STAT1 acetylation to block PD-L1 for colorectal cancer immunotherapy
Cancer Immunology, Immunotherapy (2024)
-
Liquid biopsy techniques and pancreatic cancer: diagnosis, monitoring, and evaluation
Molecular Cancer (2023)