Abstract
Transient receptor potential (TRP) channels are multifunctional signalling molecules with many roles in sensory perception and cellular physiology. Therefore, it is not surprising that TRP channels have been implicated in numerous diseases, including hereditary disorders caused by defects in genes encoding TRP channels (TRP channelopathies). Most TRP channels are located at the cell surface, which makes them generally accessible drug targets. Early drug discovery efforts to target TRP channels focused on pain, but as our knowledge of TRP channels and their role in health and disease has grown, these efforts have expanded into new clinical indications, ranging from respiratory disorders through neurological and psychiatric diseases to diabetes and cancer. In this Review, we discuss recent findings in TRP channel structural biology that can affect both drug development and clinical indications. We also discuss the clinical promise of novel TRP channel modulators, aimed at both established and emerging targets. Last, we address the challenges that these compounds may face in clinical practice, including the need for carefully targeted approaches to minimize potential side-effects due to the multifunctional roles of TRP channels.
Similar content being viewed by others
Introduction
In 1969, a Drosophila mutant with defective light sensing was identified, which showed only a transient receptor potential (TRP) when exposed to continuous light instead of the expected sustained response1. This was later found to be caused by the lack of a functional copy of the gene coding for an ion channel, which was named trp2. However, the name ‘TRP’ channel is really a misnomer as the wild-type channel in fact causes a persistent (and not transient) current. This behaviour of TRPs is in contrast to many ion channels, which fully adapt when exposed to constant stimulation.
TRPs are multifunctional signalling molecules, expressed in many tissues and cell types3,4. Most TRPs are polymodal channels, so-called coincidence detectors that are activated by both physical (temperature, voltage, pressure and tension) and chemical stimuli4. Beyond that, few generalizations can be made about TRP channels. Some TRPs function as non-selective cation channels in the plasma membrane; others regulate Ca2+ release in intracellular organelles.
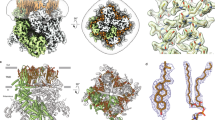
The mammalian TRP channel superfamily has 28 members (27 in humans)3. On the basis of sequence homology, the superfamily is divided into six subfamilies: canonical (also known as short TRPs, TRPC1−7), vanilloid (also known as TRP channel subfamily V, TRPV1−6), melastatin (also known as TRP channel subfamily M, TRPM1−8), ankyrin (also known as TRP channel subfamily A, TRPA1), mucolipins (TRPML1−3), and polycystins (also known as polycystic kidney disease 2-like 1 protein (PKD2L1, also termed TRPP3) and polycystin-2 (TRPP2))3,4. Because these subfamilies were created based on sequence homology and not function, members often have little in common. For example, TRPM2 is a redox sensor in macrophages5; TRPM7 provides a major Mg2+ uptake pathway in intestinal epithelial cells6; and TRPM8 detects cold and menthol in sensory neurons7,8, but regulates epithelial growth in response to androgens in the prostate9. Despite the structural similarities shared by these proteins (Fig.1; Box 1), there are enough differences to develop subtype-selective compounds.
Representative structures for each TRP channel subfamily, coloured to highlight common structural features. The S1−S4 (gold) and the S5 and S6 pore domains (cyan) are the only domains common to all subfamilies. The TRPA, TRPV, TRPM, TRPC and TRPN channels have TRP box helices (dark green). The TRPA, TRPC, TRPN and TRPV channels have amino (N)-terminal cytoplasmic ankyrin repeats (violet; the TRPA1 structure is missing about 11 repeats, as indicated by the question mark and violet shapes). The TRPC, TRPM and TRPN channels have a homologous pre-S1 helical linker (lilac) and C-terminal re-entrant loop and rib helix (brown), which is followed by a coiled coil in the TRPC and TRPM channels (blue; TRPA channels also have a carboxy (C)-terminal coiled coil). The melastatin homology regions (MHRs) of TRPM channels are shown in tan, and the NUDIX domain unique to TRPM2 is shown in red. The TRPML and TRPP channels have a homologous lumenal domain (dark grey). The pre-S1 linkers of the TRPV and TRPA channels and the C-terminal region of the TRPV channels (light grey) share no clear homology outside their respective subfamilies. The structures depicted are human TRPA1 (PDB ID 3J9P), human TRPC3 (PDB ID 6CUD), human TRPM2 (PDB ID 6MIX), Drosophila TRPN (NompC; PDB ID 5VKQ), human TRPP2 (PDB ID 5T4D), human TRPML1 (PDB ID 5JW5) and rabbit TRPV2 (PDB ID 6OO3).
Most TRPs have restricted expression patterns, but their varied tissue distribution means that the superfamily affects most cells, tissues and organs of the human body. Overall, the diverse physiological functions and regulatory mechanisms of TRPs affect how they are implicated in disease. These include both genetic and acquired channelopathies, as well as many disorders in which targeting one or more TRP channel could alleviate symptoms or provide therapeutic effects4.
Most TRPs are subjects of intensive drug discovery and development efforts. In this Review, we summarize the crucial advances of the past decade in our understanding of the complex roles that TRPs have in the development and progression of human disease. Whereas our improved understanding of the structures of TRP channels will undoubtedly aid drug discovery (Box 1), the increasingly diverse physiological roles of TRPs pose a serious challenge to drug development. Indeed, it has proved difficult to obtain sufficient specificity for clinically useful intervention without unacceptable side effects. Although developing clinically useful TRP modulator drugs is challenging, the potential rewards are enormous given the pathogenic role of TRPs in chronic pain, neurology, oncology, dermatology, pulmonology, cardiology, urology and rare diseases.
TRP channel biology and pathology
Although all TRP channels are evolutionarily highly conserved, their sensitivity to external stimuli show striking species-related differences. For example, TRPV1 is activated by capsaicin in mammals (it was originally cloned as the ‘capsaicin receptor’)10, but not in birds11. However, changing position 578 in the S4/S5 helix of cTrpv1 from alanine to glutamine renders the chicken receptor capsaicin-sensitive12. TRPV1 also shows distinct, species-dependent, heat-activation thresholds, and so TRPV1 is a noxious heat sensor in some mammals (including humans)13 but not in others (for example, camels that have evolutionarily adapted to desert heat)14. Similarly, the sensitivity of TRPA1 to cold differs between rodents and primates15. These species-dependent differences in channel sensitivity and function should be considered when selecting experimental animal models and interpreting the results.
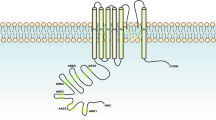
TRPV1 is a prime example of diversity in TRP channel expression and function. TRPV1 is highly expressed on primary sensory neurons as a major integrator of painful stimuli (afferent function) and a key initiator of neurogenic inflammation (efferent function)16 (Fig. 2). Moreover, TRPV1-expressing sensory neurons have been implicated in warmth sensing17,18 and itching19. In the viscera, neuronal TRPV1 triggers reflex pathways like cough, emesis, heart rate, micturition and intestinal peristalsis16. Albeit at much lower levels, TRPV1 is also expressed in various brain nuclei20 and in non-neuronal cells21.
TRPV1 is expressed on primary sensory neurons of the unmyelinated (C fibre) type. Activation of TRPV1 by agonists like capsaicin, heat and protons (and putative ‘endovanilloids’) triggers the release of pro-inflammatory neuropeptides like substance P (SP) and calcitonin gene-related peptide (CGRP), thereby initiating the biochemical cascade known as neurogenic inflammation. At the same time, an impulse is generated that is perceived in the brain as pain or itching. Protein kinase C (PKC) and nerve growth factor (NGF) lower the activation threshold of TRPV1 (sensitization). Stimulation of TRPV1 with a therapeutic dose of capsaicin results in a lasting (up to months) but fully reversible state in which the previously excited neurons remain unresponsive (‘silent’) to further challenge; traditionally, this is called desensitization. High-dose capsaicin patches and site-specific injections relieve pain by this mechanism. There is an ill-defined line between reversible desensitization and irreversible neurotoxicity achieved by therapeutic and supratherapeutic doses of capsaicin, respectively. Although both desensitization and neurotoxicity depend on capsaicin-induced Ca2+ influx through the TRPV1 channel, the downstream molecular mechanisms are still poorly understood. Mitochondrium swelling is an early ultrastructural sign of capsaicin neurotoxicity. Ca2+ is thought to sequester in mitochondria, where it triggers apoptosis using a molecular pathway that includes caspase activation. Intrathecal resiniferatoxin is used as a ‘molecular scalpel’ to achieve permanent analgesia in cancer patients with chronic, intractable pain by ablating sensory neurons. In principle, a similar strategy can be used to kill cancer cells that overexpress TRPV1 compared with normal counterparts. We note that small molecule TRPV1 antagonists prevent only TRPV1 activation by agonists, leaving other pain sensors intact.
TRPV1 is unique among drug targets in that its initial excitation by agonists is followed by a lasting refractory state (traditionally referred to as desensitization) in which TRPV1-expressing neurons are not responsive to both a repeated capsaicin challenge and to various unrelated stimuli16 (Fig. 2).
The role of TRPV1 in thermoregulation is well established22,23. In rodents, activation of TRPV1 with capsaicin initiates heat-loss behaviour at warm ambient temperatures (30–32.5 °C) and mice exhibit ‘red ear’ caused by vasodilation and seek the cool surface of the cage16. By contrast, TRPV1-deficient (Trpv1–/– or capsaicin-desensitized) mice display deficiencies in heat-loss mechanisms (such as body licking) and develop hyperthermia when exposed to 35 °C (ref.24). In humans, capsaicin may cause gustatory sweating as a mechanism of heat loss16, whereas TRPV1 antagonists can increase or decrease body temperature, or even leave it unchanged (‘thermoneutral antagonists’)25,26,27. These thermoregulatory side effects can be exploited for pharmacotherapy: TRPV1 antagonists that cause hyperthermia as a dose-limiting, on-target adverse effect may also help to restore normal body temperature after medical cooling28,29. In turn, TRPV1 agonists that cause hypothermia may protect the brain after stroke30. As reviewed elsewhere16,22,23, there is no consensus in the literature as to the exact anatomic site of the TRPV1-expressing thermoregulatory centre, or the mechanism by which it regulates body temperature.
Cryo-electron microscopy and X-ray crystallography have provided novel insights into TRP channel structure and function and highlighted several druggable sites (Fig. 3). TRPV1 has fourfold symmetry with different pore profiles for ligand-bound structures31, and a vanilloid-binding pocket deep within the membrane bilayer32 (Fig. 3b,f). TRPA1 is a sentinel for electrophilic irritants and has a distinct allosteric nexus where a covalent modification of cysteine residues regulates channel activity33 (Fig. 3c). Indeed, all known synthetic TRPA1 antagonists, such as A-967079, act as negative allosteric modulators (Fig. 3b). This is important because they can block TRPA1 (over)activation, and yet leave some level of physiological activity intact, in contrast to traditional orthosteric antagonists.
Protein colouring is the same as in Fig. 1. a−d | Schematic descriptions of TRPV (a), TRPA1 (b), TRPM (c) and TRPM1 (d) illustrate the general binding site locations identified in structural findings of TRP channels. Similar ligand shapes indicate similar ligand-binding site locations in the respective proteins, and example ligands are labelled. Black stars indicate mutations associated with human diseases that are located at the elbow connection between the S4−S5 linker and the S5 helix, and magenta stars in the S6 helix pore gate indicate mutations that lead to hypersensitive or constitutively active channels and gain-of-function phenotypes. Grey stars mark the TRPM2 D543E hypoactive mutation. e | Sample ligand-binding sites from the antagonist capsazepine bound to rat TRPV1 (PDB ID 5IS0). f | Co-agonists PIP2, Ca2+ and icilin bound to Ficedula albicollis TRPM8 (PDB ID 6NR3). g | Agonist ML-SA1 bound to human TRPML1 (PDB ID 6E7Z).
Epigenetic regulation of TRPs is an emerging area of research. In rats, histone H3 acetylation at the Trpv1 promoter region leads to increased TRPV1 protein expression in sensory neurons with concomitant visceral hyperalgesia34. In mice, SUMOylation protects TRPV1 from metabolic damage during experimental diabetes and thus delays the development of neuropathic pain35. TRPV1 from human donor tissue is also SUMOylated35. Likewise, methylation of the human TRPA1 promoter region can result in increased TRPA1 levels and altered pain perception36. Indeed, TRPA1 gene methylation is dysregulated in patients with Crohn disease, contributing to visceral pain37.
Genetic defects in TRPs — or TRP channelopathies — are increasingly recognized causes of hereditary human disease4. For example, TRPV4 channelopathy38 is linked to at least nine different diseases, ranging from autosomal dominant brachyolmia type 3 to Charcot–Marie–Tooth neuropathy type 2. Furthermore, polymorphisms in TRP genes may regulate disease risk, as exemplified by the reduced migraine incidence in carriers of rs10166942, which correlates to reduced TRPM8 gene expression39.
Pain: TRP channels as analgesic targets
Medical control of chronic pain is frequently unsatisfactory, and the current therapeutic pain market remains dominated by agents that have been around for decades. Narcotics (opioids) are effective painkillers, but, acting in the brain, they are also addictive. A logical strategy to avoid opioid side effects is to target the beginning of the pain pathway: the nociceptor where pain is generated25,40. Hence there is tremendous interest expressed by pharmaceutical companies in TRP channels that detect noxious stimuli in the periphery (Fig. 4).
Several TRP channels in free nerve endings or sensory corpuscles of sensory neurons transduce painful thermal (TRPV1, TRPA1, TRPM8 and TRPM3), chemical (TRPA1) and mechanical (TRPC1, TRPC3, TRPC6, TRPA1, TRPV2 and TRPV4) stimuli into electrophysiological excitation. TRP channels that do not participate in physiological sensation, but are recruited under pathophysiological conditions (for example, TRPC5 in mechanosensation) are of particular interest. TRP channels in keratinocytes, satellite glia and Schwann cells (TRPV1, TRPA1) also participate in transduction of painful stimuli. TRP channels in the central terminals of sensory neurons amplify and modulate neurotransmitter release in the spinal cord. TRP channels in the brain play critical parts in several physiological processes. It is likely that different TRP channels in the brain have different roles in acute nociceptive versus maladaptive chronic pain. Pain representation in the brain shifts from from nociceptive circuits in acute pain (left brain scan) to emotional circuits during chronic pain in functional magnetic resonance imaging (middle and right brain scans). Therefore, an assessment of central action or peripheral restriction of a TRP channel modulator needs to be carefully performed to maximize efficacy and therapeutic window.
The ‘capsaicin receptor’, TRPV1
Desensitization to capsaicin has a clear therapeutic potential (Fig. 2). Indeed, high-dose capsaicin patches41,42 and site-specific injections43 are clinically proven to provide meaningful pain relief in patients with osteoarthritis, post-herpetic neuralgia and diabetic polyneuropathy. Peripheral expression of TRPV1 on nociceptive fibres is predictive of the patient’s response to capsaicin therapy, which may explain the discrepant outcome in clinical trials with capsaicin in patients with diabetic polyneuropathy in whom nociceptive fibres often degenerate during advanced disease44.
Capsaicin evokes an intense initial pain reaction that limits the dose that patients can tolerate16. To reduce this adverse effect, ‘non-pungent’ TRPV1 agonists like olvanil (NE19550)45 and MRD-652 (ref.46) have been developed that differ from capsaicin in the activation kinetics of the receptor. These compounds showed promise in animal models of pain45,46, but their clinical value remains to be demonstrated.
The ultrapotent capsaicin analogue, resiniferatoxin, is undergoing clinical trials as a ‘molecular scalpel’ with which to achieve permanent analgesia in severe osteoarthritic pain47,48, and in cancer patients with chronic intractable pain49. This approach has already succeeded in the veterinary clinic, in which intrathecal resiniferatoxin provided lasting pain relief and restored ambulation in dogs with osteosarcoma50. Resiniferatoxin has been tested in a small number of female patients with cervical cancer metastatic to pelvic bone, and the results are promising so far51.
Because TRPV1 agonists that cause desensitization can be perceived as functional antagonists of the receptor (Fig. 2), it has been postulated that small molecule TRPV1 antagonists can be also pursued as therapeutic agents25,52,53. Indeed, TRPV1 is a highly druggable target52. Various antagonist chemotypes have been discovered, and many have matured into clinical lead molecules25,52,53. The efficacy of TRPV1 antagonists in preclinical pain models varied; some showed efficacy in both inflammatory (for example, complete Freund adjuvant-induced arthritic pain) and neuropathic pain models (such as the Chung model), whereas others were active only in the inflammation models25,53. We note that both the magnitude of the analgesic effect and the dose needed to demonstrate efficacy varied.
When using hypothermia by capsaicin as an on-target engagement model, many TRPV1 antagonists actually caused the opposite effect, hyperthermia25. However, this febrile reaction could be managed with simple antipyretic agents like acetaminophen and disappeared upon repeated dosing, paving the way to studies in human volunteers54. Since Trpv1–/– mice are less responsive to noxious heat55,56, and rodents desensitized to capsaicin show dramatically increased noxious heat threshold in the hot plate test16, it was unsurprising that TRPV1 antagonists impaired the cutaneous noxious heat sensation in humans, leading to burn injuries as a common adverse effect53.
Owing to these unacceptable on-target adverse effects, many first-generation TRPV1 antagonists were withdrawn from clinical trials: some, like AMG517, because of febrile reactions57 and others, like MK2295, because of the burn injuries58. Some antagonists that progressed into phase II efficacy trials failed to demonstrate analgesic activity, such as a terminated trial of AZD1386 for osteoarthritic pain59. Other clinical trials were terminated without explanation, such as those sponsored by Sanofi/Glenmark (GRC-6211) and PharmEste (PHE575). Since functional TRPV1 expression on nociceptors predicts the patient’s response to agonist (capsaicin) treatment42, one may argue that TRPV1 antagonist trial participants should also be selected on the basis of their capsaicin sensitivity. It is also worth mentioning that vitamin D was recently shown to act as a partial agonist of TRPV1 at physiologically relevant free plasma concentrations60. A partial agonist can either act as an agonist or antagonist, depending on the presence of other ligands. Such effects may contribute to the variability of TRPV1 antagonists in clinical trials.
Although these problems tempered expectations, and many companies abandoned TRPV1 as an analgesic target, there have been a few promising developments. For example, the second-generation molecule, JNJ-39439335 (mavatrep), showed significant improvement versus placebo in stair climbing-induced clinical pain in participants with knee osteoarthritis61. Another compound, NEO6860, also showed an analgesic trend, although it did not statistically outperform placebo, without affecting body temperature or heat pain perception62. It remains to be seen whether these molecules — or other ‘thermoneutral’ antagonists, like GRTE16523 (ref.63) — can demonstrate meaningful analgesic activity in the clinic. Ultimately, it will probably be easier to dissociate beneficial heat sensing from therapeutic inhibition of TRPV1 activity by targeted antagonist delivery because most, if not all, inhibitors of TRPV1 activation will inhibit heat sensing.
The role of TRPV1 phosphorylation by protein kinase-C (PKC) in the development of inflammatory hyperalgesia is well established64. Eliminating the PKC phosphorylation site S801 by CRISPR/Cas9 editing in TRPV1 reduced the ongoing pain caused by masseter muscle inflammation without blocking physiological TRPV1 functions65. This observation raises the possibility of engineering novel TRPV1 antagonists that selectively interact at the phosphorylated TRPV1.
As to future clinical testing, a promising indication for TRPV1 analgesia is gout. In experimental animals, urate crystals activate TRPV1, and the resultant pain is blocked by both genetic inactivation (Trpv1–/– mice) and pharmacological blockade (SB-366791)66. In a mouse model of gout arthritis, eucalyptol reduced both inflammation and pain by preventing the urate-induced up-regulation of TRPV1 expression in ankle tissues67. Furthermore, SB-366791 attenuates dental pain in rats68, and, as an added benefit, prevents alveolar bone loss in a rat model of periodontal disease69. Other potential indications with increased TRPV1 mRNA levels include endometriosis70 and chronic lower back pain71. However, because much of the earlier preclinical efficacy data that predicted clinical value in inflammatory pain (such as osteoarthritic pain) did not translate in patients, caution is advised for these new potential indications.
The chemical nocisensor, TRPA1
TRPA1 gene variants have been linked to paradoxical heat (painful cold) sensation72, sickle cell pain crisis73, carbamazepine-responsive cramp-fasciculation syndrome74, and familial episodic pain syndrome (FEPS)75. FEPS is a gain-of-function TRPA1 channelopathy that causes debilitating upper body pain due to increased inward currents in response to TRPA1 channel activation at normal resting potentials. From a clinical perspective, this observation is not easy to interpret as patients carry both mutant (human TRPA1 N855S) and wild-type alleles. TRPA1 is a tetramer and it is reasonable to assume that patients express various mixtures of normal and mutant channels76. Although one cannot predict the properties of these heteromultimers, the N855S mutation, like the analogous gain-of-function mutations in TRPV4 and TRPM4, is in the linker that moves during channel gating (Fig. 3b−d), explaining how it causes channels to open more readily. An antagonist that selectively blocks the mutant FEPS protein would be optimal, but companies are unlikely to invest resources into such a small market.
Genetic deletion or pharmacological blockade of TRPA1 vastly attenuate responses to many harmful chemical stimuli, ranging from formaldehyde, through acrolein (present in cigarette smoke) and diesel exhaust, to tear gases77,78. Moreover, endogenous compounds like methylglyoxal79,80 (a product of aberrant glucose metabolism) and reactive oxygen species (ROS) and nitrogen species all converge on TRPA1 (ref.81): these electrophilic compounds can activate the channel after covalently modifying a hypersensitive cysteine in the cytoplasmic domain of TRPA1 by electrophilic attack82 (Fig. 3c).
The temperature (cold) sensitivity of TRPA1 is subject to much debate and will not be discussed here, except to mention that Trpa1–/– mice show attenuated cold allodynia evoked by anticancer drugs like oxaliplatin83, and by ischaemia and reperfusion injury of the rodent hindlimb, a murine model of complex regional pain syndrome type I (CRPS-I)84. Importantly, TRPA1 antagonists recapitulated the effects of genetic Trpa1 inactivation84, indicating a novel therapeutic strategy in CRPS-I patients. Trpa1–/– mice also displayed reduced visceromotor responses to colorectal distension85, indicating a role for TRPA1 in mechanical pain; and Trpa1–/– rats showed decreased pain behaviours in response to chemical agonists of TRPA1, but normal responses to other pain stimuli, including cold, and itching86. Animal studies with TRPA1 antagonists (such as A-967079, HC-030031 and AMG0902) suggested therapeutic potential in patients with neuropathic pain87.
We note that CHEM-5861528, when given with streptozotocin to rats, ameliorated the pain and reduced the loss of intraepidermal nerve fibres88. If these results were to be confirmed in diabetic patients, this observation suggests that TRPA1 antagonists can be disease-modifying by preventing (or at least delaying) the development of diabetic polyneuropathy.
As of today, only one TRPA1 antagonist has completed phase II clinical trials, Glenmark’s GRC1753689. Although GRC17536 significantly reduced pain scores in the non-denervation group of patients with painful diabetic polyneuropathy without worrisome side effects, the compound had problems with bioavailability/pharmacokinetics and did not progress into phase III. Topical administration may help overcome such problems. Indeed, a topical HC-030031 gel (0.05%) reversed mechanical and cold allodynia in mice after ultraviolet B-induced burn injury90. This implies a therapeutic value for TRPA1 antagonist creams for patients with sunburn pain or thermal injury.
Interestingly, TRPA1 inhibition produces analgesia against modalities that are not mediated by TRPA1-expressing neurons91. This behaviour is not unprecedented, and has been observed in other TRP receptors, such as TRPV1. Although mechanosensitive nerves do not express TRPV1, resiniferatoxin desensitizes capsaicin-sensitive afferents, resulting in ameliorated mechanical hyperalgesia (and, paradoxically, cold hyperalgesia) in a murine model of arthritic pain92. The molecular underpinnings of this phenomenon are not clear. One possibility is that TRPV1 expression is plastic and nerve fibres that do not express TRPV1 under normal conditions might do so under pathological conditions, such as pain93. Similar considerations may also apply to TRPA1.
The journey of TRPA1 antagonists as pain therapeutics to the clinic was given new impetus by the recent purchase by Eli Lilly of the Hydra Biosciences molecules94. As of today, TRPA1 small molecule patents have been filed by Ajinomoto Co., Algomedix Inc, Almirall S.A., Boehringer Ingelheim, EA Pharm Co., Eli Lilly, Galderma, Genentech/Roche, Glenmark, Mandom Corp and Orion Corp95.
The ‘cold and menthol receptor’, TRPM8
According to a new model of sensory coding, there are two distinct neuronal populations involved in warmth perception: one population is excited by warmth, whereas the other is blocked by it18. This latter population expresses TRPM8 and displays ongoing cool-driven firing18. Accordingly, Trpm8–/– mice are incapable of distinguishing warm from cold, and show markedly attenuated cold allodynia96,97. Moreover, in humans, reduced TRPM8 gene expression is associated with a reduced risk for migraine, and reduced sensitivity to cold and cold pain39. These observations imply a therapeutic potential for small molecule TRPM8 antagonists in the management of cold-induced pain and migraine98.
Potent and selective TRPM8 antagonists with acceptable clinical safety profiles have been discovered98, many of which bind competitively to the menthol or icilin binding site in the S1−S4 sensor domain (Fig. 3g). Accordingly, these antagonists demonstrated a clear-cut exposure−efficacy relationship in preclinical models, including icilin-induced wet dog shakes in rats, cold pressor test in rodents, and menthol challenge in guinea pigs; these findings predict target engagement for therapeutic dose in humans98.
Interestingly, like TRPV1, TRPM8 displayed a basal activation tone99. TRPM8 antagonists evoked a mild hypothermic response, but this was not considered a hurdle to clinical use100. Some TRPM8 antagonists (such as AMG-333 and PF-05105679)100,101, have already progressed into clinical studies, in which they reduced pain in the cold pressor test. Although PF-05105679 did not cause perceptible hypothermia, some volunteers reported a ‘hot feeling’ in the perioral area that was deemed to be non-tolerable by two study subjects100. AMG-333 also caused a few grade 1 adverse effects related to TRPM8 antagonism101.
Although most of the attention was focused on antagonists, TRPM8 agonists (such as WS-12 and di-isopropyl-phosphinoyl-alkane, DIPA) also have an analgesic potential. For instance, DIPA was shown to reduce spontaneous painful contractions in the human distal colon102.
Fading and emerging TRP targets for pain relief
The classical pain targets of TRPV1, TRPA1 and TRPM8 are all expressed in nociceptors25,40. The expression pattern of novel TRP targets for pain relief is more diverse, ranging from sensory neurons (TRPM3) through brain nuclei involved in pain processing (TRPC4 and TRPC5) to the epithelium (TRPV3 and TRPV4) and immune cells (TRPM2).
Gain-of-function mutations in TRPV3 were discovered in patients with Olmstedt disease103,104, erythromelalgia104, and painful plantar keratoderma105. As with TRPA1 above, many of these TRPV3 gain-of-function mutations are at the elbow connecting the S4−S5 linker and the S5 helix (Fig. 3b). Interest in TRPV3 as an analgesic target was first raised by the observation that tissue damage upregulates TRPV3 expression in human sensory ganglia or skin106. Several companies developed potent and selective TRPV3 antagonists107. In 2014, the Sanofi-Aventis/Glenmark compound GRC15300 failed a phase II trial in patients with peripheral neuropathy, and the programme was terminated.
Genetic deletion or knockdown of Trpv4 in mice leads to attenuated pain behaviour in various preclinical models. According to these studies, TRPV4 may play a pivotal part in visceral pain108. An interesting approach is to use TRPA1/TRPV4 dual inhibitors for chemotherapy-associated neuropathic pain109.
The molecular mechanisms that underlie the transition from acute to chronic neuropathic pain are largely unknown, hampering drug development. In this transition, TRPV1, TRPA1 and TRPM2 are emerging as important players110,111. For instance, acute pancreatitis has a large component of neurogenic inflammation that can be attenuated by TRPV1 and TRPA1 antagonists112,113. These antagonists also prevented the spouting of pancreatic sensory nerve fibres during the acute pancreatitis attacks, and thereby averted the development of chronic neuropathic pain. As for TRPM2, it is highly expressed in immune cells — including macrophages and microglia — that contribute to inflammatory and neuropathic pain114. In a murine model of neuropathic pain, Trpm2 knockdown prevented the development of pain-related behaviour following chronic constriction injury114.
TRPM3 has a central role in noxious heat sensing115, making the ‘triad of TRPV1/TRPA1/TRPM3’ a potential multireceptor pain target116. The TRPM3 agonist CIM0216 induced heat hypersensitivity in wild-type mice but not in Trpm3–/– mice117. Conversely, TRPM3 antagonists reduced pain responses in several protocols in preclinical studies118,119,120; again, these results were validated in Trpm3–/– animals117.
TRPC4 and TRPC5 are non-selective cation channels that can form homomers and heteromers and are expressed mostly in the amygdala and hippocampus (Fig. 4), but also in peripheral sensory neurons both in rodents121 and humans122. Studies on Trpc4–/– rats showed tolerance to visceral pain responses, whereas somatic pain responses were unaffected123. Furthermore, the non-selective TRPC4/TRPC5 antagonist 4-methyl-2-(1-piperidinyl)quinoline (ML-204) inhibited visceral pain responses in wild-type rats124, confirming the role of TRPC4 in visceral pain. Local application of ML-204 to amygdala attenuated neuropathic pain behaviour in rats with spared nerve injury124. Genetic inactivation (Trpc5–/– mice) or pharmacological blockade of TRPC5 by the small molecule antagonist AC1903 prevented the development of mechanical hypersensitivity and persistent spontaneous or tactile pain in a wide range of murine pain models, including that induced by skin incision, chemotherapy and complete Freund adjuvant injection122. We note that these pain conditions are associated with elevated lysophosphatidylcholine (LPC) levels, and exogenous LPS triggers TRPC5-dependent pain behaviour in naive mice122; LPS also causes intensive itching during cholestasis both in rodents and nonhuman primates by activating TRPV4 in skin keratinocytes125. Because TRPC5 also regulates prolactin release126, TRPC5 antagonists may have enhanced analgesic efficacy in females given that prolactin promotes pain only in that sex. These findings suggest that centrally acting TRPC4 and TRPC5 antagonists could relieve visceral and neuropathic pain, or at least make it more tolerable127.
Respiratory disease
TRP channels are attractive targets for the treatment of respiratory diseases128 given their widespread expression in the lung, both in immune and structural cells, and their central role in evoking respiratory symptoms like bronchospasm and cough.
TRPV1 in respiratory disease
Inhaled capsaicin evokes coughing in both guinea pigs and humans by activating TRPV1 on C-fibre afferents129. Indeed, capsaicin-containing sprays and gas canisters are used by individuals for personal protection and by law enforcement for crowd control16. Increased cough reflex sensitivity to inhaled capsaicin has been observed in patients with asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and chronic idiopathic cough. These findings suggested that TRPV1 is a credible target to treat both chronic idiopathic cough and cough from inflammatory lung disease130. The increased TRPV1 activity may be explained by elevated levels of inflammatory mediators (such as prostaglandin E2, neurotrophins and bradykinin) in the airways of patients with asthma and with COPD. These endogenous substances activate airway sensory nerves, causing coughing131. Moreover, neurons undergo phenotypical changes in respiratory diseases both in experimental animals and human challenge models. For instance, in guinea pigs and rats challenged with ovalbumin, or in guinea pigs treated with neurotrophins, the number of TRPV1-positive neurons (particularly of the nodose-originating Aδ subtype) increases, suggesting that changes in neural pathways influenced by their local environment may result in exaggerated functional responses132,133. Moreover, TRPV1 single nucleotide polymorphisms (SNPs) have been associated with coughing, suggesting that TRPV1 variants may enhance susceptibility to coughing in smokers and in subjects with a history of workplace exposure134.
Clinical studies tested the postulated causal role of TRPV1 in patients with chronic idiopathic cough and in chronic cough associated with COPD in patient groups with increased cough sensitivity to capsaicin. In patients with chronic idiopathic cough, the TRPV1 antagonist SB-705498 caused a small but statistically significant inhibition of capsaicin-evoked coughing, but no effect on spontaneous coughing frequency135. In subsequent studies, XEN-D0501 (a TRPV1 antagonist with superior efficacy and potency) did not improve spontaneous cough frequency in patients with refractory coughing136, nor did it affect spontaneous coughing in patients with COPD137, which effectively rules out TRPV1 as a relevant therapeutic target for chronic cough in these patient groups, but not in other respiratory diseases associated with exaggerated coughing.
There is widespread non-neuronal TRPV1 expression in the lung, in both structural (such as fibroblast) and immune cells (such as alveolar macrophages)138. For example, TRPV1 was detected in human bronchial epithelial cells, with increased expression in patients with refractory asthma139. In bronchial epithelium, TRPV1 activation mediates mucin secretion induced by acid and particules140, as well as the release of cytokines141 and ATP142. In a preclinical model of cigarette smoke exposure used to mimic the inflammatory response seen in COPD, the TRPV1 inhibitor JNJ17203212 reduced cigarette smoke-induced ATP release from human bronchial epithelial cells142. Furthermore, bronchiolar lavage fluid collected from Trpv1–/– mice following cigarette smoke exposure had diminished ATP release and neutrophilia compared with that of wild-type mice142. Conversely, whole-lung homogenates from patients with COPD showed increased TRPV1 mRNA expression compared with samples from smokers without COPD and healthy non-smokers, suggesting an association between TRPV1 expression and disease pathophysiology142.
The role of TRPV1 in asthma is controversial; some studies show no impact whereas others show protection through TRPV1 inhibition by antagonists or genetic inactivation. For instance, in rodent ovalbumin challenge models, TRPV1 blockade improved standard end points, including eosinophilia, airway hyperresponsiveness and the late asthmatic response143. Furthermore, TRPV1 inhibition reduced the interleukin (IL)-13-driven asthma phenotype in mice, and blocked airway hyperresponsiveness to histamine in guinea pigs following ovalbumin exposure144. Similarly, data from a murine house-dust mite model suggested that TRPV1, but not TRPA1, inhibition reduced airway cellular inflammation and airway hyperresponsiveness143. Although many of the effects seen in this study were not statistically significant, there was a consistent suppression across the functional end points measured, suggesting a role for TRPV1 in CD4+-dependent allergic asthma models.
In summary, the reasons for the observed differences in studies investigating a role for TRPV1 in allergic asthma are unclear, but could be due to differences in species, strains, antigens and interventions used to dissect TRPV1 biology.
TRPA1 antagonists
TRPV1 and TRPA1 are often co-expressed in sensory neurons that innervate the airways, but they are also found separately128,138,145. TRPA1 agonists (like cinnamaldehyde) evoke human vagus nerve depolarization and induce coughing in both guinea pigs and human volunteers146. Conversely, TRPA1 antagonists (such as GRC-17536 and HC-030031) are potential anti-tussive agents147,148. In the lung, TRPA1 is also expressed in bronchial epithelial cells and fibroblasts149.
TRPA1 is an interesting respiratory target because it is activated by known lung irritants including natural products, such as allyl isothiocyanate, allicin and cannabinol150, found in mustard oil, garlic and cannabis, and by environmental irritants, such as acrolein151, that are present in air pollution and cigarette smoke152. These electrophilic agonists covalently attach to a hypersensitive cysteine in the cytoplasmic domain of TRPA1 (ref.82) (Fig. 3c). TRPA1 is also activated by reactive and electrophilic by-products of oxidative stress (such as ROS), and electrophiles like hypochlorite and hydrogen peroxide81,153. Diesel exhaust particles can also activate airway C-fibre afferents via TRPA1 (ref.154). These particles contain polycyclic aromatic hydrocarbons that stimulate mitochondrial ROS production by interacting at the aryl hydrocarbon receptor. This observation links diesel exposure to respiratory symptoms154.
In addition to environmental irritants, TRPA1 is also activated indirectly by inflammatory mediators151 (such as bradykinin, PGE2 and prostaglandin D2), which are elevated in the bronchoalveolar lavage fluid of patients with asthma and COPD155. We note that the exhaled breath of patients with inflammatory airway disease is more acidic than that of healthy volunteers156. This is interesting because inhalation of low pH solutions, such as citric acid, causes coughing in both guinea pigs and humans. In fact, inhaled citric acid is used in the clinic to evaluate cough reflex sensitivity. Citric acid was thought to evoke cough by virtue of its low pH and subsequent activation of TRPV1 and acid-sensing ion channels (ASIC)157,158. However, TRPA1 antagonists (such as GRC-17536) also inhibited citric acid-induced coughing in conscious guinea pig models147. It is not yet known whether the low pH component of citric acid or the resulting increased osmolarity is responsible for activating TRPA1 (or TRPV1) to evoke coughing.
TRPA1 has been implicated in the pathophysiology of allergic asthma159,160. In allergic individuals, exposure to relevant antigens can lead to an early asthmatic response followed, in certain patients, by a corticosteroid-sensitive, late asthmatic response. Despite its widespread use in the clinical assessment of new therapeutic entities, the mechanisms underlying late asthmatic response remain unclear. Following allergen challenge, activation of TRPA1 stimulates vagal broncho-pulmonary C-fibres and this induces late asthmatic response in the Brown Norway rat asthma model159. The mechanism of action is unclear, but the allergen probably stimulates the channel indirectly via the release of endogenous TRPA1 activators, possibly mast cell products like tryptase.
TRPA1 may also have a key role in the airway hyperresponsiveness characteristic of asthma. Indeed, the TRPA1 antagonist, HC-030031, reverses airway hyperresponsiveness induced by acetylcholine in an ovalbumin mouse model159. Toluene di-isocyanate (TDI), a reactive compound used in the manufacture of polymeric derivatives and known to activate TRPA1 (ref.161), can also evoke respiratory symptoms, including late asthmatic response, in exposed workers162. Non-allergic airway hyperresponsiveness can be induced by a single exposure of hypochlorite (a known TRPA1 agonist) in ovalbumin-exposed wild-type mice but not in Trpa1–/– mice163. Ovalbumin-challenged wild-type rats showed signs of airway inflammation86, whereas Trpa1–/– rats did not, which again suggests that TRPA1 inhibition has therapeutic potential for asthma.
Lower respiratory tract infections are a leading cause of death in adults and pneumonia is the single largest cause of death in children164. Coughing is the main method of spreading bacteria from a human host into the environment. However, the mechanisms of coughing in patients with lower respiratory tract infections are unknown. We note that lipopolysaccharide, which is found in the outer membrane of Gram-negative bacteria, has been identified as a TRPA1 activator that exerts fast excitatory actions via TRPA1 independent of Toll-like receptor-4 (TLR4) activation165. This finding implicates TRPA1 as a driver of respiratory symptoms following bacterial infections and warrants further investigation.
Although associations have been found between TRPA1 SNPs and susceptibility to airway disease166, the evidence linking TRPA1 dysfunction to respiratory disease pathophysiology is still rudimentary. Drug discovery efforts have been hampered by the limited bioavailability of TRPA1 antagonists167,168. Recently, a potent, selective and orally bioavailable small molecule TRPA1 antagonist, GDC-0334, with good target engagement in human volunteers, has been reported169. In preclinical models of respiratory disease, GDC-0334 inhibited cough response, airway smooth muscle hyperreactivity and oedema formation in several species169. It is hoped that clinical studies with GDC-0332 (or other compounds with good bioavailability) will answer the question of whether blocking TRPA1 in respiratory disorders has therapeutic promise168.
TRPV4 antagonists
TRPV4 is expressed in a wide range of non-neuronal cell types in human airways, including airway and vascular smooth muscle, epithelial cells, fibroblasts and inflammatory cells (such as macrophages and neutrophils)4. Multiple endogenous pro-inflammatory and environmental stimuli act in concert to activate TRPV4 (ref.4). Accordingly, TRPV4 activation is implicated in the pathophysiology of chronic lung diseases170,171,172.
TRPV4 was referred to as ‘the gatekeeper of pulmonary capillary permeability’. Its contribution to respiratory disease is probably best understood in the development of acute lung injury173. TRPV4 is involved in disrupting the epithelial and endothelial barrier function173, which suggests an important role in lung oedema formation associated with inflammation and tissue injury174. In the rodent lung, TRPV4 blockade, or knockdown, inhibited ventilator- and acid-induced lung injury, by significantly reducing the infiltration of inflammatory cells (including neutrophils and macrophages) and lung injury scores175,176. These data suggest a broad-spectrum inhibition of acute lung injury regardless of causality. Indeed, TRPV4 inhibitors are developed by the National Institute of Health (NIH) as countermeasures against chemical threats177.
High pulmonary venous pressure is a major cause of heart failure. TRPV4 antagonists inhibit pulmonary oedema associated with heart failure in animal models178, paving the way towards clinical trials. On the basis of these observations, calls have been made to evaluate the effect of TRPV4 inhibitors in COVID-19 patients at risk of lung oedema179 (Box 2).
TRPV4 is also implicated in chronic respiratory diseases such as asthma, COPD and chronic refractory cough170,171,172. Its role in diverse pathologies across respiratory disease has been explained by TRPV4-induced ATP release in a pannexin 1-dependent manner with resultant activation of purinoceptors180,181. Consistent with this hypothesis, TRPV4-induced ATP release was demonstrated in human airway epithelial cells142,182 and smooth muscle cells181. TRPV4 and purinoreceptor P2X3 antagonists were shown to inhibit Aδ sensory afferent nerve fibre activation and coughing induced by TRPV4 agonists or hypo-osmolar solutions (known to activate TRPV4)183. This study identified the TRPV4−ATP−P2X3 axis as a driver of airway sensory nerve reflexes such as coughing, and clinical trials with P2X3 receptor antagonists, such as AF-219, would support the role of ATP as a driver of the cough reflex183.
In the context of asthma, TRPV4 agonists can induce a mast cell-dependent, contractile response in human bronchial and guinea pig tracheal airway smooth muscle in vitro. This effect is attributed to TRPV4-induced ATP release from airway smooth muscle181, which activates P2X4 receptors on mast cells to release cysteinyl leukotrienes (Cys LT); this, in turn, activates the Cys LT-1 receptor to evoke contraction184.
COPD is an inflammatory lung disease associated with cigarette smoking. Smoke exposure was reported to cause a dose-dependent increase in ATP release — which may drive the airway inflammation — from primary human bronchial epithelial cells, and this effect was attenuated by blockers of TRPV1, TRPV4 and pannexin-1 channels142. Interestingly, lung tissue from patients with COPD shows an increase in TRPV4 mRNA expression compared with healthy smokers and non-smokers142.
Genetic variants of TRPV4 have been associated with asthma185 and COPD186. For example, the loss-of-function variant, TRPV4-P19S, was linked to childhood asthma185, and a genome-wide association study highlighted seven TRPV4 SNPs that conferred increased susceptibility to COPD186. It was postulated that TRPV4 modulators could influence COPD pathophysiology, but this hypothesis has yet to be tested.
TRPV4 activity is upregulated in lung fibroblasts derived from patients with IPF187. In IPF models, Trpv4–/– mice are protected from fibrosis187. TRPV4 modulates transforming growth factor (TGF)-β1-dependent actions in a SMAD-independent manner with enhanced actomyosin remodelling and increased nuclear translocation of the α-smooth muscle actin-transcription co-activator, myocardin-related transcription factor (MRTF)-A188,189. These data point to TRPV4 inhibition as a potential therapeutic approach in pulmonary fibrosis189. Interestingly, like asthma and COPD, increased amounts of ATP have been detected in the airways of patients with IPF190, but currently there is no evidence linking the TRPV4/pannexin/ATP axis to IPF.
TRPM8
TRPM8 is a cold-responsive receptor7,8 that, in principle, may mediate coughing and bronchoconstriction caused by inhalation of cold air. The role of TRPM8 activation in cough is, however, controversial because menthol, a TRPM8 agonist, paradoxically inhibits citric acid-induced cough in both guinea pigs191 and humans192, and causes a temporary decrease in capsaicin-evoked cough in healthy individuals193. Indeed, menthol inhibits sensory nerve activation and is used as an over-the-counter antitussive medication. Menthol was — and in many countries still is — often added to cigarettes to inhibit irritancy in the airways, which may promote nicotine addiction. Therefore, menthol is now banned from cigarettes in the USA.
There is still a big question mark over whether the beneficial effects of menthol in the lung are mediated via activation of TRPM8. This is likely to be confounded by the lack of selective tools. For example, in addition to their effects on TRPM8, both menthol and icilin activate and then block TRPA1 (ref.194) (Fig. 3c,d,g). Several recently developed TRPM8 inhibitors should help to elucidate the role of this channel in respiratory disease biology and pathophysiology.
The genito-urinary system
In the neurogenic bladder, the TRPV1-expressing C fibre-driven micturition reflex, which is inactive under physiological conditions, resumes control of the bladder functions16. Intravesical administration of a large enough dose of TRPV1 agonist (capsaicin195 or resiniferatoxin196) to desensitize the C-fibres provides lasting relief in patients with neurogenic bladder by increasing bladder capacity and reducing the number of incontinent episodes197. Furthermore, in bladder biopsy samples taken from patients with interstitial cystitis, the density of TRPV1-expressing fibres correlated with clinical symptoms198. Yet, in a phase II clinical trial intravesical resiniferatoxin failed to meet the primary end point (symptom improvement according to the Global Response Assessment, a 7-point scale rating overall change in symptoms), faring no better than placebo after 4 weeks199. Therefore, it is likely that the increased TRPV1 expression in the bladder biopsy samples was not the cause but a consequence of the disease. Parenthetically, an interesting (and controversial) indication of topical resiniferatoxin desensitization is its application to the penis to prevent premature ejaculation200.
Animal experiments suggested a therapeutic value for TRPV1 (GRC-6211)201 and TRPM8 (RQ-00434739 (ref.202) and KRP-2529 (ref.203)) antagonists for suppressing hyperactivity in the chronically inflamed bladder, which is yet to be tested in the clinic.
In the bladder, TRPV4 is expressed both in urothelium and in the detrusor muscle, where its activation causes sustained muscle contractions204. This is consistent with the spotty incontinence phenotype of the Trpv4–/– mice205. By contrast, the TRPV4 agonist GSK1016790A evokes bladder contractions206. Of relevance, TRPV4 expression is increased in human overactive bladder mucosa207. These and other preclinical findings imply that a TRPV4 agonist may improve the underactive bladder208, whereas an antagonist could be valuable in managing the overactive bladder209.
The TRPC5 antagonist AC-1903 prevented podocyte loss in a rodent model of focal segmental glomerulosclerosis, suggesting a novel interaction for renal allograft protection210. TRPC6 is highly expressed in the kidney, but the role of TRPC6 in renal pathology is complex, which has hindered drug development. For example, BI-749327, a selective TRPC6 antagonist, ameliorates renal fibrosis and dysfunction211, whereas deletion of Trpc6 worsens glomerular injury in Akita mice212. Accordingly, a loss-of-function TRPC6 variant (G757D) is associated with focal segmental glomerulosclerosis213. These latter observations warrant caution when using TRPC6 blockers in humans.
Dermatology and ophthalmology
In the skin and the eye, TRPs are attractive pharmacological targets because they are amenable to topical therapy, probably reducing the risk of serious adverse effects. TRP channels are broadly expressed in various skin cell types (including keratinocytes, melanocytes, skin appendage cells, nerve endings and immune cells), with functions ranging from skin barrier function and hair growth through wound healing to cutaneous inflammation and itching19,78,214.
TRPA1, TRPV3, TRPV4 and, to a lesser degree, TRPV1 and TRPM8 are all promising targets to relieve itching19. TRPA1 expression is elevated in human psoriatic skin biopsies215. Accordingly, Trpa1–/– mice show reduced scratching behaviour in a murine model of chronic itching, and lack the extensive epidermal hyperplasia prevalent in psoriasis and atopic dermatitis215. Transgenic mice with skin-targeted, gain-of-function Trpv3Gly573Ser (Fig. 3b) exhibit scratching behaviour216. In humans, gain-of-function TRPV3 mutations (G568D and Q580P; Fig. 3b) were described in palmoplantar keratoderma105, and increased TRPV3 expression was reported in post-burn pruritus217. Similarly, TRPV4 is overexpressed in skin biopsy samples from patients with chronic pruritus218, and genetic deletion of Trpv4 ameliorates itching in mouse models of chronic itch219. A TRPM8 agonist (menthoxypropanediol) cream was also reported to relieve human itching220.
The TRPV1 antagonist PAC-14028 (now in phase III clinical trials) improves skin barrier function and relieves pruritus in patients with atopic dermatitis221. Although topical TRPV1 antagonists are generally regarded as harmless, a recent study suggests caution. In TRPV1-Ai32 optogenic mice, cutaneous light stimulation activated TRPV1-expressing neurons with resultant local-type 17 immune response222, in which a cascade of cytokine-mediated events leads to activation of antimicrobial responses in keratinocytes and recruitment of neutrophils to the skin. If this observation holds true in humans, patients treated with topical TRPV1 antagonists may be susceptible to cutaneous fungal and bacterial infections.
TRPA1 is also involved in ultraviolet radiation-induced burn. In mice, topical administration of the TRPA1 antagonist, HC-030031, applied after irradiation blocked the development of mechanical and thermal allodynia223.
TRPV1 and TRPV3 have been implicated in hair growth. For example, Trpv3 null mice have a thick, wavy coat of hair224 whereas mice with constitutively active Trpv3 (the DS-Nh strain) are hairless225. Indeed, TRPV3 activation has been shown to inhibit human hair growth226. Accordingly, topical TRPV3 agonists and antagonists may be beneficial in patients with hirsutism and alopecia, respectively. Nude DS-Nh animals also develop a skin condition similar to human atopic dermatitis227. Furthermore, TRPV3 activation blocks lipogenesis in human sebocytes228, suggesting a therapeutic potential for a TRPV3 antagonist in dry skin dermatoses.
TRPV1 has been implicated in psoriasis229 and TRPV4 was named as a potential target in rosacea230. Recently, gain-of-function TRPM4 mutations (I1033M and I1040T; Fig. 3d) were linked to erythrokeratodermia231. Even if their connection to TRP channels is still weak, all these diseases represent unmet medical needs.
Eye drops containing a TRPM8 agonist help to moisturize the cornea in patients with dry eye disease232. In animal experiments, the TRPV4 antagonist, HC-067047, protected against the fibrosis (stromal opacification) that develops following alkali burn injury233. Last, intraocular TRPV1 antagonist administration has been proposed to relieve allergic conjunctivitis234.
Central nervous system
TRPA1 as a therapeutic target in stroke and in multiple sclerosis
Although Trpa1–/– mice lack any obvious neurological phenotype and there is little, if any, detectable mRNA signal in the central nervous system235, functional TRPA1 seems to be present, albeit at low levels, in the brain: in glial cells (astrocytes and oligodendrocytes)236, in cortical neurons237, in cerebral endothelium238 and in Schwann cells239. This discrepancy between positive function-based TRPA1 results and weak or missing Trpa1 mRNA signals is puzzling and yet to be explained.
The potential therapeutic use of brain-permeable TRPA1 antagonists for neuroprotection has emerged from an elegant electrophysiological study240. White matter is particularly vulnerable to hypoxia associated with stroke. Glutamate release and subsequent activation of N-methyl-d-aspartate (NMDA) receptors in oligodendrocytes is thought to underlie hypoxia-induced white matter injury240. Pharmacological or genetic inactivation of TRPA1 prevented hypoxia from damaging and ultimately killing oligodendrocytes after stroke240. On a related note, TRPV1-mediated hypothermia reduced stroke volume by 50% and promoted functional recovery after ischaemic stroke in mice30.
Oligodendrocytes are also affected in multiple sclerosis. Cuprizone-induced demyelination is widely used as a multiple sclerosis model to assess the potential therapeutic efficacy of novel multiple sclerosis treatments. In this model, pharmacological blockade or genetic deletion of TRPA1 reduced oligodendrocyte apoptosis241. Furthermore, methylglyoxal, a known TRPA1 activator79, accumulated in the white matter lesions of multiple sclerosis242. Dimethyl fumarate, used to treat multiple sclerosis and psoriasis, and known to activate the antioxidant response through Nuclear factor erythroid 2-related factor 2 (NRF2), was found to activate TRPA1 in immune cells independent of NRF2 (ref.243). This novel NRF2-independent mechanism may contribute to the peripherally restricted immunosuppressive action of dimethyl fumarate.
TRPA1 in anxiety, dementia and seizures
Trpa1–/– mice show reduced anxiety-like behaviour, and this beneficial effect can be reproduced with TRPA1 antagonists in healthy, wild-type mice244. The neural circuit responsible for the antidepressant action of TRPA1 antagonism is currently unknown. Importantly, ageing Trpa1–/– mice also exhibited improved memory (fewer reference memory errors), implying a role for TRPA1 in age-related memory decline245. Together, these findings identify TRPA1 as a potential target in the pharmacotherapy of anxiety and dementia, a common combination in the elderly (for TRPA1 in capillary endothelium in the brain, and memory decline in diabetic patients, see Box 2).
However, Trpa1–/– mice also show defects in white matter myelination, with the myelin basic protein level downregulated and the number of mature oligodendrocytes reduced246. According to this observation, TRPA1 may play a critical part in the maturation process of oligodendrocytes, because the myelination process is likely to continue throughout life during new skill learning, and therefore chronic and complete block of TRPA1 in oligodendrocytes may interfere with myelination. However, robust therapeutic efficacy can probably be achieved during intermittent partial pharmacological block of TRPA1 in humans, allowing preservation of the physiological house-keeping function of TRPA1.
TRPA1 in astrocytes may constitutively regulate excitatory neurotransmission in healthy brain237. If confirmed, central TRPA1 inhibition may interfere with memory formation, contradicting the memory phenotype observed in Trpa1–/– mice245. By contrast, a phase I study of a centrally acting TRPA1 antagonist in healthy volunteers revealed no central nervous system toxicity issues at concentrations that were several-fold above the IC50 value247. One potential explanation for this discrepancy is that constitutive TRPA1 activation in brain slice astrocytes is an experimental artefact: large amounts of reactive lipid hydroperoxides and other putative TRPA1 agonists may be leaking from damaged and dead cells.
Hippocampal slices from an Alzheimer disease (AD) mouse model provided compelling evidence that TRPA1 activation by β amyloid contributes to network hyperexcitability, possibly driving silent seizures248. This is important because antiepileptic drug use in AD is associated with increased risk for stroke249. Central TRPA1 antagonism may provide a safe therapy for seizures in AD.
Parenthetically, in the triple-transgenic AD mouse model (3xTg-AD+/+), Trpv1–/– animals had better memory function and lower tau accumulation in the hippocampus compared with Trpv1 wild-type mice250. The challenge, of course, is to deliver TRPA1 and/or TRPV1 antagonists to the central nervous system without causing unacceptable systemic side effects.
TRPM2, TRPM3 and TRPM4: from stroke to bipolar disorder
TRPM2 is a well established redox sensor251 and a potential target in hypoxic-ischaemic brain injury252. TRPM2 is implicated in neuronal death by ROS251,252. Indeed, the TRPM2 antagonist JNJ-28583113 protects cells from oxidative stress-induced death253. In a mouse model of cardiac arrest and cardiopulmonary resuscitation, TRPM2 inhibition improved functional recovery following cerebral ischaemia254. In the hippocampus of patients with major depressive disorder, increased TRPM2 mRNA levels were detected, suggesting TRPM2 as a target for treating depression255. TRPM2 is also a susceptibility gene for bipolar disorder256. Trpm2–/– mice display impaired social cognition, and a subset of bipolar patients possess the hypoactive D543E TRPM2 gene variant256 (Fig. 3d). The connection between TRPM2 and bipolar disorder was further strengthened by the finding that TRPM2 regulates the phosphorylation of glycogen synthase kinase-3 (GSK3), the main target of lithium257.
In the central nervous system, TRPM3 is expressed in both glia and neurons. The discovery that primidone — a drug that has long been approved for the treatment of epilepsy and essential tremor — blocks TRPM3 at concentrations that are achieved in the brain during pharmacotherapy validates TRPM3 as a promising neurological drug target258. One may argue that excessive TRPM3 activation can drive white matter injury and thus a centrally acting TRPM3 antagonist may be disease-modifying by protecting white matter. Most recently, gain-of-function TRPM3 variants (such as V837M; Fig. 3d) have been linked to intellectual disability and epilepsy259, although it is not clear how this observation can be exploited for pharmacotherapy.
TRPM4 is a Ca2+-activated non-selective cation channel in vascular smooth muscle that has a key role in myogenic constriction of cerebral arteries260. TRPM4 is closely associated with the sulfonylurea receptor-1 (SUR1)261, which explains the sensitivity of TRPM4 to the antidiabetic compound glibenclamide, a SUR1 inhibitor. In rodent models, spinal cord injury upregulated TRPM4 during secondary haemorrhage, and the increase in haemorrhage was prevented by genetic silencing of Trpm4 by antisense in rats or in Trpm4–/– mice262. In rats, siRNA-mediated silencing of Trpm4 reduced infarct volume in a permanent stroke model, indicating that TRPM4 inhibition may improve blood–brain barrier integrity after ischaemic stroke reperfusion263. SUR1-TRPM4 forms a heteromer complex with aquaporin-4, which was shown to amplify ion−water osmotic coupling and thereby drive astrocyte swelling in stroke. These observations motivated investigation of the clinically well tolerated glibenclamide as a SUR1-TRPM4 antagonist in stroke patients264. In a small-scale study, glibenclamide was shown to improve outcome234. A phase III clinical trial with intravenous glibenclamide (BIIB093) is currently recruiting stroke patients (NCT02864953)265.
TRPM4 was also shown to mediate axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and such effects could be antagonized by glibenclamide, suggesting that TRPM4 inhibition may be a novel target for multiple sclerosis266.
Inhibition of TRPCs has anxiolytic and antidepressant effects
Behavioural studies with Trpc4–/– and Trpc5–/– mice showed positive results in models that predict anxiolytic and antidepressant action267,268. This effect could be replicated by a small molecule, a TRPC1/TRPC4/TRPC5 pan-inhibitor, HC-070, which implies a therapeutic potential to treat anxiety disorders269. Hydra Biosciences and Boehringer Ingelheim have started a phase I trial with their TRPC4/5 inhibitor270. The Trpc4–/– rats also displayed reduced cocaine self-administration without deficits in learning for natural rewards271. If this observation is confirmed in humans, a TRPC4 antagonist may prove clinically useful in addiction. TRPC5 has also been implicated in oxidative neuronal death, implying a role in neuroprotection in neurodegenerative diseases like Huntington disease272. The recent availability of potent, selective TRPC5 antagonists like NU-6027 and AC-1903 may help test this hypothesis.
TRPML1 agonists for the treatment of neurodegenerative diseases
TRPML1 is a Ca2+-permeable non-selective cation channel expressed in lysosomes273. Loss-of-function TRPML1 mutations cause type-IV mucolipidosis274, a rare genetic disease with predominantly neurological symptoms. Lysosomal Ca2+ signalling by TRPML1 regulates autophagy and lysosomal biogenesis275, and TRPML1 levels in lysosomes are controlled by the transcription factor-EB (TFEB)276. The subcellular localization and activity of TFEB is also regulated by phosphorylation mediated by Mammalian Target of Rapamycin (mTOR)275. The dephosphorylated form of TFEB translocates into the nucleus, where it induces the transcription of target genes, including TRPML1 (ref.275). TFEB has attracted recent attention for its ability to clear pathogenic molecules in mouse models of Parkinson and Alzheimer disease277. In Parkinson disease mouse models, TRPML1 regulates α-synuclein exocytosis in dopaminergic neurons278. On the basis of these observations, a small molecule TRPML1 agonist could be a disease-modifying agent in neurodegenerative diseases. A recent structure of TRPML1 with the agonist ML-SA1 bound under the pore helix279 — analogous to propofol in TRPA1 or cannabidiol (CBD) in TRPV2 — could aid in developing such agonists (Fig. 3e,f). We note that Merck purchased Calporta, a company developing TRPML1 agonists for US$576 million in November 2019 (ref.280).
Cancer, obesity and diabetes
Several TRP channels show altered expression in cancers, but it is unclear whether this is cause or consequence of the disease. The use of altered TRP protein expression for cancer diagnosis and prognostication is beyond the scope of this Review. However, such altered TRP channel expression may constitute a therapeutic target. For example, high-grade astrocytoma shows increased TRPV1 expression compared with normal brain281. High-dose capsaicin administration can kill neurons owing to the Ca2+ overload it causes (Fig. 2), and thus TRPV1 agonists may help to eradicate this brain tumour. Unfortunately, it is unclear how to deliver capsaicin to the brain in doses sufficiently high to kill tumour cells without causing unacceptable side effects. Oesophageal and head-and-neck squamous cell carcinomas also overexpress TRPV1 and TRPA1 (ref.282). These cancers are more promising targets for TRPV1 and/or TRPA1 agonist therapy inasmuch as they are amenable to topical administration.
TRPV1 gene polymorphism has been linked to eating habits in children283. There is anecdotal evidence that dietary capsaicin may help maintain a healthy body weight (‘exercise in a pill’)284. Even if true, it is unclear whether this is an on-target effect of capsaicin on TRPV1, or whether is due to capsaicin-induced changes in the gut microbiota285, as experiments with Trpv1–/– mice provided conflicting reports78.
TRPM5 is a calcium-activated cation channel activated downstream of taste receptors and other chemosensory receptors. Unlike wild-type mice, which overeat chocolate and become obese, Trpm5–/– animals maintain their normal weight when on a carbohydrate-rich diet286. Interestingly, these animals also consume less alcohol287, implying value for a TRPM5 antagonist in obesity and/or alcohol use disorder. By contrast, Trpm8–/– mice are obese owing to daytime hyperphagia288, whereas the TRPM8 agonist, icilin, increases energy expenditure, and reduces body weight, in mice289. These observations identify TRPM8 as another potential target in diet-induced obesity.
Type 2 diabetes mellitus (T2DM) has reached pandemic proportions. To date, no disease-modifying treatment is available for T2DM patients. Accumulating evidence suggests that the sensory nervous system is involved in the progression of T2DM by maintaining a low-grade inflammation via TRPV1 (ref.290). Indeed, oral glucose tolerance and glucose-stimulated insulin secretion were improved by both genetic inactivation (Trpv1−/− mice) and pharmacological blockade of TRPV1 by the small molecule antagonist BCTC291. The TRPV1 antagonist, XEN-D0501, is currently undergoing phase II clinical trials in T2DM patients with good tolerability and safety292. A combined TRPV1 and TRPA1 antagonist may also protect sensory nerves and prevent cognitive decline in T2DM patients (Box 2).
Conclusions
The huge interest of pharmaceutical companies in TRPV1 antagonists was based on two premises: first, the animal models in which capsaicin desensitization inhibited pain dramatically, and second, the belief that expression of TRPV1 was restricted to nociceptive fibres. Unfortunately, neither has turned out to be true. Those initial animal models are now recognized as poor predictors of clinical efficacy, and TRPV1 expression is much broader than initially thought. Despite these disappointments, the TRP family remains an exciting and potentially rewarding group of therapeutic targets for a broad range of diseases. Clearly, the one-size-fits-all approach is not feasible and tests need to be developed to identify patient subgroups that may benefit from the therapy. Current TRP channel-modulator drug discovery efforts are guided by human genetics to allow identification of specific indications and patient groups that could benefit from pharmacotherapy (Box 3). Furthermore, appropriate target engagement and safety biomarker studies should help define clinically meaningful doses and therapeutic windows. Central unwanted side effects may be minimized by using peripherally restricted antibodies, antibody−drug conjugates, and engineered proteins, whereas systemic adverse effects can be avoided by targeted, site-specific therapy (Box 4). We predict that novel treatment modalities, such as engineered proteins, oligonucleotides and gene-based therapies, will be increasingly explored to treat human TRP channel-associated diseases in the future.
References
Cosens, D. J. & Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285–287 (1969). This article represents the birth of TRP channels with the discovery of a mutant fruit fly that responds to sustained light stimulation with a transient current (‘transient receptor potential’) instead of the usual sustained response.
Montell, C. & Rubin, G. M. Molecular characterization of the drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323 (1989).
Wu, L. J., Sweet, T. B. & Clapham, D. E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 62, 381–404 (2010).
Nilius, B. & Szallasi, A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol. Rev. 66, 676–814 (2014).
Kashio, M. et al. Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc. Natl Acad. Sci. USA 109, 6745–6750 (2012).
Mittermeier, L. et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl Acad. Sci. USA 116, 4706–4715 (2019).
McKemy, D. D., Neuhausser, W. M. & Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002).
Peier, A. M. et al. A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002). McKemy et al. (2002) and Peier et al. (2002) describe the discovery of the cold-responsive TRP channel, TRPM8.
Bidaux, G. et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr. Relat. Cancer 12, 367–382 (2005).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997). This article describes the cloning of the long-sought-after capsaicin (vanilloid) receptor, which turned out to be a heat-activated channel.
Jordt, S. E. & Julius, D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108, 421–430 (2002).
Chu, Y., Cohen, B. E. & Chuang, H. H. A single amino acid controls species sensitivity to capsaicin. Sci. Rep. 10, 8038 (2020).
Mishra, S. K., Tisel, S. M., Orestes, P., Bhangoo, S. K. & Hoon, M. A. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 30, 582–593 (2011).
Laursen, W. J., Schneider, E. R., Merriman, D. K., Bagriantsev, S. N. & Gracheva, E. O. Low-cost functional plasticity of TRPV1 supports heat tolerance in squirrels and camels. Proc. Natl Acad. Sci. USA 113, 11342–11347 (2016).
Laursen, W. J., Anderson, E. O., Hoffstaetter, L. J., Bagriantsev, S. N. & Gracheva, E. O. Species-specific temperature sensitivity of TRPA1. Temperature 11, 214–226 (2015).
Szallasi, A. & Blumberg, P. M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 51, 160–211 (1999).
Yarmolinsky, D. A. et al. Coding and plasticity in the mammalian thermosensory system. Neuron 92, 1079–1092 (2016).
Paricio-Montesinos, R. et al. The sensory coding of warm perception. Neuron 106, 830–841 (2020). This paper identifies two distinct neuronal populations that signal ‘warm’: warmth perception excites one population and, conversely, suppresses another with ongoing cool-drive firing.
Tóth, B. I., Szallasi, A. & Bíró, T. Transient receptor potential channels and itch: how deep should we scratch? Handb. Exp. Pharmacol. 226, 89–133 (2015).
Mezey, E. et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl Acad. Sci. USA 97, 3655–3660 (2000).
Fernandes, E. S., Fernandes, M. A. & Keeble, J. E. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br. J. Pharmacol. 166, 510–521 (2012).
Romanovsky, A. A. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol. Rev. 61, 228–261 (2009).
Szolcsányi, J. Effect of capsaicin on thermoregulation: an update with new aspects. Temperature 2, 277–296 (2015).
Yonghak, P., Miyata, S. & Kuganov, E. TRPV1 is crucial for thermal homeostasis in the mouse by heat loss behaviors under warm ambient temperature. Sci. Rep. 10, 8799 (2020).
Szallasi, A., Cortright, D. N., Blum, C. A. & Eid, S. R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 6, 357–372 (2007).
Garami, A. et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: insights from mathematical modeling and meta-analysis. Pharmacol. Ther. 208, 107474 (2020).
Garami, A. et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol. 223, e13038 (2018).
Garami, A. et al. Transient receptor potential vanilloid 1 antagonists prevent anesthesia-induced hypothermia and decrease postincisional opioid dose requirements in rodents. Anesthesiology 127, 813–823 (2017).
Catalina Pharma. A pharmacological way to treat perioperative hypothermia https://www.catalinapharma.com (2017).
Cao, Z. et al. TRPV1-mediated pharmacological hypothermia promotes improved functional recovery following ischemic stroke. Sci. Rep. 7, 17685 (2017). This preclinical study suggests that TRPV1 agonist may be beneficial in patients with ischaemic stroke by inducing pharmacological hypothermia.
Benítez-Angeles, M., Morales-Lázaro, S. L., Juárez-González, E. & Rosenbaum, T. TRPV1: structure, endogenous agonists, and mechanisms. Int. J. Mol. Sci. 21, 3421 (2020).
Gao, Y., Cao, E., Julius, D. & Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351 (2016).
Lin King, J. V. et al. A cell-penetrating scorpion toxin enables mode-specific modulation of TRPA1 and pain. Cell 178, 1362–1374 (2020).
Hong, S., Zheng, G. & Wiley, J. W. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology 148, 148–157 (2015).
Agarwal, N. et al. SUMOylation of enzymes and ion channels in sensory neurons protects against metabolic dysfunction, neuropathy, and sensory loss in diabetes. Neuron 107, 1141–1159.e7 (2020).
Bell, J. T. et al. Differential methylation of the TRPA1 promoter in pain sensitivity. Nat. Commun. 5, 2978 (2014). This article demonstrates for the first time that epigenetic regulation of a TRP channel can affect pain response.
Gombert, S. et al. Transient receptor potential ankyrin 1 promoter methylation and peripheral pain sensitivity in Crohn’s disease. Clin. Epigenet. 12, 1 (2019).
White, J. P. M. et al. TRPV4: molecular conductor of a diverse orchestra. Physiol. Rev. 96, 911–973 (2016).
Gavva, N. R. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci. Rep. 9, 19655 (2019). This article reveals TRPM8 to be a promising drug target in migraine.
Patapoutian, A., Tate, S. & Woolf, C. J. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 8, 55–68 (2009).
Noto, C., Pappagallo, M. & Szallasi, A. NGX-4010, a high-concentration capsaicin dermal patch for lasting relief of peripheral neuropathic pain. Curr. Opin. Investig. Drugs 10, 702–710 (2009).
Bonezzi, C. et al. Capsaicin 8% dermal patch in clinical practice: an expert opinion. Exp. Opin. Pharmacother. 21, 1377–1387 (2020).
Chung, M. K. & Campbell, J. N. Use of capsaicin to treat pain: mechanistic and therapeutic considerations. Pharmaceuticals 9, 66 (2016).
Sidenius, P. The axonopathy of diabetic neuropathy. Diabetes 31, 356–363 (1982).
Brand, L. et al. NE-19550: a novel, orally active anti-inflammatory agent. Drugs Exp. Clin. Res. 13, 259–265 (1987).
Ann, J. et al. Discovery of nonpungent transient potential receptor vanilloid 1 (TRPV1) agonist as strong topical analgesic. J. Med. Chem. 63, 418–424 (2020).
Brown, D. C. Resiniferatoxin: the evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals 9, 47 (2016).
US National Library of Medicine. A phase 3 study to evaluate the efficacy and safety of resiniferatoxin for pain due to osteoarthritis of the knee. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04044742 (2019).
US National Library of Medicine. Resiniferatoxin to treat severe pain associated with advanced cancer. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00804154 (2008).
Brown, D. C. et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 103, 1052–1059 (2005). This articles is the first preclinical study that paved the way to clinical trials using intrathecal resiniferatoxin to achieve permanent analgesia in patients with cancer pain.
Heiss, N. et al. A phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer. http://sorrentotherapeutics.com/wp-content/uploads/2013/10/APS-poster-042514-Final.pdf (NIH, 2014).
Appendino, G. & Szallasi, A. Clinically useful vanilloid receptor TRPV1 antagonists: just around the corner (or too early to tell)? Prog. Med. Chem. 44, 145–180 (2006).
Moran, M. M. & Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br. J. Pharmacol. 175, 2185–2203 (2018).
Gavva, N. R. et al. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J. Pharmacol. Exp. Ther. 323, 128–137 (2007).
Caterina, M. J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000).
Davis, J. B. et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 (2000).
Gavva, N. R. et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136, 202–210 (2008).
Eid, S. Therapeutic targeting of TRP channels: the TR(i)P to pain relief. Curr. Top. Med. Chem. 11, 2118–2130 (2011).
Miller, F., Björnsson, M., Svensson, O. & Karlsten, R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp. Clin. Trials 37, 189–199 (2014).
Long, W. et al. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J. Physiol. 598, 4321–4338 (2020).
Manitpisitkul, P. et al. A multiple-dose, double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics, and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep). Scand. J. Pain. 18, 151–164 (2018). This paper reports the first clinical study demonstrating analgesic potential for a TRPV1 antagonist.
Arsenault, P. et al. NEO6860, a modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritic knee pain. Pain. Rep. 3, e696 (2018).
Damann, N. et al. In vitro characterization of the thermoneutral transient receptor potential vanilloid-1 (TRPV1) receptor inhibitor GRTE16523. Eur. J. Pharmacol. 871, 172934 (2020).
Gu, Y., Li, G. & Huang, L.-Y. M. Inflammation induces Epac-protein kinase C alpha and epsilon signaling in TRPV1-mediated hyperalgesia. Pain 159, 2383–2393 (2018).
Joseph, J. et al. Phosphorylation of the TRPV1 S801 contributes to modality-specific hyperalgesia in mice. J. Neurosci. 39, 9954–9966 (2019).
Hoffmeister, C. et al. Participation of TRPV1 receptor in the development of acute gout attacks. Rheumatology 53, 240–249 (2014).
Yin, C. et al. Eucalyptol alleviates inflammation and pain responses in a mouse model of gout arthritis. Br. J. Pharmacol. 177, 2042–2057 (2020).
Urata, K. et al. Involvement of TRPV1 and TRPA1 in incisional intraoral and extraoral pain. J. Dent. Res. 94, 446–454 (2015).
Ossola, C. A. et al. A new target to ameliorate the damage of periodontal disease: the role of transient receptor potential vanilloid type-1 in contrast to that of specific cannabinoid receptors in rats. J. Periodontol. 90, 1325–1335 (2019).
Bohonyi, N. et al. Local upregulation of transient receptor potential ankyrin-1 and transient receptor potential vanilloid-1 channels in rectosigmoid deep infiltrating endometriosis. Mol. Pain. 13, 1744806917705564 (2017).
Ramesh, D., D’Agata, A., Starkweather, A. R. & Young, E. E. Contribution of endocannabinoid gene expression and genotype on low back pain susceptibility and chronicity. Clin. J. Pain. 34, 8–14 (2018).
Kim, H., Mittal, D. P., Iadarola, M. J. & Dionne, R. A. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J. Med. Genet. 43, e40 (2006).
Jhun, E. H. et al. Transient receptor potential polymorphism and haplotype associate with crisis pain in sickle cell disease. Pharmacogenetics 19, 401–411 (2018).
Nirenberg, M. J., Chaouni, R., Biller, T. M., Gilbert, R. M. & Paisán-Ruiz, C. A novel TRPA1 variant is associated with carbamazepine-responsive cramp-fasciculation syndrome. Clin. Genet. 93, 164–168 (2018).
Kremeyer, B. et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66, 671–680 (2010). This article described the first genetic evidence implying a causative role for TRPA1 in human pain.
Naert, R., Talavera, A., Startek, J. B. & Talavera, K. TRPA1 gene variants hurting our feelings. Pflügers Arch. 472, 953–960 (2020).
Bessac, B. F. & Jordt, S.-E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology 23, 360–370 (2008).
Moran, M. M., McAlexander, M. A., Bíró, T. & Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 10, 601–620 (2011).
Eberhardt, M. J. et al. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): a possible mechanism of metabolic neuropathies. J. Biol. Chem. 287, 28291–28306 (2012). This study provides a mechanistic explanation for the neuropathic pain that develops in patients with long-standing diabetes, and pinpoints TRPA1 as a potential preventive target.
Ohkawara, S., Tanaka-Kagawa, T., Furukawa, Y. & Jinno, H. Methylglyoxal activates the human transient receptor potential ankyrin 1 channel. J. Toxicol. Sci. 37, 831–835 (2012).
Shimizu, S., Takahashi, N. & Mori, Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). Handb. Exp. Pharmacol. 223, 767–794 (2014).
Hinman, A., Chuang, H. H., Bautista, D. M. & Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl Acad. Sci. USA 103, 19564–19568 (2006).
Nassini, R. et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 152, 1621–1631 (2011).
De Logu, F. et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav. Immun. 88, 535–546 (2020).
Vermeulen, W. et al. The role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur. J. Pharmacol. 698, 404–412 (2013).
Reese, R. M. et al. Behavioral characterization of CRISPR-generated TRPA1 knockout rat in models of pain, itch, and asthma. Sci. Rep. 10, 979 (2020).
Koivisto, A., Jalava, N., Bratty, R. & Pertovaara, A. TRPA1 antagonists for pain relief. Pharmaceuticals 11, 117 (2018).
Koivisto, A. et al. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol. Res. 65, 149–158 (2012). This preclinical study implies that pharmacological blockade of TRPA1 may protect sensory nerves and prevent the development of peripheral diabetic neuropathy.
A phase 2, 4 week randomized, double-blind, parallel group, placebo controlled proof of concept study to evaluate efficacy, safety and tolerability of GRC 17536 in patients with painful diabetic peripheral neuropathy. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-002320-33/results (2021).
de David Antoniazzi, C. T. et al. Topical treatment with a transient receptor potential ankyrin 1 (TRPA1) antagonist reduced nociception and inflammation in a thermal lesion model in rats. Eur. J. Pharm. Sci. 125, 28–38 (2018).
Petrus, M. et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain. 3, 40 (2007).
Kissin, I., Davison, N. & Bradley, E. L. Jr. Perineural resiniferatoxin prevents hyperalgesia in a rat model of postoperative pain. Anesth. Analg. 100, 774–780 (2005).
Szallasi, A. Vanilloid-sensitive neurons: a fundamental subdivision of the peripheral nervous system. J. Periph. Nerv. Syst. 1, 6–18 (1996).
Lilly. Lilly to acquire pre-clinical pain program from Hydra Biosciences. https://prnewswire.com/news-releases/lilly-to-acquire-pre-clinical-pain-program-from-hydra-biosciences-300765876.html (2018).
Chen, H. & Terrett, J. A. Transient receptor potential ankyrin 1 (TRPA1) antagonists: a patent review (2015-2019). Expert Opin. Ther. Pat. 30, 643–657 (2020).
Bautista, D. M. et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 (2007).
Knowlton, W. M., Bifolck-Fisher, A., Bautista, D. M. & McKemy, D. D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150, 340–350 (2010).
Weyer, A. D. & Lehto, S. G. Development of TRPM8 antagonists to treat chronic pain and migraine. Pharmaceuticals 10, 37 (2017).
Reimúndez, A. et al. Deletion of the cold thermoreceptor TRPM8 increases heat loss and food intake, leading to reduced body temperature and obesity in mice. J. Neurosci. 38, 3643–3656 (2018).
Winchester, W. J. et al. Inhibition of TRPM8 channels reduces pain in the cold pressor test in humans. J. Pharmacol. Exp. Ther. 351, 259–269 (2014).
Horne, D. B. et al. Discovery of TRPM8 antagonist (S)-6-(((3-fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic acid (AMG 333), a clinical candidate for the treatment of migraine. J. Med. Chem. 61, 8186–8201 (2018).
Amato, A., Terzo, S., Lentini, L., Marchesa, P. & Mulé, F. TRPM8 channel activation reduces the spontaneous contractions in distal human colon. Int. J. Mol. Sci. 21, 5403 (2020).
Lin, Z. et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 90, 558–564 (2012). This article reports the identification of a TRPV3 gene defect that causes a human disease.
Duchatelet, S. et al. A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol. 150, 303–306 (2014).
Peters, F., Kopp, J., Fischer, J. & Tantcheva-Poór, I. Mutation in TRPV3 causes painful focal plantar keratoderma. J. Acad. Eur. Dermatol. Venereol. 34, e620–e622 (2020).
Facer, P. et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4, and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 7, 11 (2007).
Broad, L. M. et al. TRPV3 in drug development. Pharmaceuticals 9, 55 (2016).
Cenac, N. et al. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135, 937–946 (2008).
Kanju, P. et al. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci. Rep. 6, 26894 (2016).
Schwatz, E. S. et al. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J. Neurosci. 33, 5603–5611 (2013).
Wang, H., Song, T., Wang, W. & Zhang, Z. TRPM2 participates the transformation of acute pain to chronic pain during injury-induced neuropathic pain. Synapse 73, e22117 (2019).
Wick, E. C. et al. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G959–G969 (2006).
Schwartz, E. S. et al. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic pain in chronic pancreatitis. J. Neurosci. 33, 5603–5611 (2013).
Haraguchi, K. et al. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation pronociceptive inflammatory reponses in mice. J. Neurosci. 32, 3931–3941 (2012).
Vriens, J. et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482–494 (2011).
Vandewauw, I. et al. A TRP channel trio mediates acute noxious heat sensing. Nature 555, 662–666 (2018). This article describes how, in mice, eliminating pain responses to harmful heat requires a triple knockout of the TRPA1, TRPV1 and TRPM3 genes, suggesting a high degree of redundancy; the triple knockout mouse retains noxious cold and mechanical sensing and preference for moderate temperatures.
Su, S., Yudin, Y., Kim, N., Tao, Y.-X. & Rohacs, T. TRPM3 channels play roles in heat hypersensitivity and spontaneous pain after nerve injury. J. Neurosci. 41, 3457–2474 (2021).
Straub, I. et al. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol. Pharmacol. 84, 736–750 (2013).
Jia, S., Zhang, Y. & Yu, J. Antinociceptive effects of isosakuranetin in a rat model of peripheral neuropathy. Pharmacology 100, 201–207 (2017).
Yao, Q., Lin, M.-T., Zhu, Y.-D., Xu, H.-L. & Zhao, Y.-Z. Recent trends in potential therapeutic applications of the dietary flavonoid didymin. Molecules 23, 2547 (2018).
Buniel, M., Wisnoskey, B., Glazebrook, P. A., Schilling, W. P. & Kunze, D. L. Distribution of TRPC channels in the visceral sensory pathway. Novartis Found. Symp. 258, 236–243 (2004).
Sadler, K. E. et al. Transient receptor potential canonical 5 mediates inflammatory mechanical and spontaneous pain in mice. Sci. Transl. Med. 13, eabd7702 (2021). This article reports TRPC5 to be a promising pain target.
Westlund, K. N. et al. A rat knockout model implicates TRPC4 in visceral pain sensation. Neuroscience 262, 165–175 (2014).
Miller, M. et al. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J. Biol. Chem. 286, 33436–33446 (2011).
Chen, Y. et al. Epithelia-sensory neuron cross talk underlies cholestatic itch induced by lysophosphatidylcholine. Gastroenterology 161, 301–317.e16 (2021).
Blum, T. et al. Trpc5 deficiency causes hypoprolactinemia and altered functions of oscillatory dopamine neurons in the arcuate nucleus. Proc. Natl Acad. Sci. USA 116, 15236–15243 (2019).
Wei, H., Sagalajev, B., Yüzer, M. A., Koivisto, A. & Pertovaara, A. Regulation of neuropathic pain behavior by amygdaloid TRPC4/C5 channels. Neurosci. Lett. 608, 12–17 (2015).
Belvisi, M. G. & Birrell, M. A. The emerging role of transient receptor potential channels in chronic lung disease. Eur. Respir. J. 50, 1601357 (2017).
Collier, J. G. & Fuller, R. W. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br. J. Pharmacol. 81, 113–117 (1984).
Belvisi, M. G. et al. Neurophenotypes in airway diseases. Insights from translational cough studies. Am. J. Respir. Crit. Care Med. 193, 1364–1372 (2016).
Grace, M., Birrell, M. A., Dubuis, E., Maher, S. A. & Belvisi, M. G. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67, 891–900 (2012).
Zhang, G., Lin, R.-L., Wiggers, M., Snow, D. M. & Lee, L.-Y. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J. Physiol. 586, 5771–5786 (2008).
Lieu, T. M., Myers, A. C., Meeker, S. & Undem, B. J. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L941–L948 (2012).
Smit, L. A. M. et al. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir. Res. 13, 26 (2012).
Khalid, J. et al. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind, randomized. controlled trial. J. Allergy Clin. Immunol. 134, 56–62 (2014).
Belvisi, M. G. et al. XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am. J. Respir. Crit. Care Med. 196, 1255–1263 (2017).
Smith, J. A. et al. TRPV1 antagonism with XEN-D0501 in chronic obstructive pulmonay disease: translation from pre-clinical model to clinical trial. Am. J. Respir. Crit. Care Med. 195, A6339 (2017).
Grace, M. S., Baxter, M., Dubuis, E., Birrell, M. A. & Belvisi, M. G. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br. J. Pharmacol. 171, 2593–2607 (2014).
McGarvey, L. P. et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J. Allergy Clin. Immunol. 133, 704–712 (2014).
Yu, H., Li, Q., Kolosov, V. P., Perelman, J. M. & Zhou, X. Regulation of particulate matter-induced mucin secretion by transient receptor potential vanilloid 1 receptors. Inflammation 35, 1851–1859 (2012).
Reilly, C. A. et al. Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. J. Biochem. Mol. Toxicol. 19, 266–275 (2005).
Baxter, M. et al. Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung. Thorax 69, 1080–1089 (2014).
Baker, K. et al. Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma. Respir. Res. 17, 67 (2016).
Delescluse, I., Mace, H. & Adcock, J. J. Inhibition of airway hyper-responsiveness by TRPV1 antagonists (SB-705498 and PF-04065463) in the unanesthesized, ovalbumin-sensitized guinea pig. Br. J. Pharmacol. 166, 1822–1832 (2012).
Bessac, B. F. et al. TRPA1 is a major oxygen sensor in murine airway sensory neurons. J. Clin. Invest. 118, 1899–1990 (2008).
Birrell, M. A. et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am. J. Respir. Crit. Care Med. 180, 1042–1047 (2009).
Mukhopadhyay, I. et al. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS ONE 9, e97005 (2014).
Andre, E. et al. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br. J. Pharmacol. 158, 1621–1628 (2009).
Mukhopadhyay, I. et al. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J. Recept. Signal. Tranduct. Res. 31, 350–358 (2011).
Bandell, M. et al. Noxious cold ion channel is activated by pungent compounds and bradykinin. Neuron 41, 848–857 (2004).
Bautista, D. M. et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 (2006).
Andre, E. et al. Cigarette smoke-induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118, 2574–2582 (2008).
Taylor-Clark, T. E. Role of reactive oxygen species and TRP channels in the cough reflex. Cell Calcium 60, 155–162 (2016).
Robinson, R. K. et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J. Allergy Clin. Immunol. 141, 1074–1084 (2018).
Profita, M. et al. Increased prostaglandin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J. Allergy Clin. Immunol. 112, 709–716 (2003).
MacNee, W. et al. Evaluation of exhaled breath condensate pH as a biomarker for COPD. Respir. Med. 105, 1037–1045 (2011).
Tevisani, M. et al. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax 59, 769–772 (2004).
Kollarik, M., Ru, F. & Undem, B. Acid-sensitive vagal sensory pathways and cough. Pulm. Pharmacol. Ther. 20, 402–411 (2007).
Raemdonck, K. et al. A role for sensory nerves in the late asthmatic response. Thorax 67, 19–25 (2012).
Caceres, A. I. et al. A sensory neuronal ion channel essential for airway inflammationand hyperreactivity in asthma. Proc. Natl Acad. Sci. USA 106, 9099–9104 (2009).
Taylor-Clark, T. E., Kiros, F., Carr, M. J. & McAlexander, M. A. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am. J. Respir. Cell. Mol. Biol. 40, 756–762 (2009).
Fabbri, L. M. et al. Prednisone inhibits late asthmatic reactions and the associated increase in airway responsiveness induced by toluene-diisocyanate in sensitized subjects. Am. Rev. Respir. Dis. 132, 1010–1014 (1985).
Hox, V. et al. Crucial role of transient receptor potential ankyrin 1 and mast cells in induction of nonallergic airway hyperreactivity in mice. Am. J. Respir. Crit. Care Med. 187, 486–493 (2013).
WHO. Pneumonia. https://www.who.int/en/news-room/fact-sheets/detail/pneumonia (2019).
Meseguer, V. et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 5, 3125 (2014).
Gallo, V. et al. TRPA1 gene polymorphisms and childhood asthma. Pediatr. Allergy Immunol. 28, 191–198 (2017).
Preti, D., Saponaro, G. & Szallasi, A. Transient receptor potential ankyrin 1 (TRPA1) antagonists. Pharm. Pat. Anal. 4, 75–94 (2015).
Mukhopadhyay, I., Kulkarni, A. & Khairatkar-Joshi, N. Blocking TRPA1 in respiratory disorders: does it hold a promise? Pharmaceuticals 9, 70 (2016).
Balestrini, A. et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 218, e20201637 (2021). This article reports a potent and orally bioavailable TRPA1 antagonist with good target engagement in humans that effectively blocks cough response, airway hyperreactivity and edema formation in preclinical models of asthma.
Dietrich, A. Modulators of transient receptor potential (TRP) channels as therapeutic options in lung disease. Pharmaceuticals 12, 23 (2019).
Scheraga, R. G., Southern, B. D., Grove, L. M. & Olman, M. A. The role of transient receptor potential vanilloid 4 in pulmonary inflammatory diseases. Front. Immunol. 8, 503 (2017).
Grace, M. S., Bonvini, S. J., Belvisi, M. G. & McIntyre, P. Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol. Ther. 177, 9–22 (2017).
Alvarez, D. F. et al. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ. Res. 99, 988–995 (2006). This is one of the first articles in which TRPV4 is revealed as a key player in acute lung injury.
Simonsen, U., Wandall-Frosthom, C., Viguera-Oliván, A. & Köhler, R. Emerging roles of calcium-activated K channels and TRPV4 channels in lung edema and pulmonary circulatory collapse. Acta Physiol. 219, 176–187 (2017).
Hamanaka, K. et al. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L353–L362 (2010).
Balakrishna, S. et al. TRPV4 inhibition counteracts edema and inflammation and imrproves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L158–L172 (2014).
Yeung, D. T., Harper, J. R. & Platoff, G. E.Jr The National Institutes of Health Countermeasures Research Program (NIH CCRP): a collaborative opportunity to develop effective and accessible chemical medical countermeasures for the American people. Drug Dev. Res. 81, 907–910 (2020).
Thorneloe, K. S. et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 4, 159ra148 (2012).
Kuebler, W. M., Jordt, S. E. & Liedtke, W. B. Urgent reconsideration of lung edema as a preventable outcome in COVID-19: inhibition of TRPV4 represents a promising and feasible approach. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L1239–L1243 (2020).
Bonvini, S. J. et al. Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: role of adenosine triphosphate. J. Allergy Clin. Immunol. 138, 249–261 (2016).
Bonvini, S. J. et al. Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. Eur. Respir. J. 56, 1901458 (2020).
Seminario-Vidal, L. et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 286, 26277–26286 (2011).
Abdulqawi, L. et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomized, double-blind, placebo-controlled phase 2 study. Lancet 385, 1198–1205 (2015).
McAlexander, M. A., Luttmann, M. A., Hunsberger, G. E. & Undem, B. J. Transient receptor potential vanilloid 4 activation constricts the human bronchus via the release of cysteinyl leukotrienes. J. Pharmacol. Exp. Ther. 349, 118–125 (2014).
Cantero-Recasens, G. et al. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J. Biol. Chem. 285, 27532–27535 (2010).
Zhu, G. et al. Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum. Mol. Genet. 18, 2053–2062 (2009).
Rahaman, S. O. et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Invest. 124, 5225–5238 (2014).
Al-Azzam, N. et al. Transient receptor vanilloid channel regulates fibroblast differentiation and airway remodelling by modulating redox signals through NADPH oxydase 4. Sci. Rep. 10, 9827 (2020).
Zhan, L. & Li, J. The role of TRPV4 in fibrosis. Gene 642, 1–8 (2018).
Riteau, N. et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 182, 774–783 (2010).
Plevkova, J. et al. The role of trigeminal nasal TRPM8-expressing sensory neurons in the antitussive effects of menthol. J. Appl. Physiol. 115, 268–274 (2013).
Morice, A. H., Marshall, A. E., Higgins, K. S. & Grattan, T. J. Effect of inhaled menthol on citric acid-induced cough in normal subjects. Thorax 49, 1024–1026 (1994).
Wise, P. M., Breslin, P. A. S. & Dalton, P. Sweet taste and menthol increase cough reflex thresholds. Pulm. Pharmacol. Ther. 25, 236–241 (2012).
Karashima, Y. et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 27, 9874–9884 (2007).
Cruz, F. et al. Desensitization of bladder sensory fibers by intravesical capsaicin has long lasting clinical and urodynamic effects in patients with hyperactive or hypersensitive bladder dysfunction. J. Urol. 157, 585–589 (1997).
Silva, C., Rio, M. E. & Cruz, F. Desensitization of bladder sensory fibers by intravesical resiniferatoxin, a capsaicin analog: long-term results for the treatment of detrusor hyperreflexia. Eur. Urol. 38, 444–452 (2000).
Phé, V. et al. Intravesical vanilloids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: a systematic review and meta-analysis. A report from the Neuro-Urology Promotion Committee of the International Continence Society (ICS). Neurourol. Urodyn. 37, 67–82 (2018).
Liu, B. L. et al. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol. Int. 92, 202–208 (2014).
Payne, S. K. et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J. Urol. 173, 1590–1594 (2005).
Shi, B. et al. Resiniferatoxin for the treatment of lifelong premature ejaculation: a preliminary study. Int. J. Urol. 21, 923–926 (2014).
Charrua, A. et al. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J. Urol. 181, 379–386 (2009).
Aizawa, N. et al. RQ-00434739, a novel TRPM8 antagonist, inhibits prostaglandin E2-induced hyperactivity of the primary bladder afferent nerves in rats. Life Sci. 218, 89–95 (2019).
Aizawa, N. et al. KPR-2579, a novel TRPM8 antagonist, inhibits acetic acid-induced bladder afferent hyperactivity in rats. Neurourol. Urodyn. 37, 1633–1640 (2018).
Birder, L. et al. Activation of urothelial transient receptor potential vanilloid 4 by 4α-phorbol 12,13-didecanoate contributes to the altered bladder reflexes in the rat. J. Pharmacol. Exp. Ther. 323, 227–235 (2007).
Gevaert, T. et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 117, 3453–3462 (2007).
Thorneloe, K. S. et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J. Pharmacol. Exp. Ther. 326, 432–442 (2008).
Roberts, M. W. G. et al. TRPV4 receptor as a functional sensory molecule in bladder urothelium: stretch-independent, tissue-specific actions and pathological implications. FASEB J. 34, 263–286 (2020).
Deruyver, Y. et al. Intravesical activation of the cation channel TRPV4 improves bladder function in a rat model for detrusor underactivity. Eur. Urol. 74, 336–345 (2018).
Everaerts, W. et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl Acad. Sci. USA 107, 19084–19089 (2010).
Zhou, Y. et al. A small molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358, 1332–1336 (2017). This study provides a proof-of-principle that chemical inhibition of TRPC5 channel activity can provide a therapeutic benefit in a rodent model of focal segmental glomerulosclerosis (FSGS).
Lin, B. L. et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl Acad. Sci. USA 116, 10156–10161 (2019).
Wang, L., Chang, J. H., Buckley, A. F. & Spurney, R. F. Knockout of TRPC6 promotes insulin resistance and exacerbates glomerular injury in Akita mice. Kidney Int. 95, 321–332 (2019).
Riehle, M. et al. TRPC6 G757D loss-of-function mutation associates with FSGS. J. Am. Soc. Nephrol. 27, 2771–2783 (2016).
Caterina, M. J. & Pang, Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals 9, 77 (2016).
Zhou, Y. et al. Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced psoriasiform dermal inflammation in mice. J. Cell. Mol. Med. 23, 4819–4828 (2019).
Yoshioka, T. et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J. Invest. Dermatol. 129, 714–722 (2009).
Kim, H. O. et al. Increased activity of TRPV3 in keratinocytes in hypertrophic burn scars with postburn pruritus. Wound Repair. Regen. 24, 841–850 (2016).
Luo, J. et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and non-allergic chronic itch. J. Allergy Clin. Immunol. 141, 608–619 (2018).
Akiyama, T. et al. Involvement of TRPV4 in serotonin-evoked scrathcing. J. Invest. Dermatol. 136, 154–160 (2016).
Misery, L. et al. Real-life study of anti-itching effects of a cream containing menthoxypronaediol, a TRPM8 agonist, in atopic dermatitis patients. J. Eur. Acad. Dermatol. Venereol. 33, e67–e69 (2019).
Lee, Y. W. et al. Efficacy and safety of PAC-14028 cream, a novel, topical, non-steroidal, selective TRPV1 antagonist in patients with mild- to moderate atopic dermatitis: a phase IIb randomized trial. Br. J. Dermatol. 180, 1030–1038 (2019).
Cohen, J. A. et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–932 (2019).
Fialho, M. F. P. et al. Topical transient receptor potential ankyrin 1 antagonist treatment attenuates nociception and inflammation in ultraviolet B radiation-induced burn model in mice. J. Dermatol. Sci. 97, 135–142 (2020).
Cheng, X. et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–343 (2010).
Asakawa, M. et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J. Invest. Dermatol. 126, 2664–2672 (2006).
Borbíró, I. et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Invest. Dermatol. 131, 1605–1614 (2011).
Imura, K., Yoshioka, T., Hirasawa, T. & Sakata, T. Role of TRPV3 in immune response to development of dermatitis. J. Inflamm. 6, 17 (2009).
Szántó, M. et al. Activation of TRPV3 inhibits lipogenesis and stimulates production of inflammatory mediators in human sebocytes: a putative contributor to dry skin dermatoses. J. Invest. Dermatol. 139, 250–253 (2019).
Zhou, Y. et al. TRPV1 mediates inflammation and hyperplasia in imiquimod (IMQ)-induced psoriasiform dermatitis (PsD) in mice. J. Dermatol. Sci. 92, 264–271 (2018).
Chen, Y. et al. TRPV4 moves toward centerfold in rosacea pathogenesis. J. Invest. Dermatol. 137, 801–804 (2017).
Wang, H. et al. Gain-of-function mutations in TRPM4 activation gate cause progressive symmetric erythrokeratodermia. J. Invest. Dermatol. 139, 1089–1097 (2019).
Yang, J. M., Wei, E. T., Kim, S. J. & Yoon, K. C. TRPM8 channels and dry eye. Pharmaceuticals 11, 125 (2018).
Okada, Y. et al. Loss of TRPV4 function suppresses inflammatory fibrosis induced by alkali-burning mouse corneas. PLoS ONE 11, e0167200 (2016).
Kwon, J. Y., Lee, H. S. & Joo, C.-K. TRPV1 antagonist suppresses allergic conjunctivitis in a murine model. Ocul. Immunol. Inflamm. 26, 440–448 (2018).
Jang, Y. et al. Quantitative analysis of TRP channel genes in mouse organs. Arch. Pharm. Res. 35, 1823–1830 (2012).
Shigetomi, E., Jackson-Weaver, O., Huckstepp, R. T., O’Dell, T. J. & Khakh, B. S. TRPA1 channels are regulators of astrocyte basal calcium levels and long term potentiation via constitutive D-serine release. J. Neurosci. 33, 10143–10153 (2013).
Kheradpezhouh, E., Tang, M. F., Mattingley, J. B. & Arabzadeh, E. Enhanced sensory coding in mouse vibrissal and visual cortex through TRPA1. Cell Rep. 32, 107935 (2020).
Wagner Pires, P. & Earley, S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. eLife 7, e35316 (2018). This paper shows that the TRPA1 agonist cinnamaldehyde reduced infarct in wild-type mice, whereas Trpa1 deletion in endothelial cells increased cerebral infarcts and eliminated the effects of cinnamaldehyde, revealing the therapeutic potential of TRPA1 activation to reduce ischaemic brain damage.
De Logu, F. et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 8, 1887 (2017).
Hamilton, N. B., Kolodziejczyk, K., Kougioumtzigou, E. & Attwell, D. Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischemia. Nature 529, 523–527 (2016).
Sághy, É. et al. TRPA1 deficiency is protective in cuprizone-induced demyelination — a new target against oligodendrocyte apoptosis. Glia 64, 2166–2180 (2016).
Wetzels, S. et al. Methylglyoxal-derived advanced glycation endproducts accumulate in multiple sclerosis lesions. Front. Immunol. 10, 855 (2019).
Herrmann, A. K. et al. Dimethyl fumarate alters intracellular Ca2+ handling in immune cells by redox-mediated pleiotropic effects. Free Radic. Biol. Med. 141, 338–347 (2019).
Cavalcante de Moura, J. et al. The blockade of transient receptor potential ankyrin 1 (TRPA1) signalling mediates antidepressant and anxiolytic-like actions in mice. Br. J. Pharmacol. 171, 4289–4299 (2014).
Borbély, É., Payrits, M., Hunyady, Á., Mező, G. & Pintér, E. Important regulatory function of transient receptor potential ankyrin-1 receptors in age-related learning and memory alterations in mice. Geroscience 41, 643–654 (2019).
Lee, K. I., Lin, H. C., Lee, H. T., Tsai, F. C. & Lee, T. S. Loss of transient receptor potential ankyrin 1 channel deregulates emotion, learning and memory, cognition, and social behavior in mice. Mol. Neurobiol. 54, 3606–3617 (2017).
US National Library of Medicine. Safety, tolerability, pharmacokinetic and pharmacodynamic effects of ODM-108: in healthy male volunteers (FIMTRIP). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02432664 (2017).
Payrits, M. et al. Genetic deletion of TRPA1 receptor attenuates amyloid beta-1-42 (Aβ1-42)-induced neurotoxicity in the mouse basal forebrain in vivo. Mech. Ageing Dev. 189, 111268 (2020).
Sarycheva, T. et al. Antiepileptic drug use and the risk of stroke among community dwelling people with Alzheimer disease: a matched control study. J. Am. Heart Assoc. 7, e009742 (2018).
Kim, J. et al. Ca2+-permeable TRPV1 pain receptor knockout recuses memory deficits and reduces amyloid-β and tau in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 29, 228–237 (2020).
Sakaguchi, R. & Mori, Y. Transient receptor potential (TRP) channels: biosensors for redox environmental stimuli anc cellular status. Free. Radic. Biol. Med. 146, 36–44 (2020).
Zhan, K.-Y., Yu, P. L., Liu, C.-H., Luo, J. H. & Yang, W. Detrimental or beneficial: the role of TRPM2 in ischemia/reperfusion injury. Acta Pharmacol. Sin. 27, 4–12 (2016).
Fourgeaud, L. et al. Pharmacology of JNJ-28583113: a novel TRPM2 antagonist. Eur. J. Pharmacol. 853, 299–307 (2019).
Dietz, R. M. et al. Reversal of global ischemia-induced cognitive dysfunction by delayed inhibition of TRPM2 ion channels. Transl. Stroke Res. 11, 254–266 (2020).
Ko, S. Y. et al. Transient receptor potential melastatin 2 governs stress-induced depressive-like behaviors. Proc. Natl Acad. Sci. USA 116, 1770–1775 (2019).
Xu, C. et al. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am. J. Med. Genet. B 141B, 36–43 (2006).
Jang, Y. et al. TRPM2, a susceptibility gene for bipolar disorder, regulates glycogen synthase kinase-3 activity in the brain. J. Neurosci. 35, 11811–11823 (2015).
Krügel, U., Straub, I., Beckmann, H. & Schaefer, M. Primidone inhibits TRPM3 and attenuates thermal nociception in vivo. Pain 158, 856–867 (2017). This paper demonstrates that primidone, a drug used to treat essential tremor and seizures, blocks TRPM3 at clinically relevant doses.
Dyment, D. A. et al. De novo substitutions of TRPM3 causes intellectual disability and epilepsy. Eur. J. Hum. Genet. 27, 1611–1618 (2019).
Earley, S., Waldron, B. J. & Brayden, J. E. Critical role of transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ. Res. 95, 922–929 (2004). This is the first paper to demonstrate that TRPM4 regulates constriction of cerebral arteries.
Woo, S. K., Kwon, M. S., Ivanov, A., Gerzanich, V. & Simard, J. M. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (TRPM4) channel. J. Biol. Chem. 288, 3655–3667 (2013).
Gerzanich, V. et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat. Med. 15, 185–191 (2009).
Loh, K. P. et al. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflügers Arch. 466, 563–576 (2014).
Vorasayan, P. et al. Intravenous glibenclamide reduces lesional water uptake in large hemisphere infarction. Stroke 50, 3021–3027 (2019).
US National Library of Medicine. Phase 3 study to evaluate the efficacy and safety of intravenous BIIB093 (Glibenclamide) for severe cerebral edema following large hemispheric infarction (CHARM). ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02864953 (2021).
Schattling, B. et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 18, 1805–1811 (2012).
Riccio, A. et al. Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. J. Neurosci. 34, 3653–3667 (2014).
Riccio, A. et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137, 761–772 (2009).
Just, S. et al. Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS ONE 13, e0191225 (2018). Riccio et al., Just et al. and Boehringer Ingelheim show that, as suggested by gene deletion studies, pharmacological inhibition of TRPC4 and TRPC5 channels is benefical in murine models of anxiety and antidepression.
Boehringer Ingelheim. Hydra Biosciences and Boehringer Ingelheim announce worldwide collaboration to develop small-molecule inhibitors for the treatment of central nervous system diseases and disorders. https://www.boehringer-ingelheim.pt/press-release/hydra-biosciences-and-boehringer-ingelheim-announce-worldwide-collaboration-develop (2021).
Rasmus, K. C., O’Neill, C. E., Bachtell, R. K. & Cooper, D. C. Cocaine self-administration in rats lacking a functional trpc4 gene. F1000Research 2, 110 (2013).
Hong, C. et al. TRPC5 channel instability induced by depalmitoylation protects striatal neurons against oxidative stress in Huntington’s disease. Biochim. Biophys. Acta Mol. Cell. Res. 1867, 118620 (2020).
Zeevi, D. A., Frumkin, A. & Bach, G. TRPML and lysosomal function. Biochim. Biophys. Acta 1772, 851–858 (2007).
Sun, M. et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 9, 2471–2478 (2000).
Wang, W. et al. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl Acad. Sci. USA 112, E1373–E1381 (2015).
Cortes, C. J. & La Spada, A. R. TFEB dysregulation as a driver of autophagy dysfunction in neurodegenerative disease: molecular mechanisms, cellular processes, and emerging therapeutic options. Neurobiol. Dis. 122, 83–93 (2019).
Song, J. X., Liu, J., Jiang, Y., Wang, Z. Y. & Li, M. Transciption factor EB: an emerging drug target for neurodegenerative disorders. Drug Discov. Today 26, 164–172 (2021).
Tsunemi, T. et al. Increased lysosomal exocytosis induced by lysosomal Ca2+ channel agonists protects human dopaminergic neurons from α-synuclein toxicity. J. Neurosci. 39, 5760–5772 (2019).
Schmiege, P., Fine, M. & Li, X. The regulatory mechanism of mammalian TRPMLs revealed by Cryo-EM. FEBS J. 285, 2579–2585 (2018).
Chemical & Engineering News. Merck acquires Calporta Therapeutics for its autophagy-boosting molecules. https://cen.acs.org/business/mergers-&-acquisitions/Merck-acquires-Calporta-Therapeutics-autophagy/97/i45 (2018).
Stock, K. et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat. Med. 18, 1232–1238 (2012).
Kiss, F., Pohóczky, K., Szallasi, A. & Helyesi, Z. Transient receptor potential (TRP) channels in head-and-neck squamous cell carcinomas: diagnostic, prognostic, and therapeutic potentials. Int. J. Mol. Sci. 21, E6374 (2020).
Chamoun, E. et al. The relationship between single nucleotide polymorphisms in taste receptor genes, taste function and dietary intake in pre-school aged children and adults in the Guelph family health study. Nutrients 10, 990 (2018).
Bray, M. Using capsaicin to lose weight: how it works. https://pepperscale.com/capsaicin-to-lose-weight/ (2019).
Wang, Y., Tang, C., Tang, Y., Yin, H. & Liu, X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr. Res. 64, https://doi.org/10.29219/fnr.v64.3525 (2020).
Larsson, M. H., Håkansson, P., Jansen, F. P., Magnell, K. & Brodin, P. Ablation of TRPM5 in mice results in reduced body weight gain and improved glucose tolerance and protects from excessive consumption of sweet palatable food when fed high caloric diets. PLoS ONE 10, e0138373 (2015).
Blednov, Y. A. et al. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes. Brain Behav. 7, 1–13 (2008).
Reimúndez, A. et al. Deletion of the cold thermoreceptor TRPM8 increases heat loss and food intake leading to reduced body temperature and obesity in mice. J. Neurosci. 38, 3643–3656 (2018).
Clemmensen, C. et al. Coordinated targeting of cold and nicotinic receptors synergistically improves obesity and type 2 diabetes. Nat. Commun. 9, 4304 (2018).
Gram, D. X., Holst, J. J. & Szallasi, A. TRPV1: a potential therapeutic target in type 2 diabetes and comorbidities? Trends Mol. Med. 23, 1002–1013 (2017).
Gram, D. X. et al. TRPV1 antagonists as novel anti-diabetic agents: regulation of oral glucose tolerance and insulin secretion through reduction of low-grade inflammation? Med. Sci. 7, 82 (2019).
European Medicines Agency. A randomised, double-blind, placebo-controlled, parallel-group trial investigating the effect of 4 weeks bi-daily dosing of XEN-D0501 on blood glucose reduction as add-on to metformin in patients with diabetes type 2. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-001880-22/LT (2021).
Gaudet, R. A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 4, 372–379 (2008).
Huang, Y., Fliegert, R., Guse, A. H., Lu, W. & Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 85, 102111 (2020).
Li, J. et al. The structure of TRPC ion channels. Cell Calcium 80, 25–28 (2019).
Jin, P. et al. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547, 118–122 (2017).
Nahama, A., Ramachandran, R., Cisternas, A. F. & Ji, H. The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: a review. Med. Drug Discov. 5, 100033 (2020).
Chao, Y.-K., Chang, S.-Y. & Grimm, C. Endo-lysosomal cation channels and infectious diseases. Rev. Physiol. Biochem. Pharmacol. https://doi.org/10.1007/112_2020_31 (2020).
Thakore, P. et al. Brain endothelial TRPA1 channels initiate neurovascular coupling. Preprint at bioRxiv https://doi.org/10.1101/2020.09.14.295600 (2020).
Tarantini, S. et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J. Cereb. Blood Flow. Metab. 35, 1871–1881 (2015).
Diogo, D. et al. Phenome-wide association studies across large population cohorts support drug target validation. Nat. Commun. 9, 4285 (2018).
Oh, S. J. et al. Ultrasonic neuromodulation via astrocytic TRPA1. Curr. Biol. 29, 3386–3401 (2019).
Rezayat, E. & Toostani, I. G. Review paper: a review on brain stimulation using low-intensity focused ultrasound. Basic Clin. Neurosci. 7, 187–194 (2016).
Huffer, K. E., Aleksandrova, A. A., Jara-Oseguera, A., Forrest, L. R. & Swartz, K. J. Global alignment and assessment of TRP transmembrane domain structures to explore functional mechanisms. Preprint at bioRxiv https://doi.org/10.1101/2020.05.14.096792v1 (2020). A recent comprehensive analysis of the structural similarities and differences of the transmembrane regions of TRP channels that reveals hot spots for interactions with modulatory chemical agents, including natural agonists, antagonists, tool compounds and drugs.
Acknowledgements
We thank N. Gavva (Takeda Pharma) for useful comments. This work was funded in part by NIH grant 1R21DC018497 (to R.G.).
Author information
Authors and Affiliations
Contributions
All authors wrote the article. A.S. outlined the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Drug Discovery thanks Thomas Voets, Boyi Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Coincidence detector
-
A process by which a neuron can detect and converge separate signals into one input (such as an action potential).
- Gustatory sweating
-
Perspiration in the head-and-neck area after eating hot spicy food.
- Allosteric nexus
-
A substructure in a protein that serves as a shared regulatory site, binding to chemical ligands or responding to physiological stimuli, and resulting in changes in the shape and activity of the protein.
- SUMOylation
-
Covalent modification by the small ubiquitin-related modifier peptide.
- Brachyolmia type 3
-
A form of severe skeletal dysplasia characterized by an abnormal curve of the spine (kyphoscoliosis) and flattened cervical vertebrae.
- Charcot−Marie−Tooth neuropathy type 2
-
A genetic defect that causes decreased heat, cold and touch sensations mostly in the hands and feet owing to axon damage.
- Freund adjuvant-induced arthritic pain
-
A frequently used rodent model for screening analgesics against inflammatory pain induced by local injection of dead mycobacteria.
- Chung model
-
A frequently used model for analgesic screening against neuropathic pain induced by unilateral spinal nerve ligation.
- CRISPR/Cas9 editing
-
Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) is a technology that allows genetic material to be added, removed or altered by creating a ‘guide’ RNA to target specific DNA sequences.
- Carbamazepine-responsive cramp-fasciculation syndrome
-
Peripheral nerve hyperexcitability syndrome that presents with stiffness, muscle pain, cramps and exercise intolerance.
- Familial episodic pain syndrome
-
(FEPS). Rare genetic peripheral neuropathy disorder characterized by recurrent, intense upper body or lower limb pain in response to fatigue, fasting, physical stress or cold exposure.
- Cold allodynia
-
Paradoxical burning sensation when exposed to a cold surface.
- Complex regional pain syndrome type I
-
Also known as reflex sympathetic dystrophy, this syndrome presents as continuous pain and sudomotor activity that is disproportionate to the initiating event.
- Wet dog shakes
-
Rapid and alternating head rotation in rats, an animal model used to quantify central 5-HT2A activity.
- Cold pressor test
-
Assessment of autonomic nervous system function, pain threshold and pain tolerance.
- Olmsted disease
-
Also known as mutilating palmoplantar keratoderma with periorificial keratotic plaques, this is a rare congenital disorder caused by abnormal growth of the skin; it is associated with itching, pain, skin fissures and skin cancers.
- Erythromelalgia
-
Also known as Mitchell disease, this is intense, burning pain (algia) associated with redness (erythro) that primarily affects the feet (mel).
- Painful plantar keratoderma
-
Painful, symmetric callus formation on the pressure points of the soles.
- SMAD
-
Downstream signal transducer for the receptors of the transforming growth factor β (TGFβ) superfamily.
- Neurogenic bladder
-
Urinary condition caused by impaired neuronal control (for example, by spinal cord injury or multiple sclerosis) of bladder function.
- Interstitial cystitis
-
Poorly understood clinical condition that predominantly affects young women and causes recurrent pain in the pelvic region associated with problems with urination.
- Akita mice
-
Genetic mouse model of type 1 diabetes.
- Optogenic mice
-
Genetically modified mice expressing light-sensitive ion channels in neurons; light is used to control neuronal function in vivo.
- Psoriasis
-
Disease of the skin that causes red, flaky, crusty plaques.
- Rosacea
-
A common skin condition that causes redness and visible blood vessels in the face; it may also affect the nose (rhinophyma) and the eyes (dry, irritated, swollen eyes).
- Erythrokeratodermia
-
A group of keratinization disorders that manifest in erythema (redness) and hyperkeratosis (scaling); most cases are indolent with no effect on general health.
- Multiple sclerosis
-
A neurological disease in which inflammation is thought to drive disease progress.
- Phenome-wide association study
-
A study designed to test for associations between a specific genetic variant (such as single nucleotide polymorphism, SNP) and a wide range of phenotypes or disease risks in a large cohort of individuals.
- Lithium
-
Mainstay pharmacotherapy for bipolar disorder for acute mood episodes, switch prophylaxis and suicide prevention.
- Lysozomes
-
Intracellular organelles with a key role in cellular waste handling and recycling.
- Mucolipidosis type 4
-
An autosomal recessive lysosomal storage disorder causing delayed mental and motor development and vision impairment that worsens with time; patients present with intellectual disability (absent speech), difficulty swallowing and weak muscle tone.
- Non-invasive, low-intensity, low-frequency ultrasound
-
(LILFU). A promising transcranial approach to stimulating brain pathways.
Rights and permissions
About this article
Cite this article
Koivisto, AP., Belvisi, M.G., Gaudet, R. et al. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat Rev Drug Discov 21, 41–59 (2022). https://doi.org/10.1038/s41573-021-00268-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-021-00268-4
This article is cited by
-
Matrix stiffness affects tumor-associated macrophage functional polarization and its potential in tumor therapy
Journal of Translational Medicine (2024)
-
Recent research advances in pain mechanisms in McCune–Albright syndrome thinking about the pain mechanism of FD/MAS
Journal of Orthopaedic Surgery and Research (2024)
-
TRPM channels in health and disease
Nature Reviews Nephrology (2024)
-
TRPM8 inhibits substance P release from primary sensory neurons via PKA/GSK-3beta to protect colonic epithelium in colitis
Cell Death & Disease (2024)
-
TRPM5 activation depends on a synergistic effect of calcium and PKC phosphorylation
Communications Biology (2024)