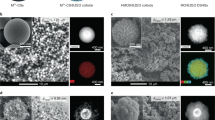
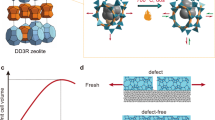
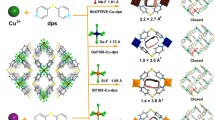
Abstract
Zeolites are a family of microporous crystalline materials, which, since the 1940s, have had an indispensable role in the chemical industry as catalysts, adsorbents and ion exchangers. Advances in synthetic methodologies and characterization techniques have enabled the fabrication of new zeolitic materials, with emerging applications in diverse areas. By tuning their porous architectures, framework compositions and crystal morphologies, coupled with the incorporation of exotic active species, zeolites and zeolite-based materials have exhibited unprecedentedly high performance in many challenging processes. In this Review, we focus on the high-efficiency catalytic production of industrially important hydrocarbons and oxygenates using non-petrochemical feedstocks, energy-efficient separations of hydrocarbon mixtures that are difficult using conventional methods and materials, and host–guest assemblies that exhibit physical properties unprecedented to either the zeolite hosts or free guest species. Finally, we provide our perspectives on future directions for the development of zeolitic materials to meet the ever-growing demands from diverse fields.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Breck, D. W. Zeolite Molecular Sieves: Structure, Chemistry, and Use (Wiley, 1973).
Xu, R., Pang, W., Yu, J., Huo, Q. & Chen, J. Chemistry of Zeolites and Related Porous Materials (Wiley, 2007).
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002).
McCusker, L. B., Liebau, F. & Engelhardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts (IUPAC Recommendations 2001). Pure Appl. Chem. 73, 381–394 (2001).
Li, Y. & Yu, J. New stories of zeolite structures: their descriptions, determinations, predictions, and evaluations. Chem. Rev. 114, 7268–7316 (2014).
Barrer, R. M. 435. Syntheses and reactions of mordenite. J. Chem. Soc. https://doi.org/10.1039/JR9480002158 (1948).
Breck, D. W. Crystalline zeolite Y. US Patent 3130007 (1964).
Argaucer, R. J. & Landolt, G. R. Crystalline zeolite ZSM-5 and method of preparing the same. US Patent 3702886 (1972).
Flanigen, E. M. et al. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature 271, 512–516 (1978).
Wilson, S. T., Lok, B. M., Messina, C. A., Cannan, T. R. & Flanigen, E. M. Aluminophosphate molecular sieves: a new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 104, 1146–1147 (1982).
Karge, H. G. & Weitkamp, J. Adsorption and Diffusion (Springer, 2008).
Kaneko, K. & Rodríguez-Reinoso, F. Nanoporous Materials for Gas Storage (Springer, 2019).
Moliner, M., Martínez, C. & Corma, A. Synthesis strategies for preparing useful small pore zeolites and zeotypes for gas separations and catalysis. Chem. Mater. 26, 246–258 (2014).
Rangnekar, N., Mittal, N., Elyassi, B., Caro, J. & Tsapatsis, M. Zeolite membranes – a review and comparison with MOFs. Chem. Soc. Rev. 44, 7128–7154 (2015).
Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 97, 2373–2420 (1997).
Čejka, J., Corma, A. & Zones, S. Zeolites and Catalysis: Synthesis, Reactions and Applications (Wiley, 2010).
Niwa, M., Katada, N. & Okumura, K. Characterization and Design of Zeolite Catalysts: Solid Acidity, Shape Selectivity and Loading Properties (Springer, 2010).
Martínez, C. & Corma, A. Inorganic molecular sieves: preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 255, 1558–1580 (2011).
Moliner, M., Martínez, C. & Corma, A. Multipore zeolites: synthesis and catalytic applications. Angew. Chem. Int. Ed. 54, 3560–3579 (2015).
Hagen, J. Industrial Catalysis: A Practical Approach (Wiley, 2015).
Chai, Y. et al. Nobel metal particles confined in zeolites: synthesis, characterization, and applications. Adv. Sci. 6, 1900299 (2019).
Li, Y., Cao, H. & Yu, J. Toward a new era of designed synthesis of nanoporous zeolitic materials. ACS Nano 12, 4096–4104 (2018).
Wang, N., Sun, Q. & Yu, J. Ultrasmall metal nanoparticles confined within crystalline nanoporous materials: a fascinating class of nanocatalysts. Adv. Mater. 31, 1803966 (2019).
Bai, R., Song, Y., Li, Y. & Yu, J. Creating hierarchical pores in zeolite catalysts. Trends Chem. 1, 601–611 (2019).
Li, Y., Li, L. & Yu, J. Applications of zeolites in sustainable chemistry. Chem 3, 928–949 (2017).
Zhang, Q., Yu, J. & Corma, A. Applications of zeolites to C1 chemistry: recent advances, challenges, and opportunities. Adv. Mater. 32, 2002927 (2020).
Yarulina, I., Chowdhury, A. D., Meirer, F., Weckhuysen, B. M. & Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 1, 398–411 (2018).
Ruddy, D. A. et al. Methanol to high-octane gasoline within a market-responsive biorefinery concept enabled by catalysis. Nat. Catal. 2, 632–640 (2019).
Snyder, B. E. R. et al. The active site of low-temperature methane hydroxylation in iron-containing zeolites. Nature 536, 317–321 (2016).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197 (2020).
Shan, J., Li, M., Allard, L. F., Lee, S. & Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 551, 605–608 (2017).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Bereciartua, P. J. et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 358, 1068–1071 (2017).
Jeon, M. Y. et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 543, 690–694 (2017).
Kumar, P. et al. One-dimensional intergrowths in two-dimensional zeolite nanosheets and their effect on ultra-selective transport. Nat. Mater. 19, 443–449 (2020).
Li, H. et al. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 367, 667–671 (2020).
Fenwick, O. et al. Tuning the energetics and tailoring the optical properties of silver clusters confined in zeolites. Nat. Mater. 15, 1017–1022 (2016).
Grandjean, D. et al. Origin of the bright photoluminescence of few-atom silver clusters confined in LTA zeolites. Science 361, 686–690 (2018).
Liu, J. et al. Carbon dots in zeolites: a new class of thermally activated delayed fluorescence materials with ultralong lifetimes. Sci. Adv. 3, e1603171 (2017).
Farrusseng, D. & Tuel, A. in Encapsulated Catalysts 335–386 (Elsevier, 2017).
Zhang, J. et al. Sinter-resistant metal nanoparticle catalysts achieved by immobilization within zeolite crystals via seed-directed growth. Nat. Catal. 1, 540–546 (2018).
Wu, S.-M., Yang, X.-Y. & Janiak, C. Confinement effects in zeolite-confined noble metals. Angew. Chem. Int. Ed. 58, 12340–12354 (2019).
Cheng, W.-C. et al. in Handbook of Heterogeneous Catalysis (eds Ertl, G., Knözinger, H., Schüth, F. & Weitkamp, J.) 2741–2778 (Wiley, 2008).
Friedel, R. A. & Anderson, R. B. Composition of synthetic liquid fuels. I. Product distribution and analysis of C5—C8 paraffin isomers from cobalt catalyst. J. Am. Chem. Soc. 72, 1212–1215 (1950).
Li, J. et al. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nat. Catal. 1, 787–793 (2018).
Ahn, J. H., Temel, B. & Iglesia, E. Selective homologation routes to 2,2,3-trimethylbutane on solid acids. Angew. Chem. Int. Ed. 48, 3814–3816 (2009).
Hazari, N., Iglesia, E., Labinger, J. A. & Simonetti, D. A. Selective homogeneous and heterogeneous catalytic conversion of methanol/dimethyl ether to triptane. Acc. Chem. Res. 45, 653–662 (2012).
Huber, G. W., Iborra, S. & Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 (2006).
Khodakov, A. Y., Chu, W. & Fongarland, P. Advances in the development of novel cobalt Fischer–Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 107, 1692–1744 (2007).
Wei, J. et al. Directly converting CO2 into a gasoline fuel. Nat. Commun. 8, 15174 (2017).
Gao, P. et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017).
Chang, C. D. & Lang, W. H. Process for manufacturing olefins. US Patent 4025576 (1977).
Yang, M., Fan, D., Wei, Y., Tian, P. & Liu, Z. Recent progress in methanol-to-olefins (MTO) catalysts. Adv. Mater. 31, 1902181 (2019).
Sun, Q., Xie, Z. & Yu, J. The state-of-the-art synthetic strategies for SAPO-34 zeolite catalysts in methanol-to-olefin conversion. Natl Sci. Rev. 5, 542–558 (2018).
Ferri, P. et al. Impact of zeolite framework composition and flexibility on methanol-to-olefins selectivity: confinement or diffusion? Angew. Chem. Int. Ed. 59, 19708–19715 (2020).
Olsbye, U. et al. Conversion of methanol to hydrocarbons: how zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 51, 5810–5831 (2012).
Gallego, E. M. et al. “Ab initio” synthesis of zeolites for preestablished catalytic reactions. Science 355, 1051–1054 (2017).
Li, C. et al. Synthesis of reaction-adapted zeolites as methanol-to-olefins catalysts with mimics of reaction intermediates as organic structure-directing agents. Nat. Catal. 1, 547–554 (2018).
Yarulina, I. et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 10, 804–812 (2018).
Torres Galvis, H. M. & de Jong, K. P. Catalysts for production of lower olefins from synthesis gas: a review. ACS Catal. 3, 2130–2149 (2013).
Jiao, F. et al. Selective conversion of syngas to light olefins. Science 351, 1065–1068 (2016).
Cheng, K. et al. Direct and highly selective conversion of synthesis gas into lower olefins: design of a bifunctional catalyst combining methanol synthesis and carbon–carbon coupling. Angew. Chem. Int. Ed. 55, 4725–4728 (2016).
Su, J. et al. Syngas to light olefins conversion with high olefin/paraffin ratio using ZnCrOx/AlPO-18 bifunctional catalysts. Nat. Commun. 10, 1297 (2019).
Batamack, P. T. D., Mathew, T. & Prakash, G. K. S. One-pot conversion of methane to light olefins or higher hydrocarbons through H-SAPO-34-catalyzed in situ halogenation. J. Am. Chem. Soc. 139, 18078–18083 (2017).
Chai, Y. et al. Acetylene-selective hydrogenation catalyzed by cationic nickel confined in zeolite. J. Am. Chem. Soc. 141, 9920–9927 (2019).
Zhou, X. et al. Observation of an oxonium ion intermediate in ethanol dehydration to ethene on zeolite. Nat. Commun. 10, 1961 (2019).
Zhao, P. et al. Entrapped single tungstate site in zeolite for cooperative catalysis of olefin metathesis with brønsted acid site. J. Am. Chem. Soc. 140, 6661–6667 (2018).
Wang, S. et al. Selective conversion of CO2 into propene and butene. Chem 6, 3344–3363 (2020).
Sadrameli, S. M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 173, 285–297 (2016).
Zhu, J. et al. Ultrafast encapsulation of metal nanoclusters into MFI zeolite in the course of its crystallization: catalytic application for propane dehydrogenation. Angew. Chem. Int. Ed. 59, 19669–19674 (2020).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2016).
Sun, Q. et al. Subnanometer bimetallic platinum–zinc clusters in zeolites for propane dehydrogenation. Angew. Chem. Int. Ed. 59, 19450–19459 (2020).
Niziolek, A. M., Onel, O. & Floudas, C. A. Production of benzene, toluene, and xylenes from natural gas via methanol: process synthesis and global optimization. AIChE J. 62, 1531–1556 (2016).
Cheng, K. et al. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability. Chem 3, 334–347 (2017).
Zhao, B. et al. Direct transformation of syngas to aromatics over Na-Zn-Fe5C2 and hierarchical HZSM-5 tandem catalysts. Chem 3, 323–333 (2017).
Kumar, A., Song, K., Liu, L., Han, Y. & Bhan, A. Absorptive hydrogen scavenging for enhanced aromatics yield during non-oxidative methane dehydroaromatization on Mo/H-ZSM-5 catalysts. Angew. Chem. Int. Ed. 57, 15577–15582 (2018).
Kosinov, N. et al. Reversible nature of coke formation on Mo/ZSM-5 methane dehydroaromatization catalysts. Angew. Chem. Int. Ed. 58, 7068–7072 (2019).
Kosinov, N. & Hensen, E. J. M. Reactivity, selectivity, and stability of zeolite-based catalysts for methane dehydroaromatization. Adv. Mater. 32, 2002565 (2020).
Menon, U., Rahman, M. & Khatib, S. J. A critical literature review of the advances in methane dehydroaromatization over multifunctional metal-promoted zeolite catalysts. Appl. Catal. A 608, 117870 (2020).
Zhou, Y. et al. Ethylene dehydroaromatization over Ga-ZSM-5 catalysts: nature and role of gallium speciation. Angew. Chem. Int. Ed. 59, 19592–19601 (2020).
Chowdhury, A. D. et al. Electrophilic aromatic substitution over zeolites generates Wheland-type reaction intermediates. Nat. Catal. 1, 23–31 (2018).
Chen, N. Y., Kaeding, W. W. & Dwyer, F. G. Para-directed aromatic reactions over shape-selective molecular sieve zeolite catalysts. J. Am. Chem. Soc. 101, 6783–6784 (1979).
Wang, C. et al. Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene. Nat. Commun. 10, 4348 (2019).
Zhang, J. et al. A Pd@zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores. Angew. Chem. Int. Ed. 56, 9747–9751 (2017).
Sun, Q. et al. Zeolite-encaged single-atom rhodium catalysts: highly-efficient hydrogen generation and shape-selective tandem hydrogenation of nitroarenes. Angew. Chem. Int. Ed. 58, 18570–18576 (2019).
Olivos-Suarez, A. I. et al. Strategies for the direct catalytic valorization of methane using heterogeneous catalysis: challenges and opportunities. ACS Catal. 6, 2965–2981 (2016).
Snyder, B. E. R., Bols, M. L., Schoonheydt, R. A., Sels, B. F. & Solomon, E. I. Iron and copper active sites in zeolites and their correlation to metalloenzymes. Chem. Rev. 118, 2718–2768 (2018).
Pappas, D. K. et al. The nuclearity of the active site for methane to methanol conversion in Cu-mordenite: a quantitative assessment. J. Am. Chem. Soc. 140, 15270–15278 (2018).
Kosinov, N., Liu, C., Hensen, E. J. M. & Pidko, E. A. Engineering of transition metal catalysts confined in zeolites. Chem. Mater. 30, 3177–3198 (2018).
Xu, J., Wang, Q. & Deng, F. Metal active sites and their catalytic functions in zeolites: insights from solid-state NMR spectroscopy. Acc. Chem. Res. 52, 2179–2189 (2019).
Kang, J. et al. Single-pass transformation of syngas into ethanol with high selectivity by triple tandem catalysis. Nat. Commun. 11, 827 (2020).
Wang, C. et al. Direct conversion of syngas to ethanol within zeolite crystals. Chem 6, 646–657 (2020).
Wang, X. et al. NMR-spectroscopic evidence of intermediate-dependent pathways for acetic acid formation from methane and carbon monoxide over a ZnZSM-5 zeolite catalyst. Angew. Chem. Int. Ed. 51, 3850–3853 (2012).
Wu, J.-F. et al. Mechanistic insight into the formation of acetic acid from the direct conversion of methane and carbon dioxide on zinc-modified H–ZSM-5 zeolite. J. Am. Chem. Soc. 135, 13567–13573 (2013).
Tang, Y. et al. Single rhodium atoms anchored in micropores for efficient transformation of methane under mild conditions. Nat. Commun. 9, 1231 (2018).
Chao, C. C. Process for separating nitrogen from mixtures thereof with less polar substances. US Patent 4859217 (1989).
Auerbach, S. M., Carrado, K. A. & Dutta, P. K. Handbook of Zeolite Science and Technology (Marcel Dekker, 2003).
Ockwig, N. W. & Nenoff, T. M. Membranes for hydrogen separation. Chem. Rev. 107, 4078–4110 (2007).
Pera-Titus, M. Porous inorganic membranes for CO2 capture: present and prospects. Chem. Rev. 114, 1413–1492 (2014).
Shah, M. S., Tsapatsis, M. & Siepmann, J. I. Hydrogen sulfide capture: from absorption in polar liquids to oxide, zeolite, and metal–organic framework adsorbents and membranes. Chem. Rev. 117, 9755–9803 (2017).
Gehre, M., Guo, Z., Rothenberg, G. & Tanase, S. Sustainable separations of C4-hydrocarbons by using microporous materials. ChemSusChem 10, 3947–3963 (2017).
Dakhchoune, M. et al. Gas-sieving zeolitic membranes fabricated by condensation of precursor nanosheets. Nat. Mater. 20, 362–369 (2021).
Kim, D., Jeon, M. Y., Stottrup, B. L. & Tsapatsis, M. para-xylene ultra-selective zeolite MFI membranes fabricated from nanosheet monolayers at the air-water interface. Angew. Chem. Int. Ed. 57, 480–485 (2018).
Cao, Z. et al. Ultrathin ZSM-5 zeolite nanosheet laminated membrane for high-flux desalination of concentrated brines. Sci. Adv. 4, eaau8634 (2018).
Lai, Z. et al. Microstructural optimization of a zeolite membrane for organic vapor separation. Science 300, 456–460 (2003).
Varoon, K. et al. Dispersible exfoliated zeolite nanosheets and their application as a selective membrane. Science 334, 72–75 (2011).
Wu, Y. et al. Enhanced propene/propane separation by directional decoration of the 12-membered rings of mordenite with ZIF fragments. Angew. Chem. Int. Ed. 59, 6765–6768 (2020).
Chai, Y. et al. Control of zeolite pore interior for chemoselective alkyne/olefin separations. Science 368, 1002–1006 (2020).
Liu, Y. et al. Uniform hierarchical MFI nanosheets prepared via anisotropic etching for solution-based sub–100-nm-thick oriented MFI layer fabrication. Sci. Adv. 6, eaay5993 (2020).
Swenson, P., Tanchuk, B., Bastida, E., An, W. & Kuznicki, S. M. Water desalination and de-oiling with natural zeolite membranes — potential application for purification of SAGD process water. Desalination 286, 442–446 (2012).
Kasai, P. H. Electron spin resonance studies of γ- and X-ray-irradiated zeolites. J. Chem. Phys. 43, 3322–3327 (1965).
Choi, S., Dickson, R. M. & Yu, J. Developing luminescent silver nanodots for biological applications. Chem. Soc. Rev. 41, 1867–1891 (2012).
Wang, N., Tang, Z. K., Li, G. D. & Chen, J. S. Single-walled 4 Å carbon nanotube arrays. Nature 408, 50–51 (2000).
Hamilton, B., Rimmer, J. S., Anderson, M. & Leigh, D. White light emission from C60 molecules confined in molecular cage materials. Adv. Mater. 5, 583–585 (1993).
Mac Dougall, J. E. et al. Synthesis and characterization of group III-V semiconductor clusters: gallium phosphide GaP in zeolite Y. J. Am. Chem. Soc. 111, 8006–8007 (1989).
Kim, H. S. & Yoon, K. B. Increase of third-order nonlinear optical activity of PdS quantum dots in zeolite Y by increasing cation size. J. Am. Chem. Soc. 134, 2539–2542 (2012).
Borja, M. & Dutta, P. K. Storage of light energy by photoelectron transfer across a sensitized zeolite–solution interface. Nature 362, 43–45 (1993).
Sykora, M. & Kincaid, J. R. Photochemical energy storage in a spatially organized zeolite-based photoredox system. Nature 387, 162–164 (1997).
Calzaferri, G., Huber, S., Maas, H. & Minkowski, C. Host–guest antenna materials. Angew. Chem. Int. Ed. 42, 3732–3758 (2003).
Alabarse, F. G. et al. Mechanism of H2O insertion and chemical bond formation in AlPO4-54·xH2O at high pressure. J. Am. Chem. Soc. 137, 584–587 (2015).
Bukowski, B. C., Bates, J. S., Gounder, R. & Greeley, J. Defect-mediated ordering of condensed water structures in microporous zeolites. Angew. Chem. Int. Ed. 58, 16422–16426 (2019).
Coutiño-Gonzalez, E. et al. Silver clusters in zeolites: from self-assembly to ground-breaking luminescent properties. Acc. Chem. Res. 50, 2353–2361 (2017).
Kennes, K. et al. Silver zeolite composites-based LEDs: a novel solid-state lighting approach. Adv. Funct. Mater. 27, 1606411 (2017).
Lim, S. Y., Shen, W. & Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 44, 362–381 (2015).
Mu, Y. et al. Carbogenic nanodots derived from organo-templated zeolites with modulated full-color luminescence. Chem. Sci. 7, 3564–3568 (2016).
Zhang, H. et al. Carbon dots in porous materials: host–guest synergy for enhanced performance. Angew. Chem. Int. Ed. 59, 19390–19402 (2020).
Wang, B. et al. Red room-temperature phosphorescence of CDs@zeolite composites triggered by heteroatoms in zeolite frameworks. ACS Cent. Sci. 5, 349–356 (2019).
Wang, B. et al. Carbon dots in a matrix: energy-transfer-enhanced room-temperature red phosphorescence. Angew. Chem. Int. Ed. 58, 18443–18448 (2019).
Zhang, H. et al. Carbon dots-in-zeolite via in-situ solvent-free thermal crystallization: achieving high-efficiency and ultralong afterglow dual emission. CCS Chem. 2, 118–127 (2020).
Kehr, N. S., Ergün, B., Lülf, H. & De Cola, L. Spatially controlled channel entrances functionalization of zeolites L. Adv. Mater. 26, 3248–3252 (2014).
Davis, M. E., Saldarriaga, C., Montes, C., Garces, J. & Crowdert, C. A molecular sieve with eighteen-membered rings. Nature 331, 698–699 (1988).
McCusker, L. B., Baerlocher, C. H., Jahn, E. & Bülow, M. The triple helix inside the large-pore aluminophosphate molecular sieve VPI-5. Zeolites 11, 308–313 (1991).
Arletti, R. et al. Irreversible conversion of a water–ethanol solution into an organized two-dimensional network of alternating supramolecular units in a hydrophobic zeolite under pressure. Angew. Chem. Int. Ed. 56, 2105–2109 (2017).
Pham, T. C. T. et al. Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energy Environ. Sci. 9, 1050–1062 (2016).
Chapman, S. et al. Probing the design rationale of a high-performing faujasitic zeotype engineered to have hierarchical porosity and moderated acidity. Angew. Chem. Int. Ed. 59, 19561–19569 (2020).
Sun, M. et al. Micron-sized zeolite beta single crystals featuring intracrystal interconnected ordered macro-meso-microporosity displaying superior catalytic performance. Angew. Chem. Int. Ed. 59, 19582–19591 (2020).
Jiao, Y. et al. Creation of Al-enriched mesoporous ZSM-5 nanoboxes with high catalytic activity: converting tetrahedral extra-framework al into framework sites by post treatment. Angew. Chem. Int. Ed. 59, 19478–19486 (2020).
Konnov, S. V. et al. Novel strategy for the synthesis of ultra-stable single-site Mo-ZSM-5 zeolite nanocrystals. Angew. Chem. Int. Ed. 59, 19553–19560 (2020).
Awala, H. et al. Template-free nanosized faujasite-type zeolites. Nat. Mater. 14, 447–451 (2015).
Park, S. H., Choi, W., Choi, H. J. & Hong, S. B. Organic-free synthesis of silicoaluminophosphate molecular sieves. Angew. Chem. Int. Ed. 57, 9413–9418 (2018).
Debost, M. et al. Synthesis of discrete CHA zeolite nanocrystals without organic templates for selective CO2 capture. Angew. Chem. Int. Ed. 59, 23491–23495 (2020).
Meng, X. & Xiao, F.-S. Green routes for synthesis of zeolites. Chem. Rev. 114, 1521–1543 (2014).
Sheng, N. et al. Insights of the crystallization process of molecular sieve AlPO4-5 prepared by solvent-free synthesis. J. Am. Chem. Soc. 138, 6171–6176 (2016).
Feng, G. et al. Accelerated crystallization of zeolites via hydroxyl free radicals. Science 351, 1188–1191 (2016).
Feng, G. et al. Radical-facilitated green synthesis of highly ordered mesoporous silica materials. J. Am. Chem. Soc. 140, 4770–4773 (2018).
Chen, X. et al. Gamma-ray irradiation to accelerate crystallization of mesoporous zeolites. Angew. Chem. Int. Ed. 59, 11325–11329 (2020).
Wang, J. et al. Organic-free synthesis of zeolite Y with high Si/Al ratios: combined strategy of in situ hydroxyl radical assistance and post-synthesis treatment. Angew. Chem. Int. Ed. 59, 17225–17228 (2020).
Dusselier, M., Van Wouwe, P., Dewaele, A., Jacobs, P. A. & Sels, B. F. Shape-selective zeolite catalysis for bioplastics production. Science 349, 78–80 (2015).
Gumidyala, A., Wang, B. & Crossley, S. Direct carbon-carbon coupling of furanics with acetic acid over Brønsted zeolites. Sci. Adv. 2, e1601072 (2016).
Ennaert, T. et al. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 45, 584–611 (2016).
Clatworthy, E. B. et al. Emphasis on the properties of metal-containing zeolites operating outside the comfort zone of current heterogeneous catalytic reactions. Angew. Chem. Int. Ed. 59, 19414–19432 (2020).
Gao, F., Mei, D., Wang, Y., Szanyi, J. & Peden, C. H. F. Selective catalytic reduction over Cu/SSZ-13: linking homo- and heterogeneous catalysis. J. Am. Chem. Soc. 139, 4935–4942 (2017).
Marberger, A. et al. Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13. Nat. Catal. 1, 221–227 (2018).
Khivantsev, K. et al. Achieving atomic dispersion of highly loaded transition metals in small-pore zeolite SSZ-13: high-capacity and high-efficiency low-temperature CO and passive NOx adsorbers. Angew. Chem. Int. Ed. 57, 16672–16677 (2018).
Liu, L. et al. Evolution and stabilization of subnanometric metal species in confined space by in situ TEM. Nat. Commun. 9, 574 (2018).
Wang, N. et al. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 138, 7484–7487 (2016).
Sun, Q. et al. Subnanometric hybrid Pd-M(OH)2, M = Ni, Co, clusters in zeolites as highly efficient nanocatalysts for hydrogen generation. Chem 3, 477–493 (2017).
Sun, Q., Wang, N., Xu, Q. & Yu, J. Nanopore-supported metal nanocatalysts for efficient hydrogen generation from liquid-phase chemical hydrogen storage materials. Adv. Mater. 32, 2001818 (2020).
Sun, Q. et al. Zeolite-encaged Pd–Mn nanocatalysts for CO2 hydrogenation and formic acid dehydrogenation. Angew. Chem. Int. Ed. 59, 20183–20191 (2020).
Zhou, C. et al. Efficient synthesis of dimethyl ether from methanol in a bifunctional zeolite membrane reactor. Angew. Chem. Int. Ed. 55, 12678–12682 (2016).
Van der Borght, K. et al. Insights into the reaction mechanism of ethanol conversion into hydrocarbons on H-ZSM-5. Angew. Chem. Int. Ed. 55, 12817–12821 (2016).
Liu, Y. et al. Enhancing the catalytic activity of hydronium ions through constrained environments. Nat. Commun. 8, 14113 (2017).
Shi, H., Eckstein, S., Vjunov, A., Camaioni, D. M. & Lercher, J. A. Tailoring nanoscopic confines to maximize catalytic activity of hydronium ions. Nat. Commun. 8, 15442 (2017).
Petrov, A. W. et al. Stable complete methane oxidation over palladium based zeolite catalysts. Nat. Commun. 9, 2545 (2018).
Grosso-Giordano, N. A. et al. Dynamic reorganization and confinement of TiIV active sites controls olefin epoxidation catalysis on two-dimensional zeotypes. J. Am. Chem. Soc. 141, 7090–7106 (2019).
Bregante, D. T. et al. Cooperative effects between hydrophilic pores and solvents: catalytic consequences of hydrogen bonding on alkene epoxidation in zeolites. J. Am. Chem. Soc. 141, 7302–7319 (2019).
Wang, L. et al. Controllable cyanation of carbon-hydrogen bonds by zeolite crystals over manganese oxide catalyst. Nat. Commun. 8, 15240 (2017).
Ryoo, R. et al. Rare-earth–platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585, 221–224 (2020).
Wang, C. et al. π-Interactions between cyclic carbocations and aromatics cause zeolite deactivation in methanol-to-hydrocarbon conversion. Angew. Chem. Int. Ed. 59, 7198–7202 (2020).
Zhou, J. et al. Directed transforming of coke to active intermediates in methanol-to-olefins catalyst to boost light olefins selectivity. Nat. Commun. 12, 17 (2021).
Feng, X. et al. Kr/Xe separation over a chabazite zeolite membrane. J. Am. Chem. Soc. 138, 9791–9794 (2016).
Kim, C., Cho, H. S., Chang, S., Cho, S. J. & Choi, M. An ethylenediamine-grafted Y zeolite: a highly regenerable carbon dioxide adsorbent via temperature swing adsorption without urea formation. Energy Environ. Sci. 9, 1803–1811 (2016).
Georgieva, V. M. et al. Triggered gate opening and breathing effects during selective CO2 adsorption by merlinoite zeolite. J. Am. Chem. Soc. 141, 12744–12759 (2019).
Jeong, Y. et al. An hetero-epitaxially grown zeolite membrane. Angew. Chem. Int. Ed. 58, 18654–18662 (2019).
Li, G. K. et al. Temperature-regulated guest admission and release in microporous materials. Nat. Commun. 8, 15777 (2017).
Wu, Y. et al. Decorated traditional zeolites with subunits of metal-organic frameworks for CH4/N2 separation. Angew. Chem. Int. Ed. 58, 10241–10244 (2019).
Shah, M. S., Tsapatsis, M. & Siepmann, J. I. Identifying optimal zeolitic sorbents for sweetening of highly sour natural gas. Angew. Chem. Int. Ed. 55, 5938–5942 (2016).
Li, Y.-X. et al. Enhancing oxidation resistance of Cu(I) by tailoring microenvironment in zeolites for efficient adsorptive desulfurization. Nat. Commun. 11, 3206 (2020).
Krajnc, A. et al. Superior performance of microporous aluminophosphate with LTA topology in solar-energy storage and heat reallocation. Adv. Energy Mater. 7, 1601815 (2017).
Tiriolo, R. et al. Sub-micrometer zeolite films on gold-coated silicon wafers with single-crystal-like dielectric constant and elastic modulus. Adv. Funct. Mater. 27, 1700864 (2017).
Yuan, Z. et al. A highly ion-selective zeolite flake layer on porous membranes for flow battery applications. Angew. Chem. Int. Ed. 55, 3058–3062 (2016).
Chi, X. et al. A highly stable and flexible zeolite electrolyte solid-state Li–air battery. Nature 592, 551–557 (2021).
Yu, L. et al. A tightly-bonded and flexible mesoporous zeolite-cotton hybrid hemostat. Nat. Commun. 10, 1932 (2019).
Kim, K. et al. Lanthanum-catalysed synthesis of microporous 3D graphene-like carbons in a zeolite template. Nature 535, 131–135 (2016).
Choi, C. H. et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 7, 10922 (2016).
Li, J. & Sun, J. Application of X-ray diffraction and electron crystallography for solving complex structure problems. Acc. Chem. Res. 50, 2737–2745 (2017).
Ma, Y., Oleynikov, P. & Terasaki, O. Electron crystallography for determining the handedness of a chiral zeolite nanocrystal. Nat. Mater. 16, 755–759 (2017).
Smeets, S. et al. Well-defined silanols in the structure of the calcined high-silica zeolite SSZ-70: new understanding of a successful catalytic material. J. Am. Chem. Soc. 139, 16803–16812 (2017).
Shen, B. et al. Atomic spatial and temporal imaging of local structures and light elements inside zeolite frameworks. Adv. Mater. 32, 1906103 (2019).
Liu, L. et al. Direct imaging of atomically dispersed molybdenum that enables location of aluminum in the framework of zeolite ZSM-5. Angew. Chem. Int. Ed. 59, 819–825 (2020).
Zhang, Q. et al. Electron microscopy studies of local structural modulations in zeolite crystals. Angew. Chem. Int. Ed. 59, 19403–19413 (2020).
Zheng, A., Li, S., Liu, S.-B. & Deng, F. Acidic properties and structure–activity correlations of solid acid catalysts revealed by solid-state NMR spectroscopy. Acc. Chem. Res. 49, 655–663 (2016).
Xu, J., Wang, Q., Li, S. & Deng, F. Solid-State NMR in Zeolite Catalysis Vol. 103 (Springer, 2019).
Heard, C. J. et al. Fast room temperature lability of aluminosilicate zeolites. Nat. Commun. 10, 4690 (2019).
Sushkevich, V. L., Verel, R. & van Bokhoven, J. A. Pathways of methane transformation over copper-exchanged mordenite as revealed by in situ NMR and IR spectroscopy. Angew. Chem. Int. Ed. 59, 910–918 (2020).
Pugh, S. M., Wright, P. A., Law, D. J., Thompson, N. & Ashbrook, S. E. Facile, room-temperature 17O enrichment of zeolite frameworks revealed by solid-state NMR spectroscopy. J. Am. Chem. Soc. 142, 900–906 (2020).
Li, S. et al. Recent advances of solid-state NMR spectroscopy for microporous materials. Adv. Mater. 32, 2002879 (2020).
Qi, G. et al. gem-Diol-type intermediate in the activation of a ketone on Sn-β zeolite as studied by solid-state NMR spectroscopy. Angew. Chem. Int. Ed. 59, 19532–19538 (2020).
Paolucci, C. et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 357, 898–903 (2017).
Imbao, J., van Bokhoven, J. A., Clark, A. & Nachtegaal, M. Elucidating the mechanism of heterogeneous Wacker oxidation over Pd-Cu/zeolite Y by transient XAS. Nat. Commun. 11, 1118 (2020).
Zhang, T., Chen, Z., Walsh, A. G., Li, Y. & Zhang, P. Single-atom catalysts supported by crystalline porous materials: views from the inside. Adv. Mater. 32, 2002910 (2020).
Bols, M. L. et al. Spectroscopic identification of the α-Fe/α-O active site in Fe-CHA zeolite for the low-temperature activation of the methane C–H bond. J. Am. Chem. Soc. 140, 12021–12032 (2018).
Fu, D. et al. Elucidating zeolite channel geometry–reaction intermediate relationships for the methanol-to-hydrocarbon process. Angew. Chem. Int. Ed. 59, 20024–20030 (2020).
Mahyuddin, M. H., Shiota, Y., Staykov, A. & Yoshizawa, K. Theoretical overview of methane hydroxylation by copper–oxygen species in enzymatic and zeolitic catalysts. Acc. Chem. Res. 51, 2382–2390 (2018).
Grajciar, L. et al. Towards operando computational modeling in heterogeneous catalysis. Chem. Soc. Rev. 47, 8307–8348 (2018).
Moliner, M., Román-Leshkov, Y. & Corma, A. Machine learning applied to zeolite synthesis: the missing link for realizing high-throughput discovery. Acc. Chem. Res. 52, 2971–2980 (2019).
Boyd, P. G., Lee, Y. & Smit, B. Computational development of the nanoporous materials genome. Nat. Rev. Mater. 2, 17037 (2017).
Daeyaert, F., Ye, F. & Deem, M. W. Machine-learning approach to the design of OSDAs for zeolite beta. Proc. Natl Acad. Sci. USA 116, 3413–3418 (2019).
Muraoka, K., Sada, Y., Miyazaki, D., Chaikittisilp, W. & Okubo, T. Linking synthesis and structure descriptors from a large collection of synthetic records of zeolite materials. Nat. Commun. 10, 4459 (2019).
Kim, B., Lee, S. & Kim, J. Inverse design of porous materials using artificial neural networks. Sci. Adv. 6, eaax9324 (2020).
Clayson, I. G., Hewitt, D., Hutereau, M., Pope, T. & Slater, B. High throughput methods in the synthesis, characterization, and optimization of porous materials. Adv. Mater. 32, 2002780 (2020).
Freeze, J. G., Kelly, H. R. & Batista, V. S. Search for catalysts by inverse design: artificial intelligence, mountain climbers, and alchemists. Chem. Rev. 119, 6595–6612 (2019).
Toyao, T. et al. Machine learning for catalysis informatics: recent applications and prospects. ACS Catal. 10, 2260–2297 (2020).
Baerlocher, Ch. & McCusker, L. B. Database of zeolite structures. IZA http://www.iza-structure.org/databases/ (2020).
Foster, M. D. & Treacy, M. M. J. Atlas of prospective zeolite structures. IZA http://www.hypotheticalzeolites.net/ (2018).
COD. Predicted crystallography open database. crystallography.net http://www.crystallography.net/pcod/ (2009).
Li, Y. & Yu, J. Hypothetical zeolite structures. figshare https://doi.org/10.6084/m9.figshare.c.4424417 (2019).
Li, Y. & Yu, J. AlPO structures. figshare https://doi.org/10.6084/m9.figshare.7822574 (2020).
Li, J., Yu, J. & Xu, R. ZEOBANK. Zeobank http://zeobank.jlu.edu.cn/ (2020).
Jensen, Z. et al. A machine learning approach to zeolite synthesis enabled by automatic literature data extraction. ACS Cent. Sci. 5, 892–899 (2019).
Raccuglia, P. et al. Machine-learning-assisted materials discovery using failed experiments. Nature 533, 73–76 (2016).
Acknowledgements
The authors thank the National Natural Science Foundation of China (grant nos. 21920102005, 21835002 and 21621001), the National Key Research and Development Program of China (grant no. 2016YFB0701100) and the 111 Project (grant no. B17020).
Author information
Authors and Affiliations
Contributions
J.Y. conceived the general outlook of the manuscript. Both authors wrote and edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat Rev Mater 6, 1156–1174 (2021). https://doi.org/10.1038/s41578-021-00347-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00347-3
This article is cited by
-
Modelling atomic and nanoscale structure in the silicon–oxygen system through active machine learning
Nature Communications (2024)
-
Kinetics, Isotherms, and Thermodynamics Studies on Adsorption of Methyl Green Dye Onto Different Zeolites
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Cu-based high-entropy two-dimensional oxide as stable and active photothermal catalyst
Nature Communications (2023)
-
Alkynyl-anchored silver nanoclusters in lanthanide metal-organic framework for luminescent thermometer and CO2 cycloaddition
Nano Research (2023)
-
Bimetallic clusters confined inside silicalite-1 for stable propane dehydrogenation
Nano Research (2023)