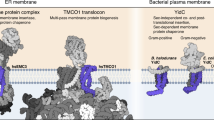
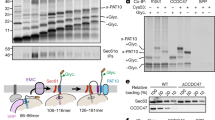
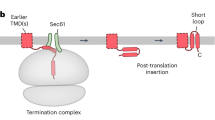
Abstract
Roughly one quarter of all genes code for integral membrane proteins that are inserted into the plasma membrane of prokaryotes or the endoplasmic reticulum membrane of eukaryotes. Multiple pathways are used for the targeting and insertion of membrane proteins on the basis of their topological and biophysical characteristics. Multipass membrane proteins span the membrane multiple times and face the additional challenges of intramembrane folding. In many cases, integral membrane proteins require assembly with other proteins to form multi-subunit membrane protein complexes. Recent biochemical and structural analyses have provided considerable clarity regarding the molecular basis of membrane protein targeting and insertion, with tantalizing new insights into the poorly understood processes of multipass membrane protein biogenesis and multi-subunit protein complex assembly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
von Heijne, G. The membrane protein universe: what’s out there and why bother? J. Intern. Med. 261, 543–557 (2007).
Xie, K. & Dalbey, R. E. Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nat. Rev. Microbiol. 6, 234–244 (2008).
Shao, S. & Hegde, R. S. Membrane protein insertion at the endoplasmic reticulum. Annu. Rev. Cell Dev. Biol. 27, 25–56 (2011).
Baum, D. A. & Baum, B. An inside-out origin for the eukaryotic cell. BMC Biol. 12, 76 (2014).
Wiedemann, N. & Pfanner, N. Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem. 86, 685–714 (2017).
Gupta, A. & Becker, T. Mechanisms and pathways of mitochondrial outer membrane protein biogenesis. Biochim. Biophys. Acta Bioenerg. 1862, 148323 (2021).
Kim, J., Na, Y. J., Park, S. J., Baek, S. H. & Kim, D. H. Biogenesis of chloroplast outer envelope membrane proteins. Plant. Cell Rep. 38, 783–792 (2019).
Xu, X., Ouyang, M., Lu, D., Zheng, C. & Zhang, L. Protein sorting within chloroplasts. Trends Cell Biol. 31, 9–16 (2021).
Mayerhofer, P. U. Targeting and insertion of peroxisomal membrane proteins: ER trafficking versus direct delivery to peroxisomes. Biochim. Biophys. Acta 1863, 870–880 (2016).
White, S. H. & von Heijne, G. Transmembrane helices before, during, and after insertion. Curr. Opin. Struct. Biol. 15, 378–386 (2005).
Walther, D. M., Rapaport, D. & Tommassen, J. Biogenesis of beta-barrel membrane proteins in bacteria and eukaryotes: evolutionary conservation and divergence. Cell Mol. Life Sci. 66, 2789–2804 (2009).
Noinaj, N., Gumbart, J. C. & Buchanan, S. K. The beta-barrel assembly machinery in motion. Nat. Rev. Microbiol. 15, 197–204 (2017).
Almagro Armenteros, J. J. et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37, 420–423 (2019).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 (2001).
Nyathi, Y., Wilkinson, B. M. & Pool, M. R. Co-translational targeting and translocation of proteins to the endoplasmic reticulum. Biochim. Biophys. Acta 1833, 2392–2402 (2013).
Keenan, R. J., Freymann, D. M., Stroud, R. M. & Walter, P. The signal recognition particle. Annu. Rev. Biochem. 70, 755–775 (2001).
Zhang, X. & Shan, S. O. Fidelity of cotranslational protein targeting by the signal recognition particle. Annu. Rev. Biophys. 43, 381–408 (2014).
Halic, M. et al. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature 427, 808–814 (2004).
Schaffitzel, C. et al. Structure of the E. coli signal recognition particle bound to a translating ribosome. Nature 444, 503–506 (2006).
Voorhees, R. M. & Hegde, R. S. Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. eLife https://doi.org/10.7554/eLife.07975 (2015). Cryo-EM structures of biochemically defined states of the mammalian SRP–ribosome complex are presented.
Goder, V., Crottet, P. & Spiess, M. In vivo kinetics of protein targeting to the endoplasmic reticulum determined by site-specific phosphorylation. EMBO J. 19, 6704–6712 (2000).
Guna, A. & Hegde, R. S. Transmembrane domain recognition during membrane protein biogenesis and quality control. Curr. Biol. 28, R498–R511 (2018).
Guna, A., Volkmar, N., Christianson, J. C. & Hegde, R. S. The ER membrane protein complex is a transmembrane domain insertase. Science 359, 470–473 (2018). Cell-based assays and biochemical reconstitution reveal EMC as a bona fide insertase for TA membrane proteins with moderately hydrophobic TMDs.
Keenan, R. J., Freymann, D. M., Walter, P. & Stroud, R. M. Crystal structure of the signal sequence binding subunit of the signal recognition particle. Cell 94, 181–191 (1998).
Hainzl, T., Huang, S., Merilainen, G., Brannstrom, K. & Sauer-Eriksson, A. E. Structural basis of signal-sequence recognition by the signal recognition particle. Nat. Struct. Mol. Biol. 18, 389–391 (2011).
Janda, C. Y. et al. Recognition of a signal peptide by the signal recognition particle. Nature 465, 507–510 (2010).
Wiedmann, B., Sakai, H., Davis, T. A. & Wiedmann, M. A protein complex required for signal-sequence-specific sorting and translocation. Nature 370, 434–440 (1994).
Gamerdinger, M., Hanebuth, M. A., Frickey, T. & Deuerling, E. The principle of antagonism ensures protein targeting specificity at the endoplasmic reticulum. Science 348, 201–207 (2015).
Hsieh, H. H., Lee, J. H., Chandrasekar, S. & Shan, S. O. A ribosome-associated chaperone enables substrate triage in a cotranslational protein targeting complex. Nat. Commun. 11, 5840 (2020).
Gamerdinger, M. et al. Early scanning of nascent polypeptides inside the ribosomal tunnel by NAC. Mol. Cell 75, 996–1006 e1008 (2019).
Moller, I. et al. Unregulated exposure of the ribosomal M-site caused by NAC depletion results in delivery of non-secretory polypeptides to the Sec61 complex. FEBS Lett. 441, 1–5 (1998).
Beatrix, B., Sakai, H. & Wiedmann, M. The alpha and beta subunit of the nascent polypeptide-associated complex have distinct functions. J. Biol. Chem. 275, 37838–37845 (2000).
Powers, T. & Walter, P. The nascent polypeptide-associated complex modulates interactions between the signal recognition particle and the ribosome. Curr. Biol. 6, 331–338 (1996).
Lauring, B., Sakai, H., Kreibich, G. & Wiedmann, M. Nascent polypeptide-associated complex protein prevents mistargeting of nascent chains to the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 92, 5411–5415 (1995).
Schibich, D. et al. Global profiling of SRP interaction with nascent polypeptides. Nature 536, 219–223 (2016). Selective ribosome profiling in Escherichia coli shows that SRP targets inner membrane proteins but not periplasmic or outer membrane proteins.
Chartron, J. W., Hunt, K. C. & Frydman, J. Cotranslational signal-independent SRP preloading during membrane targeting. Nature 536, 224–228 (2016). Selective ribosome profiling in yeast cells demonstrates SRP binding to nascent secretory proteins containing signal peptides or TMDs.
Costa, E. A., Subramanian, K., Nunnari, J. & Weissman, J. S. Defining the physiological role of SRP in protein-targeting efficiency and specificity. Science 359, 689–692 (2018). Acute SRP depletion and proximity-specific ribosome profiling in yeast reveal SRP-dependent targeting of nascent secretory proteins with signal peptides or TMDs.
Lee, H. C. & Bernstein, H. D. The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc. Natl Acad. Sci. USA 98, 3471–3476 (2001).
Hegde, R. S. & Keenan, R. J. Tail-anchored membrane protein insertion into the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 12, 787–798 (2011).
Shan, S. O. Guiding tail-anchored membrane proteins to the endoplasmic reticulum in a chaperone cascade. J. Biol. Chem. 294, 16577–16586 (2019).
Borgese, N., Coy-Vergara, J., Colombo, S. F. & Schwappach, B. The ways of tails: the GET pathway and more. Protein J. 38, 289–305 (2019).
Stefanovic, S. & Hegde, R. S. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell 128, 1147–1159 (2007).
Wang, F., Brown, E. C., Mak, G., Zhuang, J. & Denic, V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol. Cell 40, 159–171 (2010).
Mariappan, M. et al. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature 466, 1120–1124 (2010).
Mateja, A. et al. Protein targeting. Structure of the Get3 targeting factor in complex with its membrane protein cargo. Science 347, 1152–1155 (2015). Biochemical reconstitution and crystallographic analysis reveal how the TMD of a TA membrane protein binds to a large and dynamic hydrophobic groove in Get3.
Mateja, A. et al. The structural basis of tail-anchored membrane protein recognition by Get3. Nature 461, 361–366 (2009).
Gristick, H. B. et al. Crystal structure of ATP-bound Get3-Get4-Get5 complex reveals regulation of Get3 by Get4. Nat. Struct. Mol. Biol. 21, 437–442 (2014).
Schuldiner, M. et al. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634–645 (2008).
Yamamoto, Y. & Sakisaka, T. Molecular machinery for insertion of tail-anchored membrane proteins into the endoplasmic reticulum membrane in mammalian cells. Mol. Cell 48, 387–397 (2012).
Mock, J. Y. et al. Bag6 complex contains a minimal tail-anchor-targeting module and a mock BAG domain. Proc. Natl Acad. Sci. USA 112, 106–111 (2015).
Shao, S., Rodrigo-Brenni, M. C., Kivlen, M. H. & Hegde, R. S. Mechanistic basis for a molecular triage reaction. Science 355, 298–302 (2017).
Zhang, Y. et al. Ribosome-bound Get4/5 facilitates the capture of tail-anchored proteins by Sgt2 in yeast. Nat. Commun. 12, 782 (2021).
Chartron, J. W., VanderVelde, D. G. & Clemons, W. M. Jr Structures of the Sgt2/SGTA dimerization domain with the Get5/UBL4A UBL domain reveal an interaction that forms a conserved dynamic interface. Cell Rep. 2, 1620–1632 (2012).
Cho, H. & Shan, S. O. Substrate relay in an Hsp70-cochaperone cascade safeguards tail-anchored membrane protein targeting. EMBO J. https://doi.org/10.15252/embj.201899264 (2018).
Rao, M. et al. Multiple selection filters ensure accurate tail-anchored membrane protein targeting. eLife https://doi.org/10.7554/eLife.21301 (2016).
Lin, K. F., Fry, M. Y., Saladi, S. M. & Clemons, W. M. Jr Molecular basis of tail-anchored integral membrane protein recognition by the cochaperone Sgt2. J. Biol. Chem. https://doi.org/10.1016/j.jbc.2021.100441 (2021).
Chio, U. S., Chung, S., Weiss, S. & Shan, S. O. A chaperone lid ensures efficient and privileged client transfer during tail-anchored protein targeting. Cell Rep. 26, 37–44 e37 (2019).
O’Donnell, J. P. et al. The architecture of EMC reveals a path for membrane protein insertion. eLife https://doi.org/10.7554/eLife.57887 (2020). Structural and biochemical analysis of human EMC identifies a TMD-binding site in the cytosolic domain of EMC, and potential routes into the bilayer.
Pleiner, T. et al. Structural basis for membrane insertion by the human ER membrane protein complex. Science 369, 433–436 (2020). Cryo-EM structure and functional analysis of human EMC demonstrate the importance of the membrane-embedded hydrophilic vestibule of EMC3 for TA protein insertion.
Itakura, E. et al. Ubiquilins chaperone and triage mitochondrial membrane proteins for degradation. Mol. Cell 63, 21–33 (2016).
Abell, B. M., Rabu, C., Leznicki, P., Young, J. C. & High, S. Post-translational integration of tail-anchored proteins is facilitated by defined molecular chaperones. J. Cell Sci. 120, 1743–1751 (2007).
Rabu, C., Wipf, P., Brodsky, J. L. & High, S. A precursor-specific role for Hsp40/Hsc70 during tail-anchored protein integration at the endoplasmic reticulum. J. Biol. Chem. 283, 27504–27513 (2008).
Aschtgen, M. S., Zoued, A., Lloubes, R., Journet, L. & Cascales, E. The C-tail anchored TssL subunit, an essential protein of the enteroaggregative Escherichia coli Sci-1 type VI secretion system, is inserted by YidC. Microbiologyopen 1, 71–82 (2012).
Peschke, M. et al. SRP, FtsY, DnaK and YidC are required for the biogenesis of the E. coli tail-anchored membrane proteins DjlC and Flk. J. Mol. Biol. 430, 389–403 (2018).
Lee, J., Kim, D. H. & Hwang, I. Specific targeting of proteins to outer envelope membranes of endosymbiotic organelles, chloroplasts, and mitochondria. Front. Plant. Sci. 5, 173 (2014).
Becker, T., Song, J. & Pfanner, N. Versatility of preprotein transfer from the cytosol to mitochondria. Trends Cell Biol. 29, 534–548 (2019).
Bykov, Y. S., Rapaport, D., Herrmann, J. M. & Schuldiner, M. Cytosolic events in the biogenesis of mitochondrial proteins. Trends Biochem. Sci. 45, 650–667 (2020).
White, S. H. & Wimley, W. C. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365 (1999).
Wimley, W. C. & White, S. H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 3, 842–848 (1996).
Engelman, D. M., Steitz, T. A. & Goldman, A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Biophys Chem. 15, 321–353 (1986).
Anghel, S. A., McGilvray, P. T., Hegde, R. S. & Keenan, R. J. Identification of Oxa1 homologs operating in the eukaryotic endoplasmic reticulum. Cell Rep. 21, 3708–3716 (2017). Reports the discovery of Get1, EMC3 and TMCO1 as remote Oxa1 homologues in the ER, defining the evolutionarily ancient Oxa1 superfamily.
Lewis, A. J. O. & Hegde, R. S. A unified evolutionary origin for SecY and YidC. Preprint at bioRxiv https://doi.org/10.1101/2020.12.20.422553 (2020).
Wu, X. & Rapoport, T. A. Translocation of proteins through a distorted lipid bilayer. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2021.01.002 (2021).
Rapoport, T. A., Li, L. & Park, E. Structural and mechanistic insights into protein translocation. Annu. Rev. Cell Dev. Biol. 33, 369–390 (2017).
Van den Berg, B. et al. X-ray structure of a protein-conducting channel. Nature 427, 36–44 (2004).
Voorhees, R. M., Fernandez, I. S., Scheres, S. H. & Hegde, R. S. Structure of the mammalian ribosome-Sec61 complex to 3.4 Å resolution. Cell 157, 1632–1643 (2014).
Li, L. et al. Crystal structure of a substrate-engaged SecY protein-translocation channel. Nature 531, 395–399 (2016). Crystal structure of SecY engaged with SecA and an engineered secretory protein segment suggests how hydrophobic signals exit the lateral gate.
Voorhees, R. M. & Hegde, R. S. Structure of the Sec61 channel opened by a signal sequence. Science 351, 88–91 (2016). Cryo-EM structure of ribosome-bound mammalian Sec61 engaged with a signal peptide suggests how hydrophobic signals access the membrane.
Liaci, A. M. et al. Structure of the human signal peptidase complex reveals the determinants for signal peptide cleavage. bioRxiv https://doi.org/10.1101/2020.11.11.378711 (2020).
Hessa, T., White, S. H. & von Heijne, G. Membrane insertion of a potassium-channel voltage sensor. Science 307, 1427 (2005).
Spiess, M. & Lodish, H. F. An internal signal sequence: the asialoglycoprotein receptor membrane anchor. Cell 44, 177–185 (1986).
High, S. et al. Sec61p is adjacent to nascent type I and type II signal-anchor proteins during their membrane insertion. J. Cell Biol. 121, 743–750 (1993).
McKenna, M., Simmonds, R. E. & High, S. Mycolactone reveals the substrate-driven complexity of Sec61-dependent transmembrane protein biogenesis. J. Cell Sci. 130, 1307–1320 (2017). Biochemical analysis shows that the effect on membrane protein biogenesis of mycolactone, a small-molecule Sec61 inhibitor, depends on how the nascent chain initially engages with Sec61.
Zong, G. et al. Ipomoeassin F binds sec61alpha to inhibit protein translocation. J. Am. Chem. Soc. 141, 8450–8461 (2019).
Tranter, D. et al. Coibamide A targets Sec61 to prevent biogenesis of secretory and membrane proteins. ACS Chem. Biol. 15, 2125–2136 (2020).
Chitwood, P. J., Juszkiewicz, S., Guna, A., Shao, S. & Hegde, R. S. EMC is required to initiate accurate membrane protein topogenesis. Cell 175, 1507–1519 e1516 (2018). Cell-based assays and biochemical reconstitution define the role of EMC as a co-translational insertase for the first TMD of many multipass membrane proteins, including G-protein-coupled receptorss.
Fons, R. D., Bogert, B. A. & Hegde, R. S. Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J. Cell Biol. 160, 529–539 (2003).
Voigt, S., Jungnickel, B., Hartmann, E. & Rapoport, T. A. Signal sequence-dependent function of the TRAM protein during early phases of protein transport across the endoplasmic reticulum membrane. J. Cell Biol. 134, 25–35 (1996).
Itskanov, S., Kuo, K. M., Gumbart, J. C. & Park, E. Stepwise gating of the Sec61 protein-conducting channel by Sec63 and Sec62. Nat. Struct. Mol. Biol. 28, 162–172 (2021).
Weng, T. H. et al. Architecture of the active post-translational Sec translocon. EMBO J. 40, e105643 (2021).
Schorr, S. et al. Identification of signal peptide features for substrate specificity in human Sec62/Sec63-dependent ER protein import. FEBS J. 287, 4612–4640 (2020).
Do, H., Falcone, D., Lin, J., Andrews, D. W. & Johnson, A. E. The cotranslational integration of membrane proteins into the phospholipid bilayer is a multistep process. Cell 85, 369–378 (1996).
Heinrich, S. U., Mothes, W., Brunner, J. & Rapoport, T. A. The Sec61p complex mediates the integration of a membrane protein by allowing lipid partitioning of the transmembrane domain. Cell 102, 233–244 (2000).
Mariappan, M. et al. The mechanism of membrane-associated steps in tail-anchored protein insertion. Nature 477, 61–66 (2011).
Wang, F., Chan, C., Weir, N. R. & Denic, V. The Get1/2 transmembrane complex is an endoplasmic-reticulum membrane protein insertase. Nature 512, 441–444 (2014). Cell-based assays and biochemical reconstitution establish the Get1–Get2 complex as a bona fide insertase that guides the TMDs of TA proteins into the lipid bilayer.
Wang, F., Whynot, A., Tung, M. & Denic, V. The mechanism of tail-anchored protein insertion into the ER membrane. Mol. Cell 43, 738–750 (2011).
Stefer, S. et al. Structural basis for tail-anchored membrane protein biogenesis by the Get3-receptor complex. Science 333, 758–762 (2011).
Rome, M. E., Chio, U. S., Rao, M., Gristick, H. & Shan, S. O. Differential gradients of interaction affinities drive efficient targeting and recycling in the GET pathway. Proc. Natl Acad. Sci. USA 111, E4929–4935 (2014).
McDowell, M. A. et al. Structural basis of tail-anchored membrane protein biogenesis by the GET insertase complex. Mol. Cell 80, 72–86 e77 (2020). Cryo-EM structures of the yeast and human GET1–GET2–GET3 complexes reveal a heterotetrameric membrane assembly with hydrophilic vestibules into the bilayer.
Zalisko, B. E., Chan, C., Denic, V., Rock, R. S. & Keenan, R. J. Tail-anchored protein insertion by a single Get1/2 heterodimer. Cell Rep. 20, 2287–2293 (2017).
Kedrov, A. et al. Elucidating the native architecture of the YidC: ribosome complex. J. Mol. Biol. 425, 4112–4124 (2013).
Wickles, S. et al. A structural model of the active ribosome-bound membrane protein insertase YidC. eLife 3, e03035 (2014).
Itoh, Y. et al. Mechanism of membrane-tethered mitochondrial protein synthesis. Science 371, 846–849 (2021).
McGilvray, P. T. et al. An ER translocon for multi-pass membrane protein biogenesis. eLife https://doi.org/10.7554/eLife.56889 (2020). Discovery that TMCO1 operates as part of a large, multi-subunit translocon involved in the biogenesis of most mammalian multipass membrane proteins.
Miller-Vedam, L. E. et al. Structural and mechanistic basis of the EMC-dependent biogenesis of distinct transmembrane clients. eLife https://doi.org/10.7554/eLife.62611 (2020). Cryo-EM structures of yeast and human EMC coupled with mutational analysis suggest how different regions of the multifuctional complex function during biogenesis of its membrane protein clients.
Bai, L., You, Q., Feng, X., Kovach, A. & Li, H. Structure of the ER membrane complex, a transmembrane-domain insertase. Nature 584, 475–478 (2020). Cryo-EM structure and functional analysis of yeast EMC implicate the hydrophilic vestibule of EMC3 in TMD insertion and highlight a potential gating role for the EMC4 subunit.
Rivera-Monroy, J. et al. Mice lacking WRB reveal differential biogenesis requirements of tail-anchored proteins in vivo. Sci. Rep. 6, 39464 (2016).
Jonikas, M. C. et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323, 1693–1697 (2009).
Volkmar, N. & Christianson, J. C. Squaring the EMC - how promoting membrane protein biogenesis impacts cellular functions and organismal homeostasis. J. Cell Sci. https://doi.org/10.1242/jcs.243519 (2020).
Chitwood, P. J. & Hegde, R. S. The role of EMC during membrane protein biogenesis. Trends Cell Biol. 29, 371–384 (2019).
Bai, L. & Li, H. Cryo-EM structures of the endoplasmic reticulum membrane complex. FEBS J. https://doi.org/10.1111/febs.15786 (2021).
Kobayashi, K. et al. Structure of a prehandover mammalian ribosomal SRP.SRP receptor targeting complex. Science 360, 323–327 (2018).
Prinz, A., Behrens, C., Rapoport, T. A., Hartmann, E. & Kalies, K. U. Evolutionarily conserved binding of ribosomes to the translocation channel via the large ribosomal RNA. EMBO J. 19, 1900–1906 (2000).
Bonnefoy, N., Chalvet, F., Hamel, P., Slonimski, P. P. & Dujardin, G. OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J. Mol. Biol. 239, 201–212 (1994).
Sundberg, E. et al. ALBINO3, an Arabidopsis nuclear gene essential for chloroplast differentiation, encodes a chloroplast protein that shows homology to proteins present in bacterial membranes and yeast mitochondria. Plant. Cell 9, 717–730 (1997).
Samuelson, J. C. et al. YidC mediates membrane protein insertion in bacteria. Nature 406, 637–641 (2000).
Hell, K., Neupert, W. & Stuart, R. A. Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 20, 1281–1288 (2001).
Moore, M., Harrison, M. S., Peterson, E. C. & Henry, R. Chloroplast Oxa1p homolog albino3 is required for post-translational integration of the light harvesting chlorophyll-binding protein into thylakoid membranes. J. Biol. Chem. 275, 1529–1532 (2000).
Kumazaki, K. et al. Crystal structure of Escherichia coli YidC, a membrane protein chaperone and insertase. Sci. Rep. 4, 7299 (2014).
Kumazaki, K. et al. Structural basis of Sec-independent membrane protein insertion by YidC. Nature 509, 516–520 (2014). The high-resolution crystal structure of bacterial YidC reveals a membrane-embedded hydrophilic vestibule proposed to function during TMD insertion.
Borowska, M. T., Dominik, P. K., Anghel, S. A., Kossiakoff, A. A. & Keenan, R. J. A YidC-like protein in the archaeal plasma membrane. Structure 23, 1715–1724 (2015).
McDowell, M. A., Heimes, M. & Sinning, I. Structural and molecular mechanisms for membrane protein biogenesis by the Oxa1 superfamily. Nat. Struct. Mol. Biol. 28, 234–239 (2021).
Shanmugam, S. K. et al. New insights into amino-terminal translocation as revealed by the use of YidC and Sec depletion strains. J. Mol. Biol. 431, 1025–1037 (2019).
Welte, T. et al. Promiscuous targeting of polytopic membrane proteins to SecYEG or YidC by the Escherichia coli signal recognition particle. Mol. Biol. Cell 23, 464–479 (2012).
Facey, S. J., Neugebauer, S. A., Krauss, S. & Kuhn, A. The mechanosensitive channel protein MscL is targeted by the SRP to the novel YidC membrane insertion pathway of Escherichia coli. J. Mol. Biol. 365, 995–1004 (2007).
Samuelson, J. C. et al. Function of YidC for the insertion of M13 procoat protein in Escherichia coli: translocation of mutants that show differences in their membrane potential dependence and Sec requirement. J. Biol. Chem. 276, 34847–34852 (2001).
von Heijne, G. & Gavel, Y. Topogenic signals in integral membrane proteins. Eur. J. Biochem. 174, 671–678 (1988).
Petriman, N. A. et al. The interaction network of the YidC insertase with the SecYEG translocon, SRP and the SRP receptor FtsY. Sci. Rep. 8, 578 (2018).
Kedrov, A. et al. Structural dynamics of the YidC:ribosome complex during membrane protein biogenesis. Cell Rep. 17, 2943–2954 (2016).
Hessa, T. et al. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 450, 1026–1030 (2007).
Meacock, S. L., Lecomte, F. J., Crawshaw, S. G. & High, S. Different transmembrane domains associate with distinct endoplasmic reticulum components during membrane integration of a polytopic protein. Mol. Biol. Cell 13, 4114–4129 (2002).
Ismail, N., Crawshaw, S. G., Cross, B. C., Haagsma, A. C. & High, S. Specific transmembrane segments are selectively delayed at the ER translocon during opsin biogenesis. Biochem. J. 411, 495–506 (2008).
Chitwood, P. J. & Hegde, R. S. An intramembrane chaperone complex facilitates membrane protein biogenesis. Nature 584, 630–634 (2020). Discovery of the PAT complex as an intramembrane chaperone that shields hydrophilic TMDs during multipass membrane protein biogenesis.
Sato, B. K., Schulz, D., Do, P. H. & Hampton, R. Y. Misfolded membrane proteins are specifically recognized by the transmembrane domain of the Hrd1p ubiquitin ligase. Mol. Cell 34, 212–222 (2009).
Inglis, A. J., Page, K. R., Guna, A. & Voorhees, R. M. Differential modes of orphan subunit recognition for the WRB/CAML complex. Cell Rep. 30, 3691–3698 e3695 (2020).
Sun, S. & Mariappan, M. C-terminal tail length guides insertion and assembly of membrane proteins. J. Biol. Chem. 295, 15498–15510 (2020).
Feige, M. J. & Hendershot, L. M. Quality control of integral membrane proteins by assembly-dependent membrane integration. Mol. Cell 51, 297–309 (2013).
Yamamoto, S. et al. Contribution of calumin to embryogenesis through participation in the endoplasmic reticulum-associated degradation activity. Dev. Biol. 393, 33–43 (2014).
Morimoto, M. et al. Bi-allelic CCDC47 variants cause a disorder characterized by woolly hair, liver dysfunction, dysmorphic features, and global developmental delay. Am. J. Hum. Genet. 103, 794–807 (2018).
Botte, M. et al. A central cavity within the holo-translocon suggests a mechanism for membrane protein insertion. Sci. Rep. 6, 38399 (2016).
Hayer-Hartl, M., Bracher, A. & Hartl, F. U. The GroEL-GroES chaperonin machine: a nano-cage for protein folding. Trends Biochem. Sci. 41, 62–76 (2016).
Martin, R. et al. Structure and dynamics of the central lipid pool and proteins of the bacterial holo-translocon. Biophys. J. 116, 1931–1940 (2019).
Sadlish, H., Pitonzo, D., Johnson, A. E. & Skach, W. R. Sequential triage of transmembrane segments by Sec61alpha during biogenesis of a native multispanning membrane protein. Nat. Struct. Mol. Biol. 12, 870–878 (2005).
Borel, A. C. & Simon, S. M. Biogenesis of polytopic membrane proteins: membrane segments assemble within translocation channels prior to membrane integration. Cell 85, 379–389 (1996).
Krogan, N. J. et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 (2006).
Gavin, A. C. et al. Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 (2006).
Babu, M. et al. Interaction landscape of membrane-protein complexes in Saccharomyces cerevisiae. Nature 489, 585–589 (2012).
Lippincott-Schwartz, J., Bonifacino, J. S., Yuan, L. C. & Klausner, R. D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell 54, 209–220 (1988).
Juszkiewicz, S. & Hegde, R. S. Quality control of orphaned proteins. Mol. Cell 71, 443–457 (2018).
McShane, E. et al. Kinetic analysis of protein stability reveals age-dependent degradation. Cell 167, 803–815 e821 (2016).
Feng, L. et al. Molecular mechanism of AHSP-mediated stabilization of alpha-hemoglobin. Cell 119, 629–640 (2004).
Kihm, A. J. et al. An abundant erythroid protein that stabilizes free alpha-haemoglobin. Nature 417, 758–763 (2002).
Pleiner, T. et al. WNK1 is an assembly factor for the human ER membrane protein complex. Mol. Cell https://doi.org/10.1016/j.molcel.2021.04.013 (2021).
Rousseau, A. & Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 19, 697–712 (2018).
Richard, M., Boulin, T., Robert, V. J., Richmond, J. E. & Bessereau, J. L. Biosynthesis of ionotropic acetylcholine receptors requires the evolutionarily conserved ER membrane complex. Proc. Natl Acad. Sci. USA 110, E1055–1063 (2013).
Pop, O. I. et al. YidC is required for the assembly of the MscL homopentameric pore. FEBS J. 276, 4891–4899 (2009).
Nishikawa, H., Kanno, K., Endo, Y. & Nishiyama, K. I. Ring assembly of c subunits of F0 F1 -ATP synthase in Propionigenium modestum requires YidC and UncI following MPIase-dependent membrane insertion. FEBS Lett. 595, 647–654 (2021).
Coelho, J. P. L. et al. A network of chaperones prevents and detects failures in membrane protein lipid bilayer integration. Nat. Commun. 10, 672 (2019).
Bonifacino, J. S. et al. Association and dissociation of the murine T cell receptor associated protein (TRAP). Early events in the biosynthesis of a multisubunit receptor. J. Biol. Chem. 263, 8965–8971 (1988).
Schwenk, J. et al. An ER assembly line of AMPA-receptors controls excitatory neurotransmission and its plasticity. Neuron 104, 680–692 e689 (2019).
Schwenk, J. et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74, 621–633 (2012).
Gu, S. et al. Brain alpha7 nicotinic acetylcholine receptor assembly requires NACHO. Neuron 89, 948–955 (2016).
Halevi, S. et al. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 21, 1012–1020 (2002).
Eimer, S. et al. Regulation of nicotinic receptor trafficking by the transmembrane Golgi protein UNC-50. EMBO J. 26, 4313–4323 (2007).
Satoh, T., Ohba, A., Liu, Z., Inagaki, T. & Satoh, A. K. dPob/EMC is essential for biosynthesis of rhodopsin and other multi-pass membrane proteins in Drosophila photoreceptors. eLife https://doi.org/10.7554/eLife.06306 (2015).
Gottschalk, A. et al. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J. 24, 2566–2578 (2005).
Talbot, B. E., Vandorpe, D. H., Stotter, B. R., Alper, S. L. & Schlondorff, J. S. Transmembrane insertases and N-glycosylation critically determine synthesis, trafficking, and activity of the nonselective cation channel TRPC6. J. Biol. Chem. 294, 12655–12669 (2019). Genome-wide screen for factors that impair surface expression of a multipass transient receptor potential channel mutant yields components of the EMC, the SND pathway, the PAT complex and the multipass translocon.
Kamat, S., Yeola, S., Zhang, W., Bianchi, L. & Driscoll, M. NRA-2, a nicalin homolog, regulates neuronal death by controlling surface localization of toxic Caenorhabditis elegans DEG/ENaC channels. J. Biol. Chem. 289, 11916–11926 (2014).
Savidis, G. et al. Identification of zika virus and dengue virus dependency factors using functional genomics. Cell Rep. 16, 232–246 (2016).
Marceau, C. D. et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 (2016).
Labeau, A. et al. A genome-wide CRISPR-Cas9 screen identifies the dolichol-phosphate mannose synthase complex as a host dependency factor for dengue virus infection. J. Virol. https://doi.org/10.1128/JVI.01751-19 (2020).
Schneider, W. M. et al. Genome-scale identification of SARS-CoV-2 and pan-coronavirus host factor networks. Cell 184, 120–132 e114 (2021).
Doms, R. W., Lamb, R. A., Rose, J. K. & Helenius, A. Folding and assembly of viral membrane proteins. Virology 193, 545–562 (1993).
Daniels, R., Kurowski, B., Johnson, A. E. & Hebert, D. N. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol. Cell 11, 79–90 (2003).
Maggioni, M. C., Liscaljet, I. M. & Braakman, I. A critical step in the folding of influenza virus HA determined with a novel folding assay. Nat. Struct. Mol. Biol. 12, 258–263 (2005).
Blobel, G. Intracellular protein topogenesis. Proc. Natl Acad. Sci. USA 77, 1496–1500 (1980).
Kemp, G. & Cymer, F. Small membrane proteins - elucidating the function of the needle in the haystack. Biol. Chem. 395, 1365–1377 (2014).
Chen, J. et al. Pervasive functional translation of noncanonical human open reading frames. Science 367, 1140–1146 (2020).
Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982).
Huber, D. et al. SecA cotranslationally interacts with nascent substrate proteins in vivo. J. Bacteriol. https://doi.org/10.1128/JB.00622-16 (2017).
Wang, S., Yang, C. I. & Shan, S. O. SecA mediates cotranslational targeting and translocation of an inner membrane protein. J. Cell Biol. 216, 3639–3653 (2017).
Wang, S. et al. The molecular mechanism of cotranslational membrane protein recognition and targeting by SecA. Nat. Struct. Mol. Biol. 26, 919–929 (2019). Cryo-EM structure and biochemical analysis provide mechanistic insight into how SecA mediates co-translational targeting of certain membrane proteins in E. coli.
Gelis, I. et al. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell 131, 756–769 (2007).
Chatzi, K. E., Sardis, M. F., Economou, A. & Karamanou, S. SecA-mediated targeting and translocation of secretory proteins. Biochim. Biophys. Acta 1843, 1466–1474 (2014).
Ast, T., Cohen, G. & Schuldiner, M. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell 152, 1134–1145 (2013).
Aviram, N. et al. The SND proteins constitute an alternative targeting route to the endoplasmic reticulum. Nature 540, 134–138 (2016).
Hassdenteufel, S. et al. hSnd2 protein represents an alternative targeting factor to the endoplasmic reticulum in human cells. FEBS Lett. 591, 3211–3224 (2017).
Phillips, B. P. & Miller, E. A. Ribosome-associated quality control of membrane proteins at the endoplasmic reticulum. J. Cell Sci. https://doi.org/10.1242/jcs.251983 (2020).
Brandman, O. & Hegde, R. S. Ribosome-associated protein quality control. Nat. Struct. Mol. Biol. 23, 7–15 (2016).
Yip, M. C. J. & Shao, S. Detecting and rescuing stalled ribosomes. Trends Biochem. Sci. https://doi.org/10.1016/j.tibs.2021.03.008 (2021).
Joazeiro, C. A. P. Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 20, 368–383 (2019).
Hessa, T. et al. Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature 475, 394–397 (2011).
Rodrigo-Brenni, M. C., Gutierrez, E. & Hegde, R. S. Cytosolic quality control of mislocalized proteins requires RNF126 recruitment to Bag6. Mol. Cell 55, 227–237 (2014).
Dederer, V. et al. Cooperation of mitochondrial and ER factors in quality control of tail-anchored proteins. Elife 8, https://doi.org/10.7554/eLife.45506 (2019).
Matsumoto, S. et al. Msp1 clears mistargeted proteins by facilitating their transfer from mitochondria to the ER. Mol. Cell 76, 191–205 e110 (2019).
Stefanovic-Barrett, S. et al. MARCH6 and TRC8 facilitate the quality control of cytosolic and tail-anchored proteins. EMBO Rep. https://doi.org/10.15252/embr.201745603 (2018).
Leto, D. E. et al. Genome-wide CRISPR analysis identifies substrate-specific conjugation modules in ER-associated degradation. Mol. Cell 73, 377–389 e311 (2019).
McKenna, M. J. et al. The endoplasmic reticulum P5A-ATPase is a transmembrane helix dislocase. Science https://doi.org/10.1126/science.abc5809 (2020).
Chen, Y. C. et al. Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. EMBO J. 33, 1548–1564 (2014).
Okreglak, V. & Walter, P. The conserved AAA-ATPase Msp1 confers organelle specificity to tail-anchored proteins. Proc. Natl Acad. Sci. USA 111, 8019–8024 (2014).
Wohlever, M. L., Mateja, A., McGilvray, P. T., Day, K. J. & Keenan, R. J. Msp1 is a membrane protein dislocase for tail-anchored proteins. Mol. Cell 67, 194–202 e196 (2017).
Wu, X. & Rapoport, T. A. Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 53, 22–28 (2018).
Christianson, J. C. & Ye, Y. Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nat. Struct. Mol. Biol. 21, 325–335 (2014).
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011).
Beck, K. et al. YidC, an assembly site for polytopic Escherichia coli membrane proteins located in immediate proximity to the SecYE translocon and lipids. EMBO Rep. 2, 709–714 (2001).
Yi, L. et al. YidC is strictly required for membrane insertion of subunits a and c of the F1F0 ATP synthase and SecE of the SecYEG translocase. Biochemistry 42, 10537–10544 (2003).
van Bloois, E., Jan Haan, G., de Gier, J. W., Oudega, B. & Luirink, J. F1F0 ATP synthase subunit c is targeted by the SRP to YidC in the E. coli inner membrane. FEBS Lett. 576, 97–100 (2004).
Celebi, N., Yi, L., Facey, S. J., Kuhn, A. & Dalbey, R. E. Membrane biogenesis of subunit II of cytochrome bo oxidase: contrasting requirements for insertion of N-terminal and C-terminal domains. J. Mol. Biol. 357, 1428–1436 (2006).
van Bloois, E., Haan, G. J., de Gier, J. W., Oudega, B. & Luirink, J. Distinct requirements for translocation of the N-tail and C-tail of the Escherichia coli inner membrane protein CyoA. J. Biol. Chem. 281, 10002–10009 (2006).
Wagner, S. et al. Biogenesis of MalF and the MalFGK2 maltose transport complex in Escherichia coli requires YidC. J. Biol. Chem. 283, 17881–17890 (2008).
Zhu, L., Kaback, H. R. & Dalbey, R. E. YidC protein, a molecular chaperone for LacY protein folding via the SecYEG protein machinery. J. Biol. Chem. 288, 28180–28194 (2013).
Nagamori, S., Smirnova, I. N. & Kaback, H. R. Role of YidC in folding of polytopic membrane proteins. J. Cell Biol. 165, 53–62 (2004).
du Plessis, D. J., Nouwen, N. & Driessen, A. J. Subunit a of cytochrome o oxidase requires both YidC and SecYEG for membrane insertion. J. Biol. Chem. 281, 12248–12252 (2006).
van der Laan, M., Bechtluft, P., Kol, S., Nouwen, N. & Driessen, A. J. F1F0 ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis. J. Cell Biol. 165, 213–222 (2004).
Serek, J. et al. Escherichia coli YidC is a membrane insertase for Sec-independent proteins. EMBO J. 23, 294–301 (2004).
Stiegler, N., Dalbey, R. E. & Kuhn, A. M13 procoat protein insertion into YidC and SecYEG proteoliposomes and liposomes. J. Mol. Biol. 406, 362–370 (2011).
Robinson, P. J. & Woolhead, C. A. Post-translational membrane insertion of an endogenous YidC substrate. Biochim. Biophys. Acta 1833, 2781–2788 (2013).
Price, C. E., Kocer, A., Kol, S., van der Berg, J. P. & Driessen, A. J. In vitro synthesis and oligomerization of the mechanosensitive channel of large conductance, MscL, into a functional ion channel. FEBS Lett. 585, 249–254 (2011).
Ernst, S., Schonbauer, A. K., Bar, G., Borsch, M. & Kuhn, A. YidC-driven membrane insertion of single fluorescent Pf3 coat proteins. J. Mol. Biol. 412, 165–175 (2011).
Serdiuk, T. et al. Insertion and folding pathways of single membrane proteins guided by translocases and insertases. Sci. Adv. 5, eaau6824 (2019).
Tian, S. et al. Proteomic analysis identifies membrane proteins dependent on the ER membrane protein complex. Cell Rep. 28, 2517–2526 e2515 (2019).
Shurtleff, M. J. et al. The ER membrane protein complex interacts cotranslationally to enable biogenesis of multipass membrane proteins. eLife https://doi.org/10.7554/eLife.37018 (2018).
Houben, E. N., ten Hagen-Jongman, C. M., Brunner, J., Oudega, B. & Luirink, J. The two membrane segments of leader peptidase partition one by one into the lipid bilayer via a Sec/YidC interface. EMBO Rep. 5, 970–975 (2004).
Sachelaru, I. et al. YidC occupies the lateral gate of the SecYEG translocon and is sequentially displaced by a nascent membrane protein. J. Biol. Chem. 288, 16295–16307 (2013).
Acknowledgements
The authors thank members of the Hegde and Keenan laboratories for productive discussions that influenced this Review. R.S.H. is funded by the UK Medical Research Council (MC_UP_A022_1007). R.J.K. is funded by grants from the US National Institutes of Health (R01 GM086487 and R01 GM130051).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Targeting sequence
-
The sequence element in a protein that directs its delivery to a specific membrane in the cell. For membrane proteins, the targeting sequence is typically a cleavable amino-terminal signal peptide or the first transmembrane domain.
- Signal peptide
-
A targeting sequence that is found at the amino terminus of secretory proteins and some membrane proteins. After they have served their targeting function, signal peptides are cleaved off by an enzyme called ‘signal peptidase’.
- Tail-anchored (TA) membrane proteins
-
Membrane proteins whose only transmembrane domain lies within ~65 amino acids of the carboxy terminus and are oriented with the amino terminus facing the cytosol. These are sometimes called ‘type IV membrane proteins’.
- Sec61 complex
-
A heterotrimeric protein complex that translocates hydrophilic polypeptide segments across the membrane through an aqueous channel and inserts hydrophobic domains into the membrane through a lateral gate. It is called the ‘SecY complex’ in prokaryotes.
- Insertases
-
Transmembrane proteins containing a hydrophilic vestibule that facilitates translocation of short polypeptide segments across the membrane concomitant with transmembrane domain insertion.
- Oxa1 superfamily
-
An evolutionarily related group of membrane protein insertases that includes Oxa1 in the inner mitochondrial membrane, YidC in the bacterial inner membrane, Ylp1 in the archaeal plasma membrane, Alb3 in the chloroplast inner membrane, and GET1, EMC3 and TMCO1 in the eukaryotic endoplasmic reticulum.
- Type I membrane proteins
-
Signal peptide-containing membrane proteins oriented with their mature amino terminus facing the lumen (a topology that is also termed ‘Nexo’) following signal peptide cleavage.
- Type II membrane proteins
-
Membrane proteins oriented with their amino terminus facing the cytosol (a topology that is also termed ‘Ncyt’).
- Type III membrane proteins
-
Membrane proteins oriented with their amino terminus facing the lumen (a topology that is also termed ‘Nexo’); these proteins typically possess a short (fewer than 50 amino acids) amino-terminal flanking region.
- Multipass membrane proteins
-
Proteins spanning the membrane more than once.
- Single-pass membrane proteins
-
Proteins spanning the membrane once.
- Intramembrane chaperone
-
A factor that promotes folding in the membrane by temporarily shielding partially hydrophilic transmembrane domains of nascent polypeptides until their successful assembly with other transmembrane domains.
- Chaperonin
-
A family of ATP-driven multimeric chaperone complexes characterized by a cylindrical structure with an internal chamber. The interior of the chaperonin cylinder provides a protected environment within which nascent proteins can fold.
- Assembly factor
-
A factor that promotes the assembly of two or more proteins, possibly by temporarily shielding their inter-subunit interfaces.
Rights and permissions
About this article
Cite this article
Hegde, R.S., Keenan, R.J. The mechanisms of integral membrane protein biogenesis. Nat Rev Mol Cell Biol 23, 107–124 (2022). https://doi.org/10.1038/s41580-021-00413-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-021-00413-2
This article is cited by
-
EMC rectifies the topology of multipass membrane proteins
Nature Structural & Molecular Biology (2024)
-
Substrate recognition mechanism of the endoplasmic reticulum-associated ubiquitin ligase Doa10
Nature Communications (2024)
-
zDHHC20-driven S-palmitoylation of CD80 is required for its costimulatory function
Acta Pharmacologica Sinica (2024)
-
Effects of Chemical Fixatives on Kinetic Measurements of Biomolecular Interaction on Cell Membrane
The Journal of Membrane Biology (2024)
-
Visualization of translation and protein biogenesis at the ER membrane
Nature (2023)