Abstract
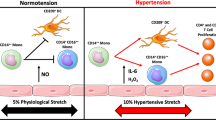
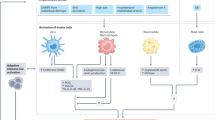
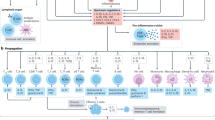
Immune mechanisms have been recognized to have a role in the pathogenesis of hypertension, vascular disease and kidney damage in humans and animals for many decades. Contemporary advances in experimentation have permitted a deeper understanding of the mechanisms by which inflammation and immunity participate in cardiovascular disease, and multiple observations have demonstrated strong correlations between the discoveries made in animals and those made in patients with hypertension. Of note, striking phenotypic similarities have been observed in the infiltration of immune cells in the kidney and the development of end-organ damage in patients and animal models with sodium-sensitive hypertension. The available data suggest that an initial salt-induced increase in renal perfusion pressure, which is likely independent of immune mechanisms, induces the infiltration of immune cells into the kidney. The mechanisms mediating immune cell infiltration in the kidney are not well understood but likely involve tissue damage, the direct influence of salt to stimulate immune cell activation, sympathetic nerve stimulation or other factors. The infiltrating cells then release cytokines, free radicals and other factors that contribute to renal damage as well as increased retention of sodium and water and vascular resistance, which lead to the further development of hypertension.
Key points
-
Individuals with hypertension, particularly those with salt sensitivity of blood pressure, have an associated increase in renal end-organ damage that is accompanied by the infiltration of macrophages and T lymphocytes into the kidney.
-
Pharmacological and genetic studies have demonstrated that immune mechanisms have a role in the development of disease in experimental models of hypertension and related kidney damage.
-
The molecular mechanisms that mediate the effects of immune cells in hypertension include the release of cytokines and other molecular species that alter physiological function and/or elicit tissue damage.
-
Evidence indicates that immune activation in hypertension occurs secondary to primary haemodynamic or other stimuli.
-
The processes that activate immunity in hypertension are unclear but may involve sympathetic stimulation, pressure-mediated tissue damage, exposure of antigens or neoantigens and other effects related to high salt intake.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260 (2012).
Poulter, N. R., Prabhakaran, D. & Caulfield, M. Hypertension. Lancet 386, 801–812 (2015).
Kearney, P. M. et al. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005).
Rapsomaniki, E. et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 383, 1899–1811 (2014).
Benjamin, E. J. et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137, e67–e492 (2018).
Muntner, P. et al. Potential U. S. population impact of the 2017 American College of Cardiology/American Heart Association high blood pressure guideline. Circulation 137, 109–118 (2017).
Kotchen, T. A., Cowley, A. W. Jr & Frohlich, E. D. Salt in health and disease-a delicate balance. N. Engl. J. Med. 368, 1229–1237 (2013).
Elijovich, F. et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 68, e7–e46 (2016).
He, J. et al. Gender differences in blood pressure response to dietary sodium intervention in the GenSalt study. J. Hypertens. 27, 48–54 (2009).
Kawasaki, T., Delea, C. S., Bartter, F. C. & Smith, H. The effect of high sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am. J. Med. 64, 193–198 (1978).
Weinberger, M. H., Miller, J. Z., Luft, F. C., Grim, C. E. & Fineberg, N. S. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 8, II127–II134 (1986).
Morimoto, A. et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350, 1734–1737 (1997).
Weinberger, M. H., Fineberg, N. S., Fineberg, S. E. & Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37, 429–432 (2001).
Hughson, M. D. et al. Associations of glomerular number and birth weight with clinicopathological features of African Americans and Whites. Am. J. Kidney Dis. 52, 18–28 (2008).
Johnson, R. J. et al. Subtle renal injury is a likely common mechanism for salt-sensitive essential hypertension. Hypertension 45, 326–330 (2005).
Johnson, R. J., Herrera-Acosta, J., Schreiner, G. F. & Rodríguez-Iturbe, B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N. Engl. J. Med. 346, 913–923 (2002).
Mattson, D. L. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am. J. Physiol. 307, F499–F508 (2014).
Rodríguez-Iturbe, B., Vaziri, N. D., Herrera-Acosta, J. & Johnson, R. J. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am. J. Physiol. 286, F606–F616 (2004).
Stewart, T., Jung, F. F., Manning, J. & Vehaskari, V. M. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 68, 2180–2188 (2005).
Rodríguez-Iturbe, B., Pons, H., Quiroz, Y., Lanaspa, M. A. & Johnson, R. J. Autoimmunity in the pathogenesis of hypertension. Nat. Rev. Nephrol. 10, 56–62 (2014).
Rodríguez-Iturbe, B. Renal infiltration of immunocompetent cells: cause and effect of sodium-sensitive hypertension. Clin. Exp. Nephrol. 14, 105–111 (2010).
Rodríguez-Iturbe, B. Autoimmunity in the pathogenesis of hypertension. Hypertension 67, 477–483 (2016).
Harrison, D. G. et al. Inflammation, immunity, and hypertension. Hypertension 57, 132–140 (2011).
Madhur, M. S. & Harrison, D. G. Synapses, signals, CDs, and cytokines: Interactions of the autonomic nervous system and immunity in hypertension. Circ. Res. 111, 1113–1116 (2012).
Norlander, A. E., Madhur, M. S. & Harrison, D. G. The immunology of hypertension. J. Exp. Med. 215, 21–33 (2018).
Ryan, M. J. An update on immune system activation in the pathogenesis of hypertension. Hypertension 62, 226–230 (2013).
Schiffrin, E. L. T lymphocytes: a role in hypertension? Curr. Opin. Nephrol. Hypertens. 19, 181–186 (2010).
Norlander, A. E. & Madhur, M. S. Inflammatory cytokines regulate renal sodium transporters: how, where, and why? Am. J. Physiol. 313, F141–F144 (2017).
Sommers, S. C., Relman, A. S. & Smithwick, R. H. Histologic studies of kidney biopsy specimens from patients with hypertension. Am. J. Pathol. 34, 685–715 (1958).
Olsen, F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Path. Microbiol. Scand. A 80, 253–256 (1972).
Paronetto, F. Immunocytochemical observations on the vascular necrosis and renal glomerular lesions of malignant nephrosclerosis. Am. J. Pathol. 46, 901–915 (1965).
Ebringer, A. & Doyle, A. E. Raised Serum IgG levels in hypertension. Br. Med. J. 5702, 146–148 (1970).
Olsen, F. Immunological factors and high blood pressure in man. Acta Path. Microbiol. Scand. A 80, 257–259 (1972).
Youn, J.-C. et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133 (2013).
Seaberg, E. C. et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19, 953–960 (2005).
Herrera, J., Ferrebuz, A., García MacGregor, E. & Rodriguez-Iturbe, B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 17, 218–225 (2006).
Ehret, G. B., O’Connor, A. A., Weder, A., Cooper, R. S. & Chakravarti, A. Follow-up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur. J. Hum. Genet. 17, 1650–1657 (2009).
Fox, E. R. et al. Association of genetic variation with systolic and diastolic blood pressure among African Americans: the candidate gene association resource study. Hum. Mol. Genet. 20, 2273–2284 (2011).
Levy, D. et al. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 41, 677–687 (2009).
Shinzawa, M. et al. Gene polymorphisms contributing to hypertension in immunoglobulin A nephropathy. Clin. Exp. Nephrol. 16, 250–258 (2012).
Poesen, R. et al. Associations of soluble CD14 and endotoxin with mortality, cardiovascular disease, and progression of kidney disease among patients with CKD. Clin. J. Am. Soc. Nephrol. 4, 1525–1533 (2015).
Devalliere, J. & Charreau, B. The Adaptor Lnk (Sh2b3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem. Pharmacol. 82, 1391–1402 (2011).
Brancato, S. K. et al. Toll-like receptor 4 signaling regulates the acute local inflammatory response to injury and the fibrosis/neovascularization of sterile wounds. Wound Repair Regen. 21, 624–633 (2013).
Triantafilou, M. & Triantafilou, K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 23, 301–304 (2002).
Irving, B. A., Chan, A. C. & Weiss, A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J. Exp. Med. 177, 1093–1103 (1993).
Itoh, Y. et al. Structural analysis of the CD3 zeta/eta locus of the rat. Expression of zeta but not eta transcripts by rat T cells. J. Immun. 151, 4705–4717 (1993).
Sussman, J. J. et al. Failure to synthesize the T cell CD3-ζ chain: structure and function of a partial T cell receptor complex. Cell 52, 85–96 (1988).
White, F. N. & Grollman, A. Autoimmune factors associated with infarction of the kidney. Nephron 1, 93–102 (1964).
Okuda, T. & Grollman, A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex. Rep. Biol. Med. 25, 257–264 (1967).
Olsen, F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol. Microbiol. Scand. C 88, 1–5 (1980).
Svendsen, U. G. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol. Microbiol. Scand. A 84, 523–528 (1976).
Khraibi, A. A., Norman, R. A. & Dzielak, D. J. Chronic immunosuppression attenuates hypertension in Okamoto spontaneously hypertensive rats. Am. J. Physiol. 247, H722–H726 (1984).
Norman, R. A. Jr., Galloway, P. G., Dzielak, D. J. & Huang, M. Mechanisms of partial infarct hypertension. J. Hypertension 6, 397–403 (1988).
Khraibi, A. A., Smith, T. L., Hutchins, P. M., Lynch, C. D. & Dusseau, J. W. Thymectomy delays the development of hypertension in Okamoto spontaneously hypertensive rats. J. Hypertens. 5, 537–541 (1987).
Khraibi, A. A. Association between disturbances in the immune system and hypertension. Am. J. Hypertens. 4, 635–641 (1991).
Ba, D., Takeichi, N., Kodama, T. & Kobayashi, H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J. Immunol. 128, 1211–1216 (1982).
Tuttle, R. S. & Boppana, D. P. Antihypertensive effect of interleukin-2. Hypertension 15, 89–94 (1990).
Bravo, Y., Quiroz, Y., Ferrebuz, A., Vaziri, N. D. & Rodríguez-Iturbe, B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am. J. Physiol. 293, F616–F623 (2007).
Rodríguez-Iturbe, B. & Johnson, R. J. The role of inflammatory cells in the kidney in the induction and maintenance of hypertension. Nephrol. Dial. Transplant 21, 260–263 (2006).
Rodríguez-Iturbe, B. et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 59, 2222–2232 (2001).
Rodríguez-Iturbe, B. et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am. J. Physiol. 282, F191–F201 (2002).
Franco, M. et al. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am. J. Physiol. 293, R251–R256 (2007).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Alvarez, V., Quiroz, Y., Nava, M., Pons, H. & Rodríguez-Iturbe, B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am. J. Physiol. 283, F1132–F1141 (2002).
Quiroz, Y. et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthase inhibition. Am. J. Physiol. 281, F38–F47 (2001).
Pons, H. et al. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am. J. Physiol. 304, F289–F299 (2013).
Itani, H. A. et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68, 123–132 (2016).
Itani, H. A. et al. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ. Res. 118, 1233–1243 (2016).
Trott, D. W. et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64, 1108–1115 (2014).
Madhur, M. S. et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010).
Kamat, N. V. et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A−/− mice. Hypertension 65, 569–576 (2015).
Norlander, A. E. et al. Interleukin-17A regulates renal sodium transporters and renal injury in Angiotensin II-induced hypertension. Hypertension 68, 167–174 (2016).
Barbaro, N. R. et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 21, 1009–1020 (2017).
Kirabo, A. et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest. 124, 4642–4656 (2014).
Kossmann, S. & Wenzel, P. Under pressure a new role for CD11c+ myeloid cells in hypertension. Hypertension 71, 557–558 (2018).
De Ciuceis, C. et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of Angiotensin II–infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in Angiotensin-induced vascular injury. Arter. Thromb. Vasc. Biol. 25, 2106–2113 (2005).
Rickard, A. J. et al. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension 54, 537–543 (2009).
Hevia, D. et al. Myeloid CD11c+ antigen-presenting cells ablation prevents hypertension in response to angiotensin II plus high-salt diet. Hypertension 71, 709–718 (2018).
Wang, L. et al. Genetic and pharmacologic inhibition of the chemokine receptor CXCR2 prevents experimental hypertension and vascular dysfunction. Circulation 134, 1353–1368 (2016).
Chan, C. T. et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66, 1023–1033 (2015).
Caillon, A. et al. γδ T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation 135, 2155–2162 (2017).
Shah, K. H. et al. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ. Res. 117, 858–869 (2015).
Barhoumi, T. et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57, 469–476 (2011).
Matrougui, K. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am. J. Pathol. 178, 434–441 (2011).
Mian, M. O., Barhoumi, T., Briet, M., Paradis, P. & Schiffrin, E. L. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J. Hypertens. 34, 97–108 (2016).
Katsuki, M., Hirooka, Y., Kishi, T. & Sunagawa, K. Decreased proportion of Foxp3+CD4+ regulatory T cells contributes to the development of hypertension in genetically hypertensive rats. J. Hypertens. 33, 773–783 (2015).
De Miguel, C., Das, S., Lund, H. & Mattson, D. L. T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Physiol. 298, R1136–R1142 (2010).
Campese, V. M. Salt sensitivity in hypertension. Hypertension 23, 531–550 (1994).
Cowley, A. W. Jr & Roman, R. J. The role of the kidney in hypertension. JAMA 275, 1581–1589 (1996).
Feldman, H. I., Klag, M. J., Chiapella, A. P. & Whelton, P. K. End-stage renal disease in US minority groups. Am. J. Kidney Dis. 19, 397–410 (1992).
Grim, C. E. et al. Blood pressure in blacks. Hypertension 15, 803–809 (1990).
Lackland, D. T. & Keil, J. E. Epidemiology of hypertension in African Americans. Semin. Nephrol. 16, 63–70 (1996).
Bigazzi, R. et al. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension 23, 195–199 (1994).
Mattson, D. L., James, L., Berdan, E. A. & Meister, C. J. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48, 149–156 (2006).
Ozawa, Y., Kobori, H., Suzaki, Y. & Navar, L. G. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am. J. Physiol. 292, F330–F339 (2007).
Mai, M. et al. Early changes in hypertension induced renal injury. Hypertension 22, 754–765 (1993).
Pechman, K. R., Basile, D. P., Lund, H. & Mattson, D. L. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am. J. Physiol. 294, R1234–R1239 (2008).
Rudemiller, N., Lund, H., Jacob, H. J., Geurts, A. M. & Mattson, D. L. CD247 modulates blood pressure by altering T lymphocyte infiltration in the kidney. Hypertension 63, 559–564 (2014).
De Miguel, C., Lund, H., Di, F. & Mattson, D. L. Infiltrating T lymphocytes in the kidney increase oxidative stress and lead to hypertension and renal disease. Am. J. Physiol. 300, F734–F742 (2011).
De Miguel, C., Lund, H. & Mattson, D. L. High dietary protein exacerbates hypertension and renal damage in Dahl salt-sensitive (SS) rats by increasing infiltrating immune cells. Hypertension 57, 269–274 (2011).
Bendich, A., Belisle, E. H. & Strausser, H. R. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem. Biophys. Res. Comm. 99, 600–607 (1981).
Geurts, A. M. et al. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol. Biol. 597, 211–225 (2010).
Geurts, A. M. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 24, 433 (2009).
Mattson, D. L. et al. Genetic mutation of recombination activating gene 1 in Dahl salt sensitive rats attenuates hypertension and renal damage. Am. J. Physiol. 304, R407–R414 (2013).
Rudemiller, N. P. et al. Mutation of SH2B3 (LNK), a GWAS candidate for hypertension, attenuates Dahl SS hypertension via inflammatory modulation. Hypertension 65, 1111–1117 (2015).
Rieux-Laucat, F. et al. Inherited and somatic CD3ζ mutations in a patient with T cell deficiency. N. Engl. J. Med. 354, 1913–1921 (2006).
Guyenet, P. G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346 (2006).
Maranon, R. O. et al. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am. J. Physiol. 308, R708–R713 (2015).
Andersson, U. & Tracey, K. J. Neural reflexes in inflammation and immunity. J. Exp. Med. 209, 1057–1068 (2012).
Marvar, P. J. et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ. Res. 107, 263–270 (2010).
Carnevale, D. et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 41, 737–752 (2014).
Mori, T. et al. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J. Am. Soc. Nephrol. 19, 1472–1482 (2008).
Evans, L. C. et al. Increased perfusion pressure drives renal T cell infiltration in the Dahl salt-sensitive rat. Hypertension 70, 543–551 (2017).
Bidani, A. K., Griffin, K. A., Williamson, G., Wang, X. & Loutzenhiser, R. Protective importance of the myogenic response in the renal circulation. Hypertension 54, 393–398 (2009).
Burke, M., Pabbidi, M. R., Farley, J. & Roman, R. J. Molecular mechanisms of renal blood flow autoregulation. Curr. Vasc. Pharmacol. 12, 845–858 (2014).
Campese, V. M., Parise, M., Karubian, F. & Bigazzi, R. Abnormal renal hemodynamics in Black salt-sensitive patients with hypertension. Hypertension 18, 805–812 (1991).
Karlsen, F. M., Andersen, C. B., Leyssac, P. P. & Holstein-Rathlou, N. H. Dynamic autoregulation and renal injury in Dahl rats. Hypertension 30, 975–983 (1997).
Takenaka, T., Forster, H., De Micheli, A. & Epstein, M. Impaired myogenic responsiveness of renal microvessels in Dahl salt sensitive rats. Circ. Res. 71, 471–480 (1992).
Loperena, R. et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc. Res. 114, 1547–1563 (2018).
Vinh, A. et al. Inhibition and genetic ablation of the B7/CD28 T cell costimulation axis prevents experimental hypertension. Circulation 122, 2529–2537 (2010).
Soos, T. J. et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70, 591–596 (2006).
Woltman, A. M. et al. Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int. 71, 1001–1008 (2007).
John, R. & Nelson, P. J. Dendritic cells in the kidney. Am. J. Soc. Nephrol 18, 2628–2635 (2007).
Westhorpe, C. L. V. et al. Effector CD4+ T cells recognize intravascular antigen presented by patrolling monocytes. Nat. Commun. 9, 747 (2018).
Devi, S. et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat. Med. 19, 107–112 (2013).
Finsterbusch, M. et al. Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc. Natl Acad. Sci. USA 113, E5172–E5181 (2016).
Macconi, D. et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J. Am. Soc. Nephrol. 20, 123–130 (2009).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Binger, K. J., Linker, R. A., Muller, D. N. & Kleinewietfeld, M. Sodium chloride, SGK1, and Th17 activation. Pflugers Arch. 467, 543–550 (2015).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Jantsch, J. et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 21, 493–501 (2015).
Titze, J. Sodium balance is not just a renal affair. Curr. Opin. Nephrol. Hypertens. 23, 101–105 (2014).
Yi, B. et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res. 166, 103–110 (2015).
O’Leary, R., Penrose, H., Miyata, K. & Satou, R. Macrophage-derived Il-6 contributes to AngII-mediated angiotensinogen stimulation in renal proximal tubular cells. Am. J. Physiol. 310, F1000–F1007 (2016).
Wade, B., Petrova, G. & Mattson, D. L. Role of immune factors in angiotensin II-induced hypertension and renal damage in Dahl salt-sensitive rats. Am. J. Physiol. 314, R323–R333 (2018).
Southcombe, J. H., Redman, C. W., Sargent, I. L. & Granne, I. Interleukin-1 family cytokines and their regulatory proteins in normal pregnancy and pre-eclampsia. Clin. Exp. Immunol. 181, 480–490 (2015).
Qi, J. et al. Targeting interleukin-1 beta to suppress sympathoexcitation in hypothalamic paraventricular nucleus in Dahl salt-sensitive hypertensive rats. Cardiovasc. Toxicol. 16, 298–306 (2016).
Zhang, J. et al. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab. 23, 360–368 (2016).
Crosswhite, P. & Sun, Z. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension 55, 1484–1491 (2010).
Lee, D. L. et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am. J. Physiol. 290, H935–H940 (2006).
Hashmat, S. et al. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am. J. Physiol. 311, F555–F561 (2016).
Norlander, A. E. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2, e92801 (2017).
Saleh, M. A., Norlander, A. E. & Madhur, M. S. Inhibition of interleukin 17-A but not interleukin-17F signaling lowers blood pressure and reduces end-organ inflammation in angiotensin II-induced hypertension. JACC Basic Transl Sci. 1, 606–616 (2016).
Sun, X.-N. et al. T cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ. Res. 120, 1584–1597 (2017).
Venegas Pont, M. et al. Tumor necrosis factor alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56, 643–649 (2010).
Zhang, J. et al. TNF-alpha produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64, 1275–1281 (2014).
Crowley, S. D. et al. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55, 99–108 (2010).
Montezano, A. C. & Touyz, R. M. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid. Redox Signal. 20, 164–182 (2014).
Cowley, A. W. Jr et al. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am. J. Physiol. 308, F179–F197 (2014).
Imig, J. D. & Ryan, M. J. Immune and inflammatory role in renal disease. Compr. Physiol. 3, 957–976 (2013).
Acknowledgements
The author’s work is supported by US National Institutes of Health grants HL137748 and HL116264 and American Heart Association grant 15SFRN2391002.
Reviewer information
Nature Reviews Nephrology thanks S. Crowley, D. Harrison and B. Rodriguez-Iturbe for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Lymphocytes
-
Immune cells that have a role in the adaptive immune system. Lymphocytes include B lymphocytes (B cells) that produce antibodies and participate in humoral immunity and T lymphocytes (T cells) that secrete cytokines and participate in cellular immunity.
- Myeloid cells
-
Cells that are derived from haematopoietic stem cells in the bone marrow and differentiate into granulocytes and monocytes. Myeloid cells form part of the innate immune system.
- DOCA-salt hypertension
-
Experimental hypertension induced in animals by administration of deoxycorticosterone acetate, a potent mineralocorticoid, and NaCl.
- Leukocyte
-
A type of blood cell derived from haematopoietic stem cells in the bone marrow. Leukocytes have a role in the immune system and include granulocytes, monocytes and lymphocytes.
- γδ T cells
-
A subset of T cells with a distinct T cell receptor. The functions of γδ T cells bridge the innate and adaptive immune systems.
Rights and permissions
About this article
Cite this article
Mattson, D.L. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 15, 290–300 (2019). https://doi.org/10.1038/s41581-019-0121-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0121-z
This article is cited by
-
VEGFC ameliorates salt-sensitive hypertension and hypertensive nephropathy by inhibiting NLRP3 inflammasome via activating VEGFR3-AMPK dependent autophagy pathway
Cellular and Molecular Life Sciences (2023)
-
Role of Female Sex Hormones and Immune Response in Salt-Sensitive Hypertension Development: Evidence from Experimental Models
Current Hypertension Reports (2023)
-
Salt sensitivity of blood pressure in childhood and adolescence
Pediatric Nephrology (2022)
-
Momordica charantia Extract Confers Protection Against Hypertension in Dahl Salt-Sensitive Rats
Plant Foods for Human Nutrition (2022)
-
HIV-positive demonstrate more salt sensitivity and nocturnal non-dipping blood pressure than HIV-negative individuals
Clinical Hypertension (2021)