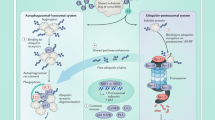
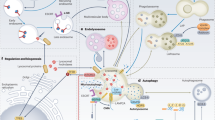
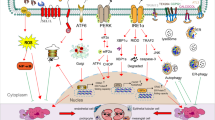
Abstract
Autophagy is a conserved lysosomal pathway for the degradation of cytoplasmic components. Basal autophagy in kidney cells is essential for the maintenance of kidney homeostasis, structure and function. Under stress conditions, autophagy is altered as part of the adaptive response of kidney cells, in a process that is tightly regulated by signalling pathways that can modulate the cellular autophagic flux — mammalian target of rapamycin, AMP-activated protein kinase and sirtuins are key regulators of autophagy. Dysregulated autophagy contributes to the pathogenesis of acute kidney injury, to incomplete kidney repair after acute kidney injury and to chronic kidney disease of varied aetiologies, including diabetic kidney disease, focal segmental glomerulosclerosis and polycystic kidney disease. Autophagy also has a role in kidney ageing. However, questions remain about whether autophagy has a protective or a pathological role in kidney fibrosis, and about the precise mechanisms and signalling pathways underlying the autophagy response in different types of kidney cells and across the spectrum of kidney diseases. Further research is needed to gain insights into the regulation of autophagy in the kidneys and to enable the discovery of pathway-specific and kidney-selective therapies for kidney diseases and anti-ageing strategies.
Key points
-
Basal autophagy is essential to the maintenance of kidney homeostasis, structure and function.
-
Autophagy is suppressed in aged kidneys, which accelerates the progression of ageing and age-related kidney diseases.
-
In the acute injury phase of acute kidney injury (AKI), autophagy is induced in proximal tubules and acts as a protective mechanism. During the recovery phase, regulated autophagy is crucial for tubular repair.
-
Persistent activation of autophagy after AKI induces phenotypic changes in proximal tubule cells that might lead to maladaptive repair, contribute to interstitial fibrosis and promote transition from AKI to chronic kidney disease.
-
Autophagy defects in kidney cells of both tubular and glomerular compartments contribute to the development of diabetic kidney disease and focal segmental glomerulosclerosis.
-
Polycystic kidney disease is associated with autophagy defects in kidney tubules that might contribute to the development and formation of kidney cysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dikic, I. & Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018).
Oku, M. & Sakai, Y. Three distinct types of microautophagy based on membrane dynamics and molecular machineries. Bioessays 40, e1800008 (2018).
Kaushik, S. & Cuervo, A. M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381 (2018).
Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42 (2019).
Morishita, H. & Mizushima, N. Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol. 35, 453–475 (2019).
Park, J. M. et al. The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy 12, 547–564 (2016).
Park, J. M. et al. ULK1 phosphorylates Ser30 of BECN1 in association with ATG14 to stimulate autophagy induction. Autophagy 14, 584–597 (2018).
Zachari, M. & Ganley, I. G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 61, 585–596 (2017).
Kihara, A., Noda, T., Ishihara, N. & Ohsumi, Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 152, 519–530 (2001).
Zhong, Y. et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468–476 (2009).
Walczak, M. & Martens, S. Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 9, 424–425 (2013).
Lorincz, P. & Juhasz, G. Autophagosome-lysosome fusion. J. Mol. Biol. 432, 2462–2482 (2020).
Gatica, D., Lahiri, V. & Klionsky, D. J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 20, 233–242 (2018).
Kroemer, G., Marino, G. & Levine, B. Autophagy and the integrated stress response. Mol. Cell 40, 280–293 (2010).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Hosokawa, N. et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981–1991 (2009).
Galluzzi, L., Bravo-San Pedro, J. M., Levine, B., Green, D. R. & Kroemer, G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 16, 487–511 (2017).
Chang, K. et al. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy https://doi.org/10.1080/15548627.2019.1704117 (2019).
Aspernig, H. et al. Mitochondrial perturbations couple mTORC2 to autophagy in C. elegans. Cell Rep. 29, 1399–1409 e1395 (2019).
Shaw, R. J. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004).
Herrero-Martin, G. et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28, 677–685 (2009).
Anderson, K. A. et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 (2008).
Lee, J. W., Park, S., Takahashi, Y. & Wang, H. G. The association of AMPK with ULK1 regulates autophagy. PLoS One 5, e15394 (2010).
Inoki, K., Zhu, T. & Guan, K. L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Gwinn, D. M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008).
Loffler, A. S. et al. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 7, 696–706 (2011).
Dunlop, E. A., Hunt, D. K., Acosta-Jaquez, H. A., Fingar, D. C. & Tee, A. R. ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy 7, 737–747 (2011).
Kim, J., Yang, G., Kim, Y., Kim, J. & Ha, J. AMPK activators: mechanisms of action and physiological activities. Exp. Mol. Med. 48, e224 (2016).
Lee, I. H. et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl Acad. Sci. USA 105, 3374–3379 (2008).
Huang, R. et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456–466 (2015).
Hariharan, N. et al. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ. Res. 107, 1470–1482 (2010).
Lan, F., Cacicedo, J. M., Ruderman, N. & Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 283, 27628–27635 (2008).
Hou, X. et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 283, 20015–20026 (2008).
Ghosh, H. S., McBurney, M. & Robbins, P. D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5, e9199 (2010).
Dai, H., Sinclair, D. A., Ellis, J. L. & Steegborn, C. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol. Ther. 188, 140–154 (2018).
Wei, Y., Pattingre, S., Sinha, S., Bassik, M. & Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678–688 (2008).
Zalckvar, E. et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 10, 285–292 (2009).
B’Chir, W. et al. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 41, 7683–7699 (2013).
Criollo, A. et al. The IKK complex contributes to the induction of autophagy. EMBO J. 29, 619–631 (2010).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Chauhan, S. et al. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol. Cell 50, 16–28 (2013).
Liu, S. et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8, 826–837 (2012).
Hartleben, B. et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 120, 1084–1096 (2010).
Kim, S. I. et al. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J. Biol. Chem. 287, 11677–11688 (2012).
Ding, Y. et al. TGF-{beta}1 protects against mesangial cell apoptosis via induction of autophagy. J. Biol. Chem. 285, 37909–37919 (2010).
Bechtel, W. et al. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrol. 24, 727–743 (2013).
Chen, J., Chen, M. X., Fogo, A. B., Harris, R. C. & Chen, J. K. mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J. Am. Soc. Nephrol. 24, 198–207 (2013).
Cina, D. P. et al. Inhibition of MTOR disrupts autophagic flux in podocytes. J. Am. Soc. Nephrol. 23, 412–420 (2012).
Oshima, Y. et al. Prorenin receptor is essential for normal podocyte structure and function. J. Am. Soc. Nephrol. 22, 2203–2212 (2011).
Godel, M. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 121, 2197–2209 (2011).
Inoki, K. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 (2011).
Narita, M. et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011).
Rong, Y. et al. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc. Natl Acad. Sci. USA 108, 7826–7831 (2011).
Bork, T. et al. Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy https://doi.org/10.1080/15548627.2019.1705007 (2019).
Alghamdi, T. A. et al. Janus kinase 2 regulates transcription factor EB expression and autophagy completion in glomerular podocytes. J. Am. Soc. Nephrol. 28, 2641–2653 (2017).
Kimura, T. et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 22, 902–913 (2011).
Livingston, M. J. et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 15, 2142–2162 (2019).
McWilliams, T. G. et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol. 214, 333–345 (2016).
Lenoir, O. et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 11, 1130–1145 (2015).
Matsuda, J. et al. Antioxidant role of autophagy in maintaining the integrity of glomerular capillaries. Autophagy 14, 53–65 (2018).
O’Sullivan, E. D., Hughes, J. & Ferenbach, D. A. Renal aging: causes and consequences. J. Am. Soc. Nephrol. 28, 407–420 (2017).
Nakamura, S. et al. Suppression of autophagic activity by Rubicon is a signature of aging. Nat. Commun. 10, 847 (2019).
Cui, J. et al. Age-related changes in the function of autophagy in rat kidneys. Age 34, 329–339 (2012).
Yamamoto-Nonaka, K. et al. Cathepsin D in podocytes is important in the pathogenesis of proteinuria and CKD. J. Am. Soc. Nephrol. 27, 2685–2700 (2016).
Chuang, P. Y. et al. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am. J. Physiol. Renal Physiol. 313, F621–F628 (2017).
Zhang, L. et al. C/EBPalpha deficiency in podocytes aggravates podocyte senescence and kidney injury in aging mice. Cell Death Dis. 10, 684 (2019).
Yamamoto, T. et al. Time-dependent dysregulation of autophagy: implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 12, 801–813 (2016).
Kaushal, G. P. & Shah, S. V. Autophagy in acute kidney injury. Kidney Int. 89, 779–791 (2016).
Jiang, M. et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 82, 1271–1283 (2012).
Takahashi, A. et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 180, 517–525 (2012).
Hsiao, H. W. et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock 37, 289–296 (2012).
Leventhal, J. S. et al. Autophagy limits endotoxemic acute kidney injury and alters renal tubular epithelial cell cytokine expression. PloS One 11, e0150001 (2016).
Mei, S. et al. Autophagy is activated to protect against endotoxic acute kidney injury. Sci. Rep. 6, 22171 (2016).
Howell, G. M. et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PloS One 8, e69520 (2013).
Ko, G. J., Bae, S. Y., Hong, Y. A., Pyo, H. J. & Kwon, Y. J. Radiocontrast-induced nephropathy is attenuated by autophagy through regulation of apoptosis and inflammation. Hum. Exp. Toxicol. 35, 724–736 (2016).
Lin, Q. et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 26, 101254 (2019).
Lei, R. et al. Mitophagy plays a protective role in iodinated contrast-induced acute renal tubular epithelial cells injury. Cell Physiol. Biochem. 46, 975–985 (2018).
Lempiainen, J. et al. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1alpha-eNOS pathway and enhanced autophagy. Acta Physiol. 208, 410–421 (2013).
Grahammer, F. et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc. Natl Acad. Sci. USA 111, E2817–E2826 (2014).
Chien, C. T., Shyue, S. K. & Lai, M. K. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation 84, 1183–1190 (2007).
Rovetta, F. et al. ER signaling regulation drives the switch between autophagy and apoptosis in NRK-52E cells exposed to cisplatin. Exp. Cell Res. 318, 238–250 (2012).
Herzog, C., Yang, C., Holmes, A. & Kaushal, G. P. zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins but impairs autophagic flux and worsens renal function. Am. J. Physiol. Renal Physiol. 303, F1239–F1250 (2012).
Jankauskas, S. S. et al. The age-associated loss of ischemic preconditioning in the kidney is accompanied by mitochondrial dysfunction, increased protein acetylation and decreased autophagy. Sci. Rep. 7, 44430 (2017).
Xie, Y. et al. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis. 9, 338 (2018).
Bhatia, D. & Choi, M. E. The emerging role of mitophagy in kidney diseases. J. Life Sci. 1, 13–22 (2019).
Wang, Y., Cai, J., Tang, C. & Dong, Z. Mitophagy in acute kidney injury and kidney repair. Cells 9, 338 (2020).
Tang, C. et al. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14, 880–897 (2018).
Tang, C. et al. Activation of BNIP3-mediated mitophagy protects against renal ischemia-reperfusion injury. Cell Death Dis. 10, 677 (2019).
Wang, Y. et al. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 9, 1113 (2018).
Zhao, C. et al. Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function. Oncotarget 8, 20988–21000 (2017).
Liu, J. X. et al. Disturbance of mitochondrial dynamics and mitophagy in sepsis-induced acute kidney injury. Life Sci. 235, 116828 (2019).
Maejima, I. et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 (2013).
Chang-Panesso, M. et al. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Invest. 129, 5501–5517 (2019).
Kusaba, T., Lalli, M., Kramann, R., Kobayashi, A. & Humphreys, B. D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl Acad. Sci. USA 111, 1527–1532 (2014).
Rosen, S. & Heyman, S. Concerning cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 94, 218 (2018).
Kumar, S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 93, 27–40 (2018).
Li, L., Wang, Z. V., Hill, J. A. & Lin, F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J. Am. Soc. Nephrol. 25, 305–315 (2014).
Cheng, H., Fan, X., Lawson, W. E., Paueksakon, P. & Harris, R. C. Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int. 88, 85–94 (2015).
Nassour, J. et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 565, 659–663 (2019).
Taji, F. et al. Autophagy induction reduces telomerase activity in HeLa cells. Mech. Ageing Dev. 163, 40–45 (2017).
Baisantry, A. et al. Autophagy induces prosenescent changes in proximal tubular S3 segments. J. Am. Soc. Nephrol. 27, 1609–1616 (2016).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Canaud, G. et al. Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci. Transl. Med. 11, eaav4754 (2019).
Li, L. et al. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J. Clin. Invest. 129, 2374–2389 (2019).
Shu, S. et al. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 37, 269–280 (2018).
Li, L., Zepeda-Orozco, D., Black, R. & Lin, F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am. J. Pathol. 176, 1767–1778 (2010).
Livingston, M. J. et al. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12, 976–998 (2016).
Yan, Q. et al. Autophagy activation contributes to lipid accumulation in tubular epithelial cells during kidney fibrosis. Cell Death Discov. 4, 2 (2018).
Hernandez-Gea, V. et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142, 938–946 (2012).
Thoen, L. F. et al. A role for autophagy during hepatic stellate cell activation. J. Hepatol. 55, 1353–1360 (2011).
Xue, X. et al. Protein kinase Calpha drives fibroblast activation and kidney fibrosis by stimulating autophagic flux. J. Biol. Chem. 293, 11119–11130 (2018).
Li, L. et al. Forkhead box O3 (FoxO3) regulates kidney tubular autophagy following urinary tract obstruction. J. Biol. Chem. 292, 13774–13783 (2017).
Yang, X. et al. WNT1-inducible signaling protein-1 mediates TGF-beta1-induced renal fibrosis in tubular epithelial cells and unilateral ureteral obstruction mouse models via autophagy. J. Cell Physiol. 235, 2009–2022 (2020).
Noh, M. R., Woo, C. H., Park, M. J., In Kim, J. & Park, K. M. Ablation of C/EBP homologous protein attenuates renal fibrosis after ureteral obstruction by reducing autophagy and microtubule disruption. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1634–1641 (2018).
Kim, W. Y. et al. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology 17, 148–159 (2012).
Xu, G. et al. Defects in MAP1S-mediated autophagy turnover of fibronectin cause renal fibrosis. Aging 8, 977–985 (2016).
Ding, Y. et al. Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J. Am. Soc. Nephrol. 25, 2835–2846 (2014).
Li, H. et al. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy 12, 1472–1486 (2016).
Peng, X. et al. ATG5-mediated autophagy suppresses NF-kappaB signaling to limit epithelial inflammatory response to kidney injury. Cell Death Dis. 10, 253 (2019).
Xu, Y., Wang, J., Xu, W., Ding, F. & Ding, W. Prohibitin 2-mediated mitophagy attenuates renal tubular epithelial cells injury by regulating mitochondrial dysfunction and NLRP3 inflammasome activation. Am. J. Physiol. Renal Physiol. 316, F396–F407 (2019).
Nam, S. A. et al. Autophagy in FOXD1 stroma-derived cells regulates renal fibrosis through TGF-beta and NLRP3 inflammasome pathway. Biochem. Biophys. Res. Commun. 508, 965–972 (2019).
Nam, S. A. et al. Autophagy attenuates tubulointerstital fibrosis through regulating transforming growth factor-beta and NLRP3 inflammasome signaling pathway. Cell Death Dis. 10, 78 (2019).
Bhatia, D. et al. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight 4, e132826 (2019).
Li, S. et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic. Biol. Med. 152, 632–649 (2020).
Alicic, R. Z., Rooney, M. T. & Tuttle, K. R. Diabetic kidney disease: challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 12, 2032–2045 (2017).
Liu, W. J. et al. Lysosome restoration to activate podocyte autophagy: a new therapeutic strategy for diabetic kidney disease. Cell Death Dis. 10, 806 (2019).
Tagawa, A. et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 65, 755–767 (2016).
Yang, D. et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol. Life Sci. 75, 669–688 (2018).
Ding, Y. & Choi, M. E. Autophagy in diabetic nephropathy. J. Endocrinol. 224, R15–R30 (2015).
Zhao, X. et al. Advanced glycation end-products suppress autophagic flux in podocytes by activating mammalian target of rapamycin and inhibiting nuclear translocation of transcription factor EB. J. Pathol. 245, 235–248 (2018).
Liu, J. et al. beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis. 7, e2183 (2016).
Wang, X. et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 86, 712–725 (2014).
Li, W. et al. FoxO1 promotes mitophagy in the podocytes of diabetic male mice via the PINK1/Parkin pathway. Endocrinology 158, 2155–2167 (2017).
Zhou, D. et al. PGRN acts as a novel regulator of mitochondrial homeostasis by facilitating mitophagy and mitochondrial biogenesis to prevent podocyte injury in diabetic nephropathy. Cell Death Dis. 10, 524 (2019).
Wang, H. et al. Podocyte-specific knockin of PTEN protects kidney from hyperglycemia. Am. J. Physiol. Renal Physiol. 314, F1096–F1107 (2018).
Liu, M. et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat. Commun. 8, 413 (2017).
Li, L. et al. Signal regulatory protein alpha protects podocytes through promoting autophagic activity. JCI Insight 5, e124747 (2019).
Zhan, M., Usman, I. M., Sun, L. & Kanwar, Y. S. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 26, 1304–1321 (2015).
Ma, Z. et al. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney. J. Clin. Invest. (in the press).
Vallon, V. et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Renal Physiol. 304, F156–F167 (2013).
Wang, Y., Zheng, Z. J., Jia, Y. J., Yang, Y. L. & Xue, Y. M. Role of p53/miR-155-5p/sirt1 loop in renal tubular injury of diabetic kidney disease. J. Transl. Med. 16, 146 (2018).
Zhang, Y. et al. MicroRNA-22 promotes renal tubulointerstitial fibrosis by targeting PTEN and suppressing autophagy in diabetic nephropathy. J. Diabetes Res. 2018, 4728645 (2018).
Huang, C. et al. Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci. Rep. 6, 29196 (2016).
Chen, K. et al. Optineurin-mediated mitophagy protects renal tubular epithelial cells against accelerated senescence in diabetic nephropathy. Cell Death Dis. 9, 105 (2018).
Brijmohan, A. S. et al. HDAC6 inhibition promotes transcription factor EB activation and is protective in experimental kidney disease. Front. Pharmacol. 9, 34 (2018).
Pontrelli, P. et al. Deregulation of autophagy under hyperglycemic conditions is dependent on increased lysine 63 ubiquitination: a candidate mechanism in the progression of diabetic nephropathy. J. Mol. Med. 96, 645–659 (2018).
Yamahara, K. et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J. Am. Soc. Nephrol. 24, 1769–1781 (2013).
Tan, J., Wang, M., Song, S., Miao, Y. & Zhang, Q. Autophagy activation promotes removal of damaged mitochondria and protects against renal tubular injury induced by albumin overload. Histol. Histopathol. 33, 681–690 (2018).
Nolin, A. C. et al. Proteinuria causes dysfunctional autophagy in the proximal tubule. Am. J. Physiol. Renal Physiol. 311, F1271–F1279 (2016).
Xu, D. et al. NIX-mediated mitophagy protects against proteinuria-induced tubular cell apoptosis and renal injury. Am. J. Physiol. Renal Physiol. 316, F382–F395 (2019).
Kitada, M. et al. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp. Diabetes Res. 2011, 908185 (2011).
Kitada, M. et al. A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia 59, 1307–1317 (2016).
Sakai, S. et al. Proximal tubule autophagy differs in type 1 and 2 diabetes. J. Am. Soc. Nephrol. 30, 929–945 (2019).
Saito, A. et al. Significance of proximal tubular metabolism of advanced glycation end products in kidney diseases. Ann. NY Acad. Sci. 1043, 637–643 (2005).
Takahashi, A. et al. Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes 66, 1359–1372 (2017).
Liu, W. J. et al. Autophagy-lysosome pathway in renal tubular epithelial cells is disrupted by advanced glycation end products in diabetic nephropathy. J. Biol. Chem. 290, 20499–20510 (2015).
Xavier, S. et al. BAMBI is expressed in endothelial cells and is regulated by lysosomal/autolysosomal degradation. PLoS One 5, e12995 (2010).
Fan, Y. et al. BAMBI elimination enhances alternative TGF-beta signaling and glomerular dysfunction in diabetic mice. Diabetes 64, 2220–2233 (2015).
Fiorentino, L. et al. Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol. Med. 5, 441–455 (2013).
Xu, L., Fan, Q., Wang, X., Zhao, X. & Wang, L. Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis. 7, e2445 (2016).
Rosenberg, A. Z. & Kopp, J. B. Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 12, 502–517 (2017).
Kumar, V. et al. Disrupted apolipoprotein L1-miR193a axis dedifferentiates podocytes through autophagy blockade in an APOL1 risk milieu. Am. J. Physiol. Cell Physiol. 317, C209–C225 (2019).
Beckerman, P. et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat. Med. 23, 429–438 (2017).
Asanuma, K. et al. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 17, 1165–1167 (2003).
Yi, M. et al. Autophagy is activated to protect against podocyte injury in adriamycin-induced nephropathy. Am. J. Physiol. Renal Physiol. 313, F74–F84 (2017).
Kawakami, T. et al. Deficient autophagy results in mitochondrial dysfunction and FSGS. J. Am. Soc. Nephrol. 26, 1040–1052 (2015).
Zeng, C. et al. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J. Pathol. 234, 203–213 (2014).
Ogawa-Akiyama, A. et al. Podocyte autophagy is associated with foot process effacement and proteinuria in patients with minimal change nephrotic syndrome. PLoS One 15, e0228337 (2020).
Zschiedrich, S. et al. Targeting mTOR signaling can prevent the progression of FSGS. J. Am. Soc. Nephrol. 28, 2144–2157 (2017).
Torres, V. E. & Harris, P. C. Progress in the understanding of polycystic kidney disease. Nat. Rev. Nephrol. 15, 70–72 (2019).
Belibi, F. et al. Hypoxia-inducible factor-1alpha (HIF-1alpha) and autophagy in polycystic kidney disease (PKD). Am. J. Physiol. Renal Physiol. 300, F1235–F1243 (2011).
Nowak, K. L. & Edelstein, C. L. Apoptosis and autophagy in polycystic kidney disease (PKD). Cell. Signal. 68, 109518 (2020).
Anvarian, Z., Mykytyn, K., Mukhopadhyay, S., Pedersen, L. B. & Christensen, S. T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 15, 199–219 (2019).
Pampliega, O. & Cuervo, A. M. Autophagy and primary cilia: dual interplay. Curr. Opin. Cell Biol. 39, 1–7 (2016).
Wang, S., Livingston, M. J., Su, Y. & Dong, Z. Reciprocal regulation of cilia and autophagy via the MTOR and proteasome pathways. Autophagy 11, 607–616 (2015).
Takiar, V. et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl Acad. Sci. USA 108, 2462–2467 (2011).
Zhu, P., Sieben, C. J., Xu, X., Harris, P. C. & Lin, X. Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum. Mol. Genet. 26, 158–172 (2017).
Cornec-Le Gall, E., Alam, A. & Perrone, R. D. Autosomal dominant polycystic kidney disease. Lancet 393, 919–935 (2019).
Rowe, I. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 (2013).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Li, A. et al. Rapamycin treatment dose-dependently improves the cystic kidney in a new ADPKD mouse model via the mTORC1 and cell-cycle-associated CDK1/cyclin axis. J. Cell Mol. Med. 21, 1619–1635 (2017).
Kou, P., Wei, S. & Xiong, F. Recent advances of mTOR inhibitors use in autosomal dominant polycystic kidney disease: is the road still open? Curr. Med. Chem. 26, 2962–2973 (2019).
Calvo-Rubio, M. et al. Dietary fat composition influences glomerular and proximal convoluted tubule cell structure and autophagic processes in kidneys from calorie-restricted mice. Aging Cell 15, 477–487 (2016).
Shavlakadze, T. et al. Short-term low-dose mTORC1 inhibition in aged rats counter-regulates age-related gene changes and blocks age-related kidney pathology. J. Gerontol. A Biol. Sci. Med. Sci. 73, 845–852 (2018).
Hong, Q. et al. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 93, 1330–1343 (2018).
Ren, H. et al. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell Endocrinol. 500, 110628 (2020).
Li, X. Y. et al. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol. Ther. Nucleic Acids 9, 48–56 (2017).
Song, G. et al. Astragaloside IV ameliorates early diabetic nephropathy by inhibition of MEK1/2-ERK1/2-RSK2 signaling in streptozotocin-induced diabetic mice. J. Int. Med. Res. 46, 2883–2897 (2018).
Guo, H. et al. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKalpha-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci. Rep. 7, 6852 (2017).
Wang, X. et al. Astragaloside IV represses high glucose-induced mesangial cells activation by enhancing autophagy via SIRT1 deacetylation of NF-kappaB p65 subunit. Drug Des. Devel. Ther. 12, 2971–2980 (2018).
Morel, E. et al. Autophagy: a druggable process. Annu. Rev. Pharmacol. Toxicol. 57, 375–398 (2017).
Panda, P. K. et al. Chemical screening approaches enabling drug discovery of autophagy modulators for biomedical applications in human diseases. Front. Cell Dev. Biol. 7, 38 (2019).
Shoji-Kawata, S. et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206 (2013).
Williams, A. et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 4, 295–305 (2008).
Lin, T. A., Wu, V. C. & Wang, C. Y. Autophagy in chronic kidney diseases. Cells 8, 61 (2019).
Pasquier, B. Autophagy inhibitors. Cell Mol. Life Sci. 73, 985–1001 (2016).
Fantus, D., Rogers, N. M., Grahammer, F., Huber, T. B. & Thomson, A. W. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat. Rev. Nephrol. 12, 587–609 (2016).
Viana, S. D., Reis, F. & Alves, R. Therapeutic use of mTOR inhibitors in renal diseases: advances, drawbacks, and challenges. Oxid. Med. Cell Longev. 2018, 3693625 (2018).
Larson-Casey, J. L., Deshane, J. S., Ryan, A. J., Thannickal, V. J. & Carter, A. B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44, 582–596 (2016).
Rinschen, M. M. et al. A multi-layered quantitative in vivo expression atlas of the podocyte unravels kidney disease candidate genes. Cell Rep. 23, 2495–2508 (2018).
Acknowledgements
The authors were supported in part by grants from the National Natural Science Foundation of China (81870474), the National Institutes of Health (DK058831, DK087843) and the US Department of Veterans Affairs (BX000319). Z.D. is a recipient of the Senior Research Career Scientist award from the US Department of Veterans Affairs.
Author information
Authors and Affiliations
Contributions
C.T. and Z.D. researched data for the article and wrote the article. All authors contributed substantially to discussion of the content, and reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks C. Edelstein, D. Koya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Vesicle nucleation
-
The process of mobilizing a small group of molecules to the phagophore assembly site to form a phagophore during autophagy.
- Phagophore
-
A cup-shaped double-membrane vesicle that expands and engulfs the components destined for digestion during autophagy.
- Autophagic flux
-
The whole process of autophagy, including autophagosome formation, maturation, fusion with lysosomes, subsequent breakdown and the release of macromolecules back into the cytosol.
- Lipofuscin
-
A yellowish brown, autofluorescent, lipid-containing pigment that accumulates in the cytoplasm of cells during ageing.
- Ischaemic preconditioning
-
(IPC). An endogenous adaptive mechanism elicited by brief ischaemia that protects against a subsequent more sustained ischaemic insult.
- Chemical chaperones
-
Small molecules that enhance protein folding and/or stability.
Rights and permissions
About this article
Cite this article
Tang, C., Livingston, M.J., Liu, Z. et al. Autophagy in kidney homeostasis and disease. Nat Rev Nephrol 16, 489–508 (2020). https://doi.org/10.1038/s41581-020-0309-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-0309-2
This article is cited by
-
Targeting PNPO to suppress tumor growth via inhibiting autophagic flux and to reverse paclitaxel resistance in ovarian cancer
Apoptosis (2024)
-
Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation
Journal of Translational Medicine (2023)
-
The expression of autophagy markers in IVIG-resistant Kawasaki disease and the establishment of prediction model
BMC Pediatrics (2023)
-
N6-methyladenosine methylation in kidney injury
Clinical Epigenetics (2023)
-
Crosstalk between autophagy and insulin resistance: evidence from different tissues
European Journal of Medical Research (2023)