Abstract
The default mode network (DMN) is a set of widely distributed brain regions in the parietal, temporal and frontal cortex. These regions often show reductions in activity during attention-demanding tasks but increase their activity across multiple forms of complex cognition, many of which are linked to memory or abstract thought. Within the cortex, the DMN has been shown to be located in regions furthest away from those contributing to sensory and motor systems. Here, we consider how our knowledge of the topographic characteristics of the DMN can be leveraged to better understand how this network contributes to cognition and behaviour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
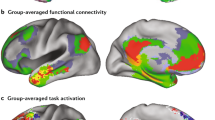
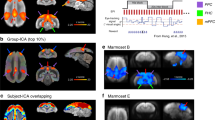
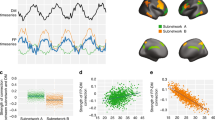
The spatial map data that support the findings of Fig. 1b are available in The Open Science Framework (https://osf.io/nz8gf/). The brain masks used to obtain the data used in Supplementary Fig. 1 are available in Neurovault (https://identifiers.org/neurovault.collection:8569). The source data for Supplementary Fig. 1 are included in Supplementary Data.
References
Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353–375 (1992).
Milner, B., Corkin, S. & Teuber, H.-L. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of HM. Neuropsychologia 6, 215–234 (1968).
Silbersweig, D. A. et al. Detection of thirty-second cognitive activations in single subjects with positron emission tomography: a new low-dose H215O regional cerebral blood flow three-dimensional imaging technique. J. Cereb. Blood Flow. Metab. 13, 617–629 (1993).
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001).
Gazzaniga, M. S. The New Cognitive Neurosciences (MIT Press, 2000).
Grill-Spector, K. & Malach, R. The human visual cortex. Annu. Rev. Neurosci. 27, 649–677 (2004).
Jack, C. R. Jr et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology 190, 85–92 (1994).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
Shulman, G. L. et al. Searching for activations that generalize over tasks. Hum. Brain Mapp. 5, 317–322 (1997).
Duncan, J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cognit. Sci. 14, 172–179 (2010).
Cole, M. W. et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 16, 1348–1355 (2013).
Buckner, R. L. & Wheeler, M. E. The cognitive neuroscience of remembering. Nat. Rev. Neurosci. 2, 624–634 (2001).
Andreasen, N. C. et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatry 152, 1576–1585 (1995).
Binder, J. R. et al. Conceptual processing during the conscious resting state: a functional MRI study. J. Cognit. Neurosci. 11, 80–93 (1999).
Kelley, W. M. et al. Finding the self? An event-related fMRI study. J. Cognit. Neurosci. 14, 785–794 (2002).
Margulies, D. S. et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12574–12579 (2016).
Hill, J. et al. Similar patterns of cortical expansion during human development and evolution. Proc. Natl Acad. Sci. USA 107, 13135–13140 (2010).
Buckner, R. L. & Krienen, F. M. The evolution of distributed association networks in the human brain. Trends Cognit. Sci. 17, 648–665 (2013).
Mesulam, M.-M. From sensation to cognition. Brain 121, 1013–1052 (1998).
Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Friston, K., Frith, C., Liddle, P. & Frackowiak, R. Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow. Metab. 13, 5–14 (1993).
Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl Acad. Sci. USA 100, 253–258 (2003).
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R. & Buckner, R. L. Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–562 (2010).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Andrews-Hanna, J. R., Smallwood, J. & Spreng, R. N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 1316, 29 (2014).
Braga, R. M. & Buckner, R. L. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron 95, 457–471 (2017).
DiNicola, L. M., Braga, R. M. & Buckner, R. L. Parallel distributed networks dissociate episodic and social functions within the individual. J. Neurophysiol. 123, 1144–1179 (2020).
Buckner, R. L. & DiNicola, L. M. The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608 (2019).
Butters, N., Pandya, D., Stein, D. & Rosen, J. A search for the spatial engram within the frontal lobes of monkeys. Acta Neurobiol. Exp. 32, 305–329 (1972).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl Acad. Sci. USA 102, 9673–9678 (2005).
Leech, R., Braga, R. & Sharp, D. J. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. 32, 215–222 (2012).
Braga, R. M. & Leech, R. Echoes of the brain: local-scale representation of whole-brain functional networks within transmodal cortex. Neuroscientist 21, 540–551 (2015).
Braga, R. M., Sharp, D. J., Leeson, C., Wise, R. J. & Leech, R. Echoes of the brain within default mode, association, and heteromodal cortices. J. Neurosci. 33, 14031–14039 (2013).
Bzdok, D. & Yeo, B. T. Inference in the age of big data: future perspectives on neuroscience. Neuroimage 155, 549–564 (2017).
de Wael, R. V. et al. BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Commun. Biol. 3, 1–10 (2020).
Rausch, A. et al. Connectivity-based parcellation of the amygdala predicts social skills in adolescents with autism spectrum disorder. J. Autism Dev. Disord. 48, 572–582 (2018).
Frith, C. D. & Frith, U. Interacting minds — a biological basis. Science 286, 1692–1695 (1999).
Addis, D. R., Wong, A. T. & Schacter, D. L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45, 1363–1377 (2007).
Tulving, E. in Principles of Frontal Lobe Function (eds Stuss, D. T. & Knight, R. T.) Ch. 20 (Oxford Univ. Press, 2002).
Hassabis, D. & Maguire, E. A. Deconstructing episodic memory with construction. Trends Cognit. Sci. 11, 299–306 (2007).
Ho, N. S. P. et al. Facing up to why the wandering mind: patterns of off-task laboratory thought are associated with stronger neural recruitment of right fusiform cortex while processing facial stimuli. NeuroImage 214, 116765 (2020).
Smallwood, J. et al. The neural correlates of ongoing conscious thought. iScience 24, 102132 (2021).
Smallwood, J., Nind, L. & O’Connor, R. C. When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Conscious. Cogn. 18, 118–125 (2009).
Konu, D. et al. A role for ventromedial prefrontal cortex in self-generated episodic social cognition. NeuroImage 218, 116977 (2020).
Spreng, R. N., Mar, R. A. & Kim, A. S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510 (2009).
Spreng, R. N. & Grady, C. L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 22, 1112–1123 (2010).
Laird, A. R. et al. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 29, 14496–14505 (2009).
Chiong, W. et al. The salience network causally influences default mode network activity during moral reasoning. Brain 136, 1929–1941 (2013).
Reniers, R. L. et al. Moral decision-making, ToM, empathy and the default mode network. Biol. Psychol. 90, 202–210 (2012).
Bzdok, D. et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 217, 783–796 (2012).
Göttlich, M., Ye, Z., Rodriguez-Fornells, A., Münte, T. F. & Krämer, U. M. Viewing socio-affective stimuli increases connectivity within an extended default mode network. NeuroImage 148, 8–19 (2017).
Vessel, E. A., Starr, G. G. & Rubin, N. Art reaches within: aesthetic experience, the self and the default mode network. Front. Neurosci. 7, 258 (2013).
Simony, E. et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat. Commun. 7, 12141 (2016).
Smallwood, R. F. et al. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J. Pain 14, 663–675 (2013).
Zhang, M., Savill, N., Margulies, D. S., Smallwood, J. & Jefferies, E. Distinct individual differences in default mode network connectivity relate to off-task thought and text memory during reading. Sci. Rep. 9, 16220 (2019).
Epstein, R. A. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn. Sci. 12, 388–396 (2008).
Rogers, R. D. et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry 55, 594–602 (2004).
Ritchey, M. & Cooper, R. A. Deconstructing the posterior medial episodic network. Trends Cogn. Sci. 24, 451–465 (2020).
Ralph, M. A. L., Jefferies, E., Patterson, K. & Rogers, T. T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 18, 42 (2017).
Schilbach, L., Eickhoff, S. B., Rotarska-Jagiela, A., Fink, G. R. & Vogeley, K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cogn. 17, 457–467 (2008).
Amodio, D. M. & Frith, C. D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 (2006).
Satpute, A. B. & Lindquist, K. A. The default mode network’s role in discrete emotion. Trends Cognit. Sci. 23, 851–864 (2019).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665 (2011).
Bzdok, D. et al. Subspecialization in the human posterior medial cortex. Neuroimage 106, 55–71 (2015).
Eickhoff, S. B., Laird, A. R., Fox, P. T., Bzdok, D. & Hensel, L. Functional segregation of the human dorsomedial prefrontal cortex. Cereb. Cortex 26, 304–321 (2016).
Murphy, C. et al. Distant from input: evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. NeuroImage 171, 393–401 (2018).
Murphy, C. et al. Modes of operation: a topographic neural gradient supporting stimulus dependent and independent cognition. NeuroImage 186, 487–496 (2019).
Konishi, M., McLaren, D. G., Engen, H. & Smallwood, J. Shaped by the past: the default mode network supports cognition that is independent of immediate perceptual input. PLoS ONE 10, e0132209 (2015).
Smallwood, J. et al. Escaping the here and now: evidence for a role of the default mode network in perceptually decoupled thought. Neuroimage 69, 120–125 (2013).
Turnbull, A. et al. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat. Commun. 10, 3816 (2019).
Sormaz, M. et al. Default mode network can support the level of detail in experience during active task states. Proc. Natl Acad. Sci. USA 115, 9318–9323 (2018).
Lanzoni, L. et al. The role of default mode network in semantic cue integration. Neuroimage 219, 117019 (2020).
Vatansever, D., Menon, D. K. & Stamatakis, E. A. Default mode contributions to automated information processing. Proc. Natl Acad. Sci. USA 114, 12821–12826 (2017).
Van Den Heuvel, M. P. & Sporns, O. Rich-club organization of the human connectome. J. Neurosci. 31, 15775–15786 (2011).
Honey, C. J. et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl Acad. Sci. USA 106, 2035–2040 (2009).
Park, B.-y. et al. Signal diffusion along connectome gradients and inter-hub routing differentially contribute to dynamic human brain function. NeuroImage 224, 117429 (2021).
Jones, E. & Powell, T. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 93, 793–820 (1970).
Fellman, D. & Van Essen, D. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Xu, T. et al. Cross-species functional alignment reveals evolutionary hierarchy within the connectome. NeuroImage 223, 117346 (2020).
Moscovitch, M., Cabeza, R., Winocur, G. & Nadel, L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67, 105–134 (2016).
Alcalá-López, D. et al. Computing the social brain connectome across systems and states. Cereb. Cortex 28, 2207–2232 (2018).
Gendron, M. & Barrett, L. F. Emotion perception as conceptual synchrony. Emot. Rev. 10, 101–110 (2018).
Kernbach, J. M. et al. Subspecialization within default mode nodes characterized in 10,000 UK Biobank participants. Proc. Natl Acad. Sci. USA 115, 12295–12300 (2018).
Dohmatob, E., Dumas, G. & Bzdok, D. Dark control: the default mode network as a reinforcement learning agent. Hum. Brain Mapp. 41, 3318–3341 (2020).
Goodale, M. A. & Milner, A. D. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25 (1992).
Penfield, W. & Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937).
Fox, K. C. et al. Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain. Nat. Hum. Behav. 4, 1039–1052 (2020).
Gonzalez-Garcia, C., Flounders, M. W., Chang, R., Baria, A. T. & He, B. J. Content-specific activity in frontoparietal and default-mode networks during prior-guided visual perception. eLife 7, 36068 (2018).
Murphy, C. et al. Hello, is that me you are looking for? A re-examination of the role of the DMN in off-task thought. PLoS ONE 14, e0216182 (2019).
Gorgolewski, K. J. et al. A correspondence between individual differences in the brain’s intrinsic functional architecture and the content and form of self-generated thoughts. PLoS ONE 9, e97176 (2014).
Davey, J. et al. Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. J. Neurosci. 35, 15230–15239 (2015).
van der Linden, M., Berkers, R., Morris, R. G. M. & Fernandez, G. Angular gyrus involvement at encoding and retrieval is associated with durable but less specific memories. J. Neurosci. 37, 9474–9485 (2017).
Bonnici, H. M., Richter, F. R., Yazar, Y. & Simons, J. S. Multimodal feature integration in the angular gyrus during episodic and semantic retrieval. J. Neurosci. 36, 5462–5471 (2016).
Wen, T., Duncan, J. & Mitchell, D. J. Hierarchical representation of multi-step tasks in multiple-demand and default mode networks. J. Neurosci. 40, 7724–7738 (2020).
Wang, X., Gao, Z., Smallwood, J. & Jefferies, E. Both default and multiple-demand regions represent semantic goal information. J. Neurosci. 41, 3679–3691 (2021).
Smallwood, J. Distinguishing how from why the mind wanders: a process–occurrence framework for self-generated mental activity. Psychol. Bull. 139, 519 (2013).
Smallwood, J. & Schooler, J. W. The science of mind wandering: empirically navigating the stream of consciousness. Annu. Rev. Psychol. 66, 487–518 (2015).
Li, Q. et al. Atypical neural topographies underpin dysfunctional pattern separation in temporal lobe epilepsy. Brain https://doi.org/10.1093/brain/awab121 (2021).
Hilgetag, C. C. & Goulas, A. ‘Hierarchy’ in the organization of brain networks. Philos. Trans. R. Soc. B 375, 20190319 (2020).
Moser, E. I., Kropff, E. & Moser, M.-B. Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci. 31, 69–89 (2008).
Boccara, C. N. et al. Grid cells in pre-and parasubiculum. Nat. Neurosci. 13, 987–994 (2010).
Fyhn, M., Hafting, T., Treves, A., Moser, M. B. & Moser, E. I. Hippocampal remapping and grid realignment in entorhinal cortex. Nature 446, 190–194 (2007).
Behrens, T. E. J. et al. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018).
Fries, P. Rhythms for cognition: communication through coherence. Neuron 88, 220–235 (2015).
Welch, G. & Bishop, G. An Introduction to the Kalman Filter (Univ. of North Carolina, 1995).
Horner, A. J., Bisby, J. A., Bush, D., Lin, W.-J. & Burgess, N. Evidence for holistic episodic recollection via hippocampal pattern completion. Nat. Commun. 6, 1–11 (2015).
Huang, Y. & Rao, R. P. Predictive coding. Wiley Interdiscip. Rev. Cognit. Sci. 2, 580–593 (2011).
Friston, K. & Kiebel, S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. B: Biol. Sci. 364, 1211–1221 (2009).
Allen, M. & Friston, K. J. From cognitivism to autopoiesis: towards a computational framework for the embodied mind. Synthese 195, 2459–2482 (2018).
Chanes, L. & Barrett, L. F. Redefining the role of limbic areas in cortical processing. Trends Cognit. Sci. 20, 96–106 (2016).
Benner, M. J. & Tushman, M. L. Exploitation, exploration, and process management: the productivity dilemma revisited. Acad. Manag. Rev. 28, 238–256 (2003).
Pearson, J. M., Hayden, B. Y., Raghavachari, S. & Platt, M. L. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr. Biol. 19, 1532–1537 (2009).
Bayer, H. M. & Glimcher, P. W. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47, 129–141 (2005).
Rudebeck, P. H. & Murray, E. A. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J. Neurosci. 31, 10569–10578 (2011).
Seghier, M. L. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61 (2013).
Braga, R. M., DiNicola, L. M., Becker, H. C. & Buckner, R. L. Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks. J. Neurophysiol. 124, 1415–1448 (2020).
Alderson-Day, B. & Fernyhough, C. Inner speech: development, cognitive functions, phenomenology, and neurobiology. Psychol. Bull. 141, 931 (2015).
Van Essen, D. C. et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79 (2013).
Botvinick, M. M. Hierarchical models of behavior and prefrontal function. Trends Cognit. Sci. 12, 201–208 (2008).
Smith, V., Mitchell, D. J. & Duncan, J. Role of the default mode network in cognitive transitions. Cereb. Cortex 28, 3685–3696 (2018).
Crittenden, B. M., Mitchell, D. J. & Duncan, J. Recruitment of the default mode network during a demanding act of executive control. eLife 4, e06481 (2015).
Krieger-Redwood, K. et al. Down but not out in posterior cingulate cortex: deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition. Neuroimage 141, 366–377 (2016).
Vatansever, D., Menon, D. K., Manktelow, A. E., Sahakian, B. J. & Stamatakis, E. A. Default mode network connectivity during task execution. Neuroimage 122, 96–104 (2015).
Gerlach, K. D., Spreng, R. N., Gilmore, A. W. & Schacter, D. L. Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage 55, 1816–1824 (2011).
Wang, X., Margulies, D. S., Smallwood, J. & Jefferies, E. A gradient from long-term memory to novel cognition: transitions through default mode and executive cortex. Neuroimage 220, 117074 (2020).
Dixon, M. L. et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl Acad. Sci. USA 115, E1598–E1607 (2018).
Davey, J. et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage 137, 165–177 (2016).
Hazy, T. E., Frank, M. J. & O’Reilly, R. C. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos. Trans. R. Soc. B Biol. Sci. 362, 1601–1613 (2007).
Olton, D. S., Becker, J. T. & Handelmann, G. E. Hippocampus, space, and memory. Behav. Brain Sci. 2, 313–322 (1979).
Huijbers, W., Pennartz, C. M., Cabeza, R. & Daselaar, S. M. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS ONE 6, e17463 (2011).
Moser, E. I. et al. Grid cells and cortical representation. Nat. Rev. Neurosci. 15, 466–481 (2014).
Constantinescu, A. O., O’Reilly, J. X. & Behrens, T. E. Organizing conceptual knowledge in humans with a gridlike code. Science 352, 1464–1468 (2016).
Paquola, C. et al. Convergence of cortical types and functional motifs in the human mesiotemporal lobe. eLife 9, e60673 (2020).
Acknowledgements
The authors thank N. Ho for help in the preparation of Supplementary Fig. 1 and R. Braga, L. Lanzoni and D. Vatansever for providing data for use in preparing the figures. The work in this Perspective was supported by consolidator grants from the European Research Council (ERC) (Award 646-927 WANDERINGMINDS to J.S., Award 866533-CORTIGRAD to D.S.M. and Award 771863-FLEXSEM to E.J.). D.B. was supported by the Canada Institute for Advanced Research (CIFAR) Artificial Intelligence Chairs programme, Google and National Institutes of Health (NIH) grant R01AG068563A. R.L. was supported by Wellcome/Engineering and Physical Sciences Research Council (EPSRC) Centre for Medical Engineering (Ref: WT 203148/Z/16/). B.B. received support from the Natural Sciences and Engineering Research Council of Canada (NSERC) (Discovery-1304413), the Canadian Institutes of Health Research (CIHR FDN-154298, PJT-174995), SickKids Foundation (NI17-039), BrainCanada (Azrieli Future Leaders) and the Tier-2 Canada Research Chairs programme.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Human Connectome Project: http://www.humanconnectomeproject.org/
Neurosynth: https://neurosynth.org/
Supplementary information
Rights and permissions
About this article
Cite this article
Smallwood, J., Bernhardt, B.C., Leech, R. et al. The default mode network in cognition: a topographical perspective. Nat Rev Neurosci 22, 503–513 (2021). https://doi.org/10.1038/s41583-021-00474-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-021-00474-4
This article is cited by
-
Human brain representations of internally generated outcomes of approximate calculation revealed by ultra-high-field brain imaging
Nature Communications (2024)
-
Spatiotemporal dynamics of hippocampal-cortical networks underlying the unique phenomenological properties of trauma-related intrusive memories
Molecular Psychiatry (2024)
-
Fractional amplitude of low-frequency fluctuation alterations in patients with cervical spondylotic myelopathy: a resting-state fMRI study
Neuroradiology (2024)
-
Effects of different modalities of transcranial magnetic stimulation on post-stroke cognitive impairment: a network meta-analysis
Neurological Sciences (2024)
-
Higher Sensory Sensitivity is Linked to Greater Expansion Amongst Functional Connectivity Gradients
Journal of Autism and Developmental Disorders (2024)