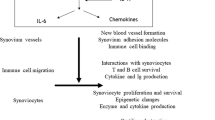
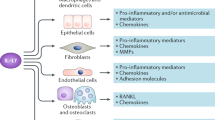
Abstract
More than any other cytokine family, the 11 members of the IL-1 family are associated with innate immune responses, which occur in acute inflammation and chronic inflammatory conditions such as rheumatic diseases. In many rheumatic diseases, the severity of the condition can result from the balance between the pro-inflammatory and anti-inflammatory members of the IL-1 family. Pro-inflammatory family members (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β and IL-36γ) are found in the articular environment during arthritis and often correlate with the degree of inflammation present. IL-1β has emerged as pivotal for promoting inflammation, particularly in autoinflammatory diseases, whereas IL-1α and the IL-36 subfamily are associated with skin diseases. IL-33 regulates T helper 2 (TH2) cell-mediated diseases, in sharp contrast to IL-18, which mainly regulates TH1 cell-mediated responses. The IL-1 family also contains four members that suppress inflammation: two specific receptor antagonists (IL-1 receptor antagonist (IL-1Ra) and IL-36 receptor antagonist (IL-36Ra)), and two members that broadly suppress innate inflammation by non-specifically reducing several cytokines and chemokines (IL-37 and IL-38). In this Review, each of the eleven IL-1 family cytokines and their receptors are discussed, along with their putative roles in rheumatic disease and therapeutic options for targeting or promoting these cytokines.
Key points
-
The IL-1 family of cytokines contains 11 members that either promote inflammation or specifically or non-specifically limit inflammation.
-
The main functions of the IL-1 family are innate immune reactions and inflammation, rather than acquired immunity.
-
IL-1β has emerged as an important cytokine in the pathogenesis of several rheumatic diseases, and can be targeted to treat these diseases and their associated co-morbidities.
-
IL-18 and IL-1β are the main targets for treating macrophage activation syndrome, a dangerous condition that can occur in several rheumatic diseases.
-
The role of the six newer members of the IL-1 family (IL-36α, IL-36β, IL-36γ, IL-36 receptor antagonist, IL-37 and IL-38) in rheumatic diseases is still being investigated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dinarello, C. A., Goldin, N. P. & Wolff, S. M. Demonstration and characterization of two distinct human leukocytic pyrogens. J. Exp. Med. 139, 1369–1381 (1974).
Dinarello, C. A., Renfer, L. & Wolff, S. M. Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proc. Natl Acad. Sci. USA 74, 4624–4627 (1977).
Auron, P. E. et al. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc. Natl Acad. Sci. USA 81, 7907–7911 (1984).
Dinarello, C. A. Biological basis for interleukin-1 in disease. Blood 87, 2095–2147 (1996).
Lomedico, P. T. et al. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature 312, 458–462 (1984).
Dayer, J. M., Robinson, D. R. & Krane, S. M. Prostaglandin production by rheumatoid synovial cells: stimulation by a factor from human mononuclear Cells. J. Exp. Med. 145, 1399–1404 (1977).
Mizel, S. B., Dayer, J. M., Krane, S. M. & Mergenhagen, S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc. Natl Acad. Sci. USA 78, 2474–2477 (1981).
Saklatvala, J. & Dingle, J. T. Identification of catabolin, a protein from synovium which induces degradation of cartilage in organ culture. Biochem. Biophys. Res. Commun. 96, 1225–1231 (1980).
Dinarello, C. A., Rosenwasser, L. J. & Wolff, S. M. Demonstration of a circulating suppressor factor of thymocyte proliferation during endotoxin fever in humans. J. Immunol. 127, 2517–2519 (1981).
Arend, W. P., Joslin, F. G. & Massoni, R. J. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J. Immunol. 134, 3868–3875 (1985).
Prieur, A. M., Kaufmann, M. T., Griscelli, C. & Dayer, J. M. Specific interleukin-1 inhibitor in serum and urine of children with systemic juvenile chronic arthritis. Lancet 2, 1240–1242 (1987).
Seckinger, P., Lowenthal, J. W., Williamson, K., Dayer, J. M. & MacDonald, H. R. A urine inhibitor of interleukin 1 activity that blocks ligand binding. J. Immunol. 139, 1546–1549 (1987).
Eisenberg, S. P. et al. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature 343, 341–346 (1990).
Cavalli, G. & Dinarello, C. A. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 9, 1157 (2018).
Okamura, H. et al. Cloning of a new cytokine that induces interferon-g. Nature 378, 88–91 (1995).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Kumar, S. et al. Identification and initial characterization of four novel members of the interleukin-1 family. J. Biol. Chem. 275, 10308–10314 (2000).
Nicklin, M. J. et al. A sequence-based map of the nine genes of the human interleukin-1 cluster. Genomics 79, 718–725 (2002).
Dinarello, C. A. et al. Suppression of innate inflammation and immunity by interleukin-37. Eur. J. Immunol. 46, 1067–1081 (2016).
Cavalli, G. & Dinarello, C. A. Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 281, 179–190 (2018).
van de Veerdonk, F. L., de Graaf, D. M., Joosten, L. A. & Dinarello, C. A. Biology of IL-38 and its role in disease. Immunol. Rev. 281, 191–196 (2018).
Garlanda, C., Dinarello, C. A. & Mantovani, A. The interleukin-1 family: back to the future. Immunity 39, 1003–1018 (2013).
Dinarello, C. A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27 (2018).
Towne, J. E. et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β, and IL-36γ) or antagonist (IL-36Ra) activity. J. Biol. Chem. 286, 42594–42602 (2011).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356, 768–774 (1992).
Cerretti, D. P. et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science 256, 97–100 (1992).
Lefrancais, E. et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl Acad. Sci. USA 109, 1673–1678 (2012).
Ainscough, J. S. et al. Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36γ. Proc. Natl. Acad. Sci. USA 114, E2748–E2757 (2017).
Zhang, M., Kenny, S. J., Ge, L., Xu, K. & Schekman, R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 4, e11205 (2015).
Carrie, A. et al. A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat. Genet. 23, 25–31 (1999).
Pavlowsky, A. et al. Neuronal JNK pathway activation by IL-1 is mediated through IL1RAPL1, a protein required for development of cognitive functions. Commun. Integr. Biol. 3, 245–247 (2010).
Bulek, K. et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J. Immunol. 182, 2601–2609 (2009).
Riva, F. et al. TIR8/SIGIRR is an Interleukin-1 receptor/Toll like receptor family member with regulatory functions in inflammation and immunity. Front. Immunol. 3, 322 (2012).
Gunther, S. et al. IL-1 family cytokines use distinct molecular mechanisms to signal through their shared co-receptor. Immunity 47, 510–523 (2017).
Tsutsumi, N. et al. The structural basis for receptor recognition of human interleukin-18. Nat. Commun. 5, 5340 (2014).
Kato, Z. et al. The structure and binding mode of interleukin-18. Nat. Struct. Biol. 10, 966–971 (2003).
Li, S. et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl Acad. Sci. USA 112, 2497–2502 (2015).
Nold-Petry, C. A. et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 16, 354–365 (2015).
Cavalli, G. et al. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology 55, 2220–2229 (2016).
Lunding, L. et al. IL-37 requires IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy 79, 366–373 (2015).
Zeng, Q. et al. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc. Natl Acad. Sci. USA 114, 1631–1636 (2017).
Boraschi, D., Italiani, P., Weil, S. & Martin, M. U. The family of the interleukin-1 receptors. Immunol. Rev. 281, 197–232 (2018).
Greenfeder, S. A. et al. Molecular cloning and characterization of a second subunit of the interleukin-1 receptor complex. J. Biol. Chem. 270, 13757–13765 (1995).
Thomas, C., Bazan, J. F. & Garcia, K. C. Structure of the activating IL-1 receptor signaling complex. Nat. Struct. Mol. Biol. 19, 455–457 (2012).
Wang, D. et al. Structural insights into the assembly and activation of IL-1β with its receptors. Nat. Immunol. 11, 905–911 (2010).
Greenfeder, S. A. et al. Insertion of a structural domain of interleukin (IL)-1β confers agonist activity to the IL-1 receptor antagonist. Implications for IL-1 bioactivity. J. Biol. Chem. 270, 22460–22466 (1995).
Colotta, F. et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 261, 472–475 (1993).
Smith, D. E. et al. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity 18, 87–96 (2003).
Hojen, J. F. et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat. Immunol. 20, 1138–1149 (2019).
Liu, X. et al. Structural insights into the interaction of IL-33 with its receptors. Proc. Natl Acad. Sci. USA 110, 14918–14923 (2013).
McDonald, G. B. et al. Predictive value of clinical findings and plasma biomarkers after fourteen days of prednisone treatment for acute graft-versus-host disease. Biol. Blood Marrow Transpl. 23, 1257–1263 (2017).
Werman, A. et al. The precursor form of IL-1α is an intracrine proinflammatory activator of transcription. Proc. Natl Acad. Sci. USA 101, 2434–2439 (2004).
Stevenson, F. T., Turck, J., Locksley, R. M. & Lovett, D. H. The N-terminal propiece of interleukin 1α is a transforming nuclear oncoprotein. Proc. Natl Acad. Sci. USA 94, 508–513 (1997).
Cohen, I. et al. IL-1α is a DNA damage sensor linking genotoxic stress signaling to sterile inflammation and innate immunity. Sci. Rep. 5, 14756 (2015).
Rider, P., Carmi, Y., Voronov, E. & Apte, R. N. Interleukin-1α. Semin. Immunol. 25, 430–438 (2013).
Di Paolo, N. C. & Shayakhmetov, D. M. Interleukin 1α and the inflammatory process. Nat. Immunol. 17, 906–913 (2016).
Rider, P. et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J. Immunol. 187, 4835–4843 (2011).
Rider, P., Voronov, E., Dinarello, C. A., Apte, R. N. & Cohen, I. Alarmins: feel the stress. J. Immunol. 198, 1395–1402 (2017).
Kim, B. et al. The interleukin-1α precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front. Immunol. 4, 391 (2013).
Kurt-Jones, E. A., Beller, D. I., Mizel, S. B. & Unanue, E. R. Identification of a membrane-associated interleukin-1 in macrophages. Proc. Natl Acad. Sci. USA 82, 1204–1208 (1985).
Kaplanski, G. et al. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood 84, 4242–4248 (1994).
Hacham, M., Argov, S., White, R. M., Segal, S. & Apte, R. N. Different patterns of interleukin-1alpha and interleukin-1beta expression in organs of normal young and old mice. Eur. Cytokine Netw. 13, 55–65 (2002).
Cohen, I. et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc. Natl Acad. Sci. USA 107, 2574–2579 (2010).
Ter Horst, R. et al. Host and environmental factors influencing individual human cytokine responses. Cell 167, 1111–1124 (2016).
Tunjungputri, R. N. et al. The inter-relationship of platelets with interleukin-1beta-mediated inflammation in humans. Thromb. Haemost. 118, 2112–2125 (2018).
Lachmann, H. J. et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360, 2416–2425 (2009).
Lachmann, H. J. et al. In vivo regulation of interleukin 1β in patients with cryopyrin-associated periodic syndromes. J. Exp. Med. 206, 1029–1036 (2009).
Zheng, Y., Humphry, M., Maguire, J. J., Bennett, M. R. & Clarke, M. C. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity 38, 285–295 (2013).
de Dieuleveult, A. L., Siemonsma, P. C., van Erp, J. B. & Brouwer, A. M. Effects of aging in multisensory integration: a systematic review. Front. Aging Neurosci. 9, 80 (2017).
Fernandes, J. C., Martel-Pelletier, J. & Pelletier, J. P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 39, 237–246 (2002).
Meulenbelt, I. et al. Association of the interleukin-1 gene cluster with radiographic signs of osteoarthritis of the hip. Arthritis Rheum. 50, 1179–1186 (2004).
Nasi, S., Ea, H. K., So, A. & Busso, N. Revisiting the role of interleukin-1 pathway in osteoarthritis: interleukin-1α and -1β, and NLRP3 inflammasome are not involved in the pathological features of the murine menisectomy model of osteoarthritis. Front. Pharmacol. 8, 282 (2017).
Gruber, J. et al. Induction of interleukin-1 in articular cartilage by explantation and cutting. Arthritis Rheum. 50, 2539–2546 (2004).
Ismail, H. M. et al. Interleukin-1 acts via the JNK-2 signaling pathway to induce aggrecan degradation by human chondrocytes. Arthritis Rheumatol. 67, 1826–1836 (2015).
Joosten, L. A. et al. Interleukin-18 promotes joint inflammation and induces interleukin-1-driven cartilage destruction. Am. J. Pathol. 165, 959–967 (2004).
Koenders, M. I. et al. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 58, 3461–3470 (2008).
Zwerina, J. et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl Acad. Sci. USA 104, 11742–11747 (2007).
Jiang, Y. et al. A multicenter, double-blind, dose-ranging, randomized, placebo- controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 43, 1001–1009 (2000).
Berda-Haddad, Y. et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc. Natl Acad. Sci. USA 108, 20684–20689 (2011).
Wakita, D. et al. Role of interleukin-1 signaling in a mouse model of Kawasaki Disease-associated abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 36, 886–897 (2016).
Campbell, A. J. & Burns, J. C. Adjunctive therapies for Kawasaki disease. J. Infect. 72(Suppl), S1–S5 (2016).
Kone-Paut, I. et al. The use of interleukin 1 receptor antagonist (anakinra) in Kawasaki disease: a retrospective cases series. Autoimmun. Rev. 17, 768–774 (2018).
Guillaume, M. P., Reumaux, H. & Dubos, F. Usefulness and safety of anakinra in refractory Kawasaki disease complicated by coronary artery aneurysm. Cardiol. Young 28, 739–742 (2018).
Tremoulet, A. H. et al. Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial). Contemp. Clin. Trials 48, 70–75 (2016).
Carrasco, D., Stecher, M., Lefebvre, G. C., Logan, A. C. & Moy, R. An open label, phase 2 study of MABp1 monotherapy for the treatment of acne vulgaris and psychiatric comorbidity. J. Drugs Dermatol. 14, 560–564 (2015).
Coleman, K. M., Gudjonsson, J. E. & Stecher, M. Open-label trial of MABp1, a true human monoclonal antibody targeting interleukin 1α, for the treatment of psoriasis. JAMA Dermatol. 151, 555–556 (2015).
Hickish, T. et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 18, 192–201 (2017).
Hong, D. S. et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 15, 656–666 (2014).
Hong, D. S. et al. Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest. New Drugs 33, 621–631 (2015).
Kanni, T. et al. MABp1 targeting IL-1alpha for moderate to severe hidradenitis suppurativa not eligible for adalimumab: a randomized study. J. Invest. Dermatol. 138, 795–801 (2018).
Tzanetakou, V. et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 152, 52–59 (2016).
Kawaguchi, Y., Hara, M. & Wright, T. M. Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J. Clin. Invest. 103, 1253–1260 (1999).
Zhang, L. et al. Association of interleukin 1 family with systemic sclerosis. Inflammation 37, 1213–1220 (2014).
Joosten, L. A. et al. Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty arthritis by reducing release and extracellular processing of IL-1β and by the induction of endogenous IL-1Ra. Ann. Rheum. Dis. 75, 1219–1227 (2016).
Joosten, L. A. et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 62, 3237–3248 (2010).
Jouvenne, P., Fossiez, F., Banchereau, J. & Miossec, P. High levels of neutralizing autoantibodies against IL-1 alpha are associated with a better prognosis in chronic polyarthritis: a follow-up study. Scand. J. Immunol. 46, 413–418 (1997).
Vincent, T., Plawecki, M., Goulabchand, R., Guilpain, P. & Eliaou, J. F. Emerging clinical phenotypes associated with anti-cytokine autoantibodies. Autoimmun. Rev. 14, 528–535 (2015).
Sugihara, T. et al. A new murine model to define the critical pathologic and therapeutic mediators of polymyositis. Arthritis Rheum. 56, 1304–1314 (2007).
Sugihara, T., Okiyama, N., Watanabe, N., Miyasaka, N. & Kohsaka, H. IL-1 and tumor necrosis factor α blockade for treatment of experimental polymyositis. Arthritis Rheum. 64, 2655–2662 (2012).
Botsios, C., Sfriso, P., Furlan, A., Punzi, L. & Dinarello, C. A. Resistant Behcet disease responsive to anakinra. Ann. Intern. Med. 149, 284–286 (2008).
Zong, M. et al. Anakinra treatment in patients with refractory inflammatory myopathies and possible predictive response biomarkers: a mechanistic study with 12 months follow-up. Ann. Rheum. Dis. 73, 913–920 (2014).
Munroe, M. E. et al. Pathways of impending disease flare in African-American systemic lupus erythematosus patients. J. Autoimmun. 78, 70–78 (2017).
Ostendorf, B. et al. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann. Rheum Dis. 64, 630–633 (2005).
Tayer-Shifman, O. E. & Ben-Chetrit, E. Refractory macrophage activation syndrome in a patient with SLE and APLA syndrome – successful use of PET-CT and Anakinra in its diagnosis and treatment. Mod. Rheumatol. 25, 954–957 (2015).
Egues Dubuc, C. A. et al. Hemophagocytic syndrome as the initial manifestation of systemic lupus erythematosus. Reumatol. Clin. 10, 321–324 (2014).
Dinarello, C. A., Simon, A. & Van Der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug. Discov. 11, 633–652 (2012).
Dinarello, C. A. et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J. Immunol. 139, 1902–1910 (1987).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Libby, P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J. Am. Coll. Cardiol. 70, 2278–2289 (2017).
Schlesinger, N. et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann. Rheum. Dis. 71, 1839–1848 (2012).
Chevalier, X. et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J. Rheumatol. 32, 1317–1323 (2005).
Larsen, C. M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Cavelti-Weder, C. et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care 35, 1654–1662 (2012).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J. Am. Coll. Cardiol. 71, 2392–2401 (2018).
Van Tassell, B. W. et al. Interleukin-1 blockade in recently decompensated systolic heart failure: Results from REDHART (Recently decompensated heart failure anakinra response trial). Circ. Heart Fail. 10, e004373 (2017).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139, 1289–1299 (2019).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Dinarello, C. A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 29, 317–329 (2010).
Lust, J. A. et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am. J. Hematol. 91, 571–574 (2016).
Andrei, C. et al. The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell. 10, 1463–1475 (1999).
Andrei, C. et al. Phospholipases C and A2 control lysosome-mediated IL-1β secretion: implications for inflammatory processes. Proc. Natl Acad. Sci. USA. 101, 9745–9750 (2004).
Gardella, S. et al. Secretion of bioactive interleukin-1β by dendritic cells is modulated by interaction with antigen specific T cells. Blood 95, 3809–3815 (2000).
Semino, C., Carta, S., Gattorno, M., Sitia, R. & Rubartelli, A. Progressive waves of IL-1β release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways. Cell Death Dis. 9, 1088–1102 (2018).
Qu, Y., Franchi, L., Nunez, G. & Dubyak, G. R. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925 (2007).
Kuriakose, T. & Kanneganti, T. D. Gasdermin D flashes an exit signal for IL-1. Immunity 48, 1–3 (2018).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44 (2018).
Brough, D., Pelegrin, P. & Nickel, W. An emerging case for membrane pore formation as a common mechanism for the unconventional secretion of FGF2 and IL-1β. J. Cell Sci. 130, 3197–3202 (2017).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Zhang, D. et al. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J. Neurosci. Res. 97, 645–660 (2019).
Xiao, J. et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLOS Biol. 16, e3000047 (2018).
Netea, M. G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113, 2324–2335 (2009).
Fantuzzi, G. et al. Response to local inflammation of IL-1 beta-converting enzyme-deficient mice. J. Immunol. 158, 1818–1824 (1997).
Joosten, L. A. et al. Inflammatory arthritis in caspase 1 gene-deficient mice: Contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 60, 3651–3662 (2009).
Kastner, D. L., Aksentijevich, I. & Goldbach-Mansky, R. Autoinflammatory disease reloaded: a clinical perspective. Cell 140, 784–790 (2010).
Manthiram, K., Zhou, Q., Aksentijevich, I. & Kastner, D. L. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 18, 832–842 (2017).
Agostini, L. et al. NALP3 forms an IL-1β processing inflammasome with increased activity in Muckle-Wells auto-inflammatory disorder. Immunity 20, 319–325 (2004).
Schett, G., Dayer, J. M. & Manger, B. Interleukin-1 function and role in rheumatic disease. Nat. Rev. Rheumatol. 12, 14–24 (2016).
Masters, S. L., Simon, A., Aksentijevich, I. & Kastner, D. L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 27, 621–668 (2009).
Chae, J. J. et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1β production. Proc. Natl Acad. Sci. USA. 103, 9982–9987 (2006).
Shoham, N. G. et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl Acad. Sci. USA 100, 13501–13506 (2003).
Drenth, J. P., van der Meer, J. W. & Kushner, I. Unstimulated peripheral blood mononuclear cells from patients with the hyper-IgD syndrome produce cytokines capable of potent induction of C-reactive protein and serum amyloid A in Hep3B cells. J. Immunol. 157, 400–404 (1996).
Gattorno, M. et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 58, 1505–1515 (2008).
Gattorno, M. et al. Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 56, 3138–3148 (2007).
Goldbach-Mansky, R. et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N. Engl. J. Med. 355, 581–592 (2006).
Giamarellos-Bourboulis, E. J. et al. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1β by mononuclear cells through a caspase 1-mediated process. Ann. Rheum. Dis. 68, 273–278 (2009).
Seibert, K. et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl Acad. Sci. USA 91, 12013–12017 (1994).
So, A., De Smedt, T., Revaz, S. & Tschopp, J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res. Ther. 9, R28 (2007).
Wang, H. J., Jiang, Y. F., Wang, X. R., Zhang, M. L. & Gao, P. J. Elevated serum interleukin-38 level at baseline predicts virological response in telbivudine-treated patients with chronic hepatitis B. World J. Gastroenterol. 22, 4529–4537 (2016).
Terkeltaub, R. et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann. Rheum. Dis. 68, 1613–1617 (2009).
Janssen, C. A. et al. Anakinra for the treatment of acute gout flares: a randomized, double-blind, placebo-controlled, active-comparator, non-inferiority trial. Rheumatology 58, 1344–1352 (2019).
Marchetti, C. et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl Acad. Sci. USA 115, E1530–E1539 (2018).
Klück, V. et al. OLT1177™, an oral NLRP3 inflammasome inhibitor, inhibits acute joint inflammation and circulating IL-1β during gout flares in humans. Ann. Rheum. Dis. 78 (Suppl 1), A69 (2019).
Jansen, T. L. et al. The first Phase 2a proof-of-concept study of a selective NLRP3 inflammasome inhibitor, dapansutrile (OLT1177™), in acute gout. Ann. Rheum. Dis. 78 (Suppl 1), A70 (2019).
Cicero, A. F. et al. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella heart study. J. Hypertens. 32, 57–64 (2014).
Athyros, V. G. & Mikhailidis, D. P. Uric acid, chronic kidney disease and type 2 diabetes: a cluster of vascular risk factors. J. Diabetes Complications 28, 122–123 (2014).
Gustafsson, D. & Unwin, R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 14, 164 (2013).
Crisan, T. O. et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 75, 755–762 (2016).
Crisan, T. O. et al. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc. Natl Acad. Sci. USA 114, 5485–5490 (2017).
Pascual, V., Allantaz, F., Arce, E., Punaro, M. & Banchereau, J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 201, 1479–1486 (2005).
Fitzgerald, A. A., Leclercq, S. A., Yan, A., Homik, J. E. & Dinarello, C. A. Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum. 52, 1794–1803 (2005).
Quartier, P. et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann. Rheum. Dis. 70, 747–754 (2011).
Horneff, G., Peitz, J., Kekow, J. & Foell, D. Canakinumab for first line steroid-free treatment in a child with systemic-onset juvenile idiopathic arthritis. Scand. J. Rheumatol. 46, 500–501 (2017).
Wulffraat, N. M. & Woo, P. Canakinumab in pediatric rheumatic diseases. Expert Opin. Biol. Ther. 13, 615–622 (2013).
Vojinovic, J. et al. Safety and efficacy of an oral histone deacetylase inhibitor in systemic onset juvenile idiopathic arthritis. Arthritis Rheum. 63, 1452–1458 (2011).
Leoni, F. et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol. Med. 11, 1–15 (2005).
Furlan, A. et al. Pharmacokinetics, safety and inducible cytokine responses during a phase 1 trial of the oral histone deacetylase inhibitor ITF2357 (givinostat). Mol. Med. 17, 353–362 (2011).
Rudinskaya, A. & Trock, D. H. Successful treatment of a patient with refractory adult-onset Still’s disease with anakinra. J. Clin. Rheumatol. 9, 330–332 (2003).
Vasques Godinho, F. M., Parreira Santos, M. J. & Canas da Silva, J. Refractory adult onset Still’s disease successfully treated with anakinra. Ann. Rheum. Dis. 64, 647–648 (2005).
Colafrancesco, S. et al. Response to interleukin-1 inhibitors in 140 Italian patients with adult-onset Still’s disease: a multicentre retrospective observational study. Front. Pharmacol. 8, 369 (2017).
Junge, G., Mason, J. & Feist, E. Adult onset Still’s disease — the evidence that anti-interleukin-1 treatment is effective and well-tolerated (a comprehensive literature review). Semin. Arthritis Rheum. 47, 295–302 (2017).
Ruscitti, P., Ursini, F., Cipriani, P., De Sarro, G. & Giacomelli, R. Biologic drugs in adult onset Still’s disease: a systematic review and meta-analysis of observational studies. Expert Rev. Clin. Immunol. 13, 1089–1097 (2017).
Parisi, F., Paglionico, A., Varriano, V., Ferraccioli, G. & Gremese, E. Refractory adult-onset Still disease complicated by macrophage activation syndrome and acute myocarditis: a case report treated with high doses (8 mg/kg/d) of anakinra. Medicine 96, e6656 (2017).
Fabiani, C. et al. Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behcet’s disease-related uveitis: a multicenter retrospective observational study. Clin. Rheumatol. 36, 191–197 (2017).
Kiltz, U. et al. Prolonged treatment with Tadekinig alfa in adult-onset Still’s disease. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2018-214496 (2018).
Gabay, C. et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann. Rheum. Dis. 77, 840–847 (2018).
Ombrello, M. J. et al. HLA-DRB1*11 and variants of the MHC class II locus are strong risk factors for systemic juvenile idiopathic arthritis. Proc. Natl Acad. Sci. USA 112, 15970–15975 (2015).
Wang, F. F. et al. A genetic role for macrophage migration inhibitory factor (MIF) in adult-onset Still’s disease. Arthritis Res. Ther. 15, R65 (2013).
Cavalli, G. et al. Identification of rare coding variants in IL-1-related pathways in patients with adult onset Still’s Disease [abstract]. Ann. Rheum. Dis. 78 (Suppl. 2), 190 (2018).
Cepika, A. M. et al. A multidimensional blood stimulation assay reveals immune alterations underlying systemic juvenile idiopathic arthritis. J. Exp. Med. 214, 3449–3466 (2017).
Kim, H. A. et al. Phase 2 enzyme inducer sulphoraphane blocks prostaglandin and nitric oxide synthesis in human articular chondrocytes and inhibits cartilage matrix degradation. Rheumatology 51, 1006–1016 (2012).
Smith, M. D., Triantafillou, S., Parker, A., Youssef, P. P. & Coleman, M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 24, 365–371 (1997).
Adams, S. B. Jr et al. Global metabolic profiling of human osteoarthritic synovium. Osteoarthritis Cartilage 20, 64–67 (2012).
Benito, M. J., Veale, D. J., FitzGerald, O., van den Berg, W. B. & Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267 (2005).
Goekoop, R. J. et al. Low innate production of interleukin-1β and interleukin-6 is associated with the absence of osteoarthritis in old age. Osteoarthritis Cartilage 18, 942–947 (2010).
Fraenkel, L. et al. The association of peripheral monocyte derived interleukin 1β (IL-1β), IL-1 receptor antagonist, and tumor necrosis factor-α with osteoarthritis in the elderly. J. Rheumatol. 25, 1820–1826 (1998).
Lee, J. K. et al. Differences in signaling pathways by IL-1β and IL-18. Proc. Natl Acad. Sci. USA 101, 8815–8820 (2004).
Jovanovic, D. et al. Effect of IL-13 on cytokines, cytokine receptors and inhibitors on human osteoarthritis synovium and synovial fibroblasts. Osteoarthritis Cartilage 6, 40–49 (1998).
Fujikawa, Y., Shingu, M., Torisu, T. & Masumi, S. Interleukin-1 receptor antagonist production in cultured synovial cells from patients with rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 54, 318–320 (1995).
Ismail, H. M. et al. JNK-2 controls aggrecan degradation in murine articular cartilage and the development of experimental osteoarthritis. Arthritis Rheumatol. 68, 1165–1171 (2016).
Kloppenburg, M. et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 78, 413–420 (2018).
Wang, S. X. et al. Safety, tolerability, and pharmacodynamics of an anti-interleukin-1α/β dual variable domain immunoglobulin in patients with osteoarthritis of the knee: a randomized phase 1 study. Osteoarthritis Cartilage 25, 1952–1961 (2017).
Fleischmann, R. M. et al. A phase II trial of lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol. 71, 1056–1069 (2019).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352 (2009).
Evans, C. H., Ghivizzani, S. C. & Robbins, P. D. Gene delivery to joints by intra-articular injection. Hum. Gene Ther. 29, 2–14 (2018).
Cohen, S. B. et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 13, R125 (2011).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17, 588–606 (2018).
Marchetti, C. et al. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res. Ther. 20, 169 (2018).
Kim, H. A., Yeo, Y., Kim, W. U. & Kim, S. Phase 2 enzyme inducer sulphoraphane blocks matrix metalloproteinase production in articular chondrocytes. Rheumatology 48, 932–938 (2009).
Ali, S. et al. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc. Natl Acad. Sci. USA 104, 18660–18665 (2007).
Lingel, A. et al. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors-insight into heterotrimeric IL-1 signaling complexes. Structure 17, 1398–1410 (2009).
Cevikbas, F. & Steinhoff, M. IL-33: a novel danger signal system in atopic dermatitis. J. Invest. Dermatol. 132, 1326–1329 (2012).
Liew, F. Y., Girard, J. P. & Turnquist, H. R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 16, 676–689 (2016).
Yang, Q. et al. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur. J. Immunol. 41, 3351–3360 (2011).
Cayrol, C. & Girard, J. P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl Acad. Sci. USA 106, 9021–9026 (2009).
Carriere, V. et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl Acad. Sci. USA 104, 282–287 (2007).
Bessa, J. et al. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J. Autoimmun. 55, 33–41 (2014).
Chen, Z., Bozec, A., Ramming, A. & Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 15, 9–17 (2019).
Biton, J. et al. In vivo expansion of activated FOXP3+ regulatory T cells and establishment of a type 2 immune response upon IL-33 treatment protect against experimental arthritis. J. Immunol. 197, 1708–1719 (2016).
Palmer, G. et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 60, 738–749 (2009).
Martin, P. et al. Disease severity in K/BxN serum transfer-induced arthritis is not affected by IL-33 deficiency. Arthritis Res. Ther. 15, R13 (2013).
Athari, S. K. et al. Collagen-induced arthritis and imiquimod-induced psoriasis develop independently of interleukin-33. Arthritis Res. Ther. 18, 143 (2016).
Shen, J. et al. IL-33 and soluble ST2 levels as novel predictors for remission and progression of carotid plaque in early rheumatoid arthritis: a prospective study. Semin. Arthritis Rheum. 45, 18–27 (2015).
Hong, Y. S. et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J. Korean Med. Sci. 26, 1132–1139 (2011).
Matsuyama, Y. et al. Sustained elevation of interleukin-33 in sera and synovial fluids from patients with rheumatoid arthritis non-responsive to anti-tumor necrosis factor: possible association with persistent IL-1β signaling and a poor clinical response. Rheumatol. Int. 32, 1397–1401 (2012).
Tang, S. et al. Increased IL-33 in synovial fluid and paired serum is associated with disease activity and autoantibodies in rheumatoid arthritis. Clin. Dev. Immunol. 2013, 985301 (2013).
Kunisch, E., Chakilam, S., Gandesiri, M. & Kinne, R. W. IL-33 regulates TNF-alpha dependent effects in synovial fibroblasts. Int. J. Mol. Med. 29, 530–540 (2012).
Rivellese, F. et al. Ability of interleukin-33- and immune complex-triggered activation of human mast cells to down-regulate monocyte-mediated immune responses. Arthritis Rheumatol 67, 2343–2353 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03469934 (2019).
Dinarello, C. A., Novick, D., Kim, S. & Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 4, 289–303 (2013).
Kaplanski, G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol. Rev. 281, 138–153 (2018).
Puren, A. J., Fantuzzi, G. & Dinarello, C. A. Gene expression, synthesis and secretion of IL-1β and IL-18 are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl Acad. Sci. USA 96, 2256–2261 (1999).
Okamura, H. et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 63, 3966–3972 (1995).
Novick, D. et al. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 10, 127–136 (1999).
Novick, D. et al. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine 14, 334–342 (2001).
Girard, C. et al. Elevated serum levels of free interleukin-18 in adult-onset Still’s disease. Rheumatology 55, 2237–2247 (2016).
Novick, D. et al. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J. Autoimmun. 34, 121–126 (2011).
Novick, D., Elbirt, D., Dinarello, C. A., Rubinstein, M. & Sthoeger, Z. M. Interleukin-18 binding protein in the sera of patients with Wegener’s granulomatosis. J. Clin. Immunol. 29, 38–45 (2009).
Ludwiczek, O. et al. Elevated systemic levels of free interleukin-18 (IL-18) in patients with Crohn’s disease. Eur. Cytokine Netw. 16, 27–33 (2005).
Mazodier, K. et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 106, 3483–3489 (2005).
Canna, S. W. et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J. Allergy Clin. Immunol. 139, 1698–1701 (2017).
Minoia, F. et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 66, 3160–3169 (2014).
Grom, A. A. Macrophage activation syndrome and reactive hemophagocytic lymphohistiocytosis: the same entities? Curr. Opin. Rheumatol. 15, 587–590 (2003).
Grom, A. A. & Mellins, E. D. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr. Opin. Rheumatol. 22, 561–566 (2011).
Grom, A. A. et al. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J. Pediatr. 142, 292–296 (2003).
Janka, G. E. Familial and acquired hemophagocytic lymphohistiocytosis. Annu. Rev. Med. 63, 233–246 (2012).
Weiss, E. S. et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 131, 1442–1455 (2018).
Gao, Z., Wang, Y., Wang, J., Zhang, J. & Wang, Z. Soluble ST2 and CD163 as potential biomarkers to differentiate primary hemophagocytic lymphohistiocytosis from macrophage activation syndrome. Mediterr. J. Hematol. Infect. Dis. 11, e2019008 (2019).
Maruyama, J. & Inokuma, S. Cytokine profiles of macrophage activation syndrome associated with rheumatic diseases. J. Rheumatol. 37, 967–973 (2010).
Crayne, C. B., Albeituni, S., Nichols, K. E. & Cron, R. Q. The immunology of macrophage activation syndrome. Front. Immunol. 10, 119 (2019).
Lin, F. C. et al. IFN-γ causes aplastic anemia by altering hematopoietic stem/progenitor cell composition and disrupting lineage differentiation. Blood 124, 3699–3708 (2014).
Canna, S. W. et al. Interferon-γ mediates anemia but is dispensable for fulminant Toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis. Arthritis Rheum. 65, 1764–1775 (2013).
Ravelli, A. et al. Expert consensus on dynamics of laboratory tests for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. RMD Open 2, e000161 (2016).
Schulert, G. S. & Grom, A. A. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu. Rev. Med. 66, 145–159 (2015).
Shimizu, M. et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology 49, 1645–1653 (2010).
Wada, T. et al. Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency. Cytokine 65, 74–78 (2014).
Canna, S. W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146 (2014).
Duncan, J. A. & Canna, S. W. The NLRC4 inflammasome. Immunol. Rev. 281, 115–123 (2018).
Moghaddas, F. et al. Autoinflammatory mutation in NLRC4 reveals a leucine-rich repeat (LRR)-LRR oligomerization interface. J. Allergy Clin. Immunol. 142, 1956–1967 (2018).
Romberg, N., Vogel, T. P. & Canna, S. W. NLRC4 inflammasomopathies. Curr. Opin. Allergy Clin. Immunol. 17, 398–404 (2017).
Ravelli, A., Grom, A. A., Behrens, E. M. & Cron, R. Q. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 13, 289–298 (2012).
Sonmez, H. E., Demir, S., Bilginer, Y. & Ozen, S. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin. Rheumatol. 37, 3329–3335 (2018).
Toldo, S. et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 306, H1025–H1031 (2014).
Fisher, C. J. Jr. et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271, 1836–1843 (1994).
Opal, S. M. et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 25, 1115–1124 (1997).
Shakoory, B. et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 44, 275–281 (2016).
Ji, J. D. & Lee, W. J. Interleukin-18 gene polymorphisms and rheumatoid arthritis: a meta-analysis. Gene 523, 27–32 (2013).
Bokarewa, M. & Hultgren, O. Is interleukin-18 useful for monitoring rheumatoid arthritis? Scand. J. Rheumatol. 34, 433–436 (2005).
Gracie, J. A. et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J. Clin. Invest. 104, 1393–1401 (1999).
Adis International. Tadekinig alfa — Merck Serono. Adis Insight https://adisinsight.springer.com/drugs/800013227 (2009).
Wu, C. Y., Yang, H. Y., Yao, T. C., Liu, S. H. & Huang, J. L. Serum IL-18 as biomarker in predicting long-term renal outcome among pediatric-onset systemic lupus erythematosus patients. Medicine 95, e5037 (2016).
Koenig, K. F. et al. Serum cytokine profile in patients with active lupus nephritis. Cytokine 60, 410–416 (2012).
Favilli, F. et al. IL-18 activity in systemic lupus erythematosus. Ann. NY Acad. Sci. 1173, 301–309 (2009).
Italiani, P. et al. IL-1 family cytokines and soluble receptors in systemic lupus erythematosus. Arthritis Res. Ther. 20, 27 (2018).
Aghdashi, M., Aribi, S. & Salami, S. Serum levels of IL-18 in Iranian females with systemic lupus erythematosus. Med. Arch. 67, 237–240 (2013).
Maczynska, I. et al. Proinflammatory cytokine (IL-1β, IL-6, IL-12, IL-18 and TNF-α) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE). Immunol. Lett. 102, 79–82 (2006).
Pan, G. et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine 13, 1–7 (2001).
Kumar, S. et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 18, 61–71 (2002).
Nold, M. F. et al. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 11, 1014–1022 (2010).
Garlanda, C., Riva, F., Bonavita, E. & Mantovani, A. Negative regulatory receptors of the IL-1 family. Semin. Immunol. 25, 4087–4415 (2013).
Molgora, M. et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 551, 110–114 (2017).
Cavalli, G. et al. Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc. Natl Acad. Sci. USA 114, 2313–2318 (2017).
Luo, Y. et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc. Natl Acad. Sci. USA 111, 15178–15183 (2014).
Ballak, D. B. et al. Interleukin-37 treatment of mice with metabolic syndrome improves insulin sensitivity and reduces pro-inflammatory cytokine production in adipose tissue. J. Biol. Chem. 293, 14224–14236 (2018).
Ballak, D. B. et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat. Commun. 5, 4711 (2014).
Pei, B. et al. Associations of the IL-1F7 gene polymorphisms with rheumatoid arthritis in Chinese Han population. Int. J. Immunogenet. 40, 199–203 (2013).
Shi, L. P., He, Y. & Liu, Z. D. Correlation between single nucleotide polymorphism of rs3811047 in IL-1 F7 gene and rheumatoid arthritis susceptibility among Han population in central plains of China. Asian Pac. J. Trop. Med. 6, 73–75 (2013).
Kang, B., Cheng, S., Peng, J., Yan, J. & Zhang, S. Interleukin-37 gene variants segregated anciently coexist during hominid evolution. Eur. J. Hum. Genet. 23, 1392–1398 (2015).
Zhao, P. W. et al. Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLOS ONE 9, e95346 (2014).
Yang, L., Zhang, J., Tao, J. & Lu, T. Elevated serum levels of interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. APMIS 123, 1025–1031 (2015).
Xia, T. et al. Plasma interleukin-37 is elevated in patients with rheumatoid arthritis: its correlation with disease activity and Th1/Th2/Th17-related cytokines. Dis. Markers 2015, 795043 (2015).
Xia, L., Shen, H. & Lu, J. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: attenuated the production of inflammatory cytokines. Cytokine 76, 553–557 (2015).
Wang, L., Wang, Y., Xia, L., Shen, H. & Lu, J. Elevated frequency of IL-37- and IL-18Rα-positive T cells in the peripheral blood of rheumatoid arthritis patients. Cytokine 110, 291–297 (2018).
Wang, M. et al. Detection of the novel IL-1 family cytokines by QAH-IL1F-1 assay in rheumatoid arthritis. Cell. Mol. Biol. 62, 31–34 (2016).
Feng, M. et al. Plasma interleukin-37 is increased and inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells in systemic juvenile idiopathic arthritis patients. J. Transl. Med. 16, 277 (2018).
El-Barbary, A. M. et al. Role of interleukin 37 as a novel proangiogenic factor in juvenile idiopathic arthritis. J. Clin. Rheumatol. 25, 85–90 (2018).
Chi, H. et al. Interleukin-37 is increased in adult-onset Still’s disease and associated with disease activity. Arthritis Res. Ther. 20, 54 (2018).
Song, L. et al. High interleukin-37 (IL-37) expression and increased mucin-domain containing-3 (TIM-3) on peripheral T cells in patients with rheumatoid arthritis. Med. Sci. Monit. 24, 5660–5667 (2018).
Ragab, D., Mobasher, S. & Shabaan, E. Elevated levels of IL-37 correlate with T cell activation status in rheumatoid arthritis patients. Cytokine 113, 305–310 (2019).
Eisenmesser, E. Z. et al. Interleukin-37 monomer is the active form for reducing innate immunity. Proc. Natl Acad. Sci. USA 116, 5514–5522 (2019).
Ellisdon, A. M. et al. Homodimerization attenuates the anti-inflammatory activity of interleukin-37. Sci. Immunol. 2, 1548 (2017).
Chen, B. et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J. Transl. Med. 13, 36 (2015).
Keermann, M. et al. Expression of IL-36 family cytokines and IL-37 but not IL-38 is altered in psoriatic skin. J. Dermatol. Sci. 80, 150–152 (2015).
Song, L. et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J. Clin. Immunol. 33, 111–117 (2013).
Ye, Z., Wang, C., Kijlstra, A., Zhou, X. & Yang, P. A possible role for interleukin 37 in the pathogenesis of Behcet’s disease. Curr. Mol. Med. 14, 535–542 (2014).
Bouali, E., Kaabachi, W., Hamzaoui, A. & Hamzaoui, K. Interleukin-37 expression is decreased in Behcet’s disease and is associated with inflammation. Immunol. Lett. 167, 87–94 (2015).
Charrad, R. et al. Anti-inflammatory activity of IL-37 in asthmatic children: correlation with inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology 221, 182–187 (2016).
Saglam, M. et al. Levels of interleukin-37 in gingival crevicular fluid, saliva, or plasma in periodontal disease. J. Periodontal Res. 50, 614–621 (2014).
Liu, W. et al. Anti-inflammatory effect of IL-37b in children with allergic rhinitis. Mediators Inflamm. 2014, 746846 (2014).
Grabherr, F. et al. Ethanol-mediated suppression of IL-37 licenses alcoholic liver disease. Liver Int. 38, 1095–1101 (2017).
Ge, G. et al. Interleukin-37 suppresses tumor growth through inhibition of angiogenesis in non-small cell lung carcinoma. J. Exp. Clin. Cancer Res. 35, 13–23 (2016).
Busfield, S. J. et al. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics 66, 213–216 (2000).
Debets, R. et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J. Immunol. 167, 1440–1446 (2001).
Lachner, J., Mlitz, V., Tschachler, E. & Eckhart, L. Epidermal cornification is preceded by the expression of a keratinocyte-specific set of pyroptosis-related genes. Sci. Rep. 7, 17446 (2017).
Onoufriadis, A. et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am. J. Hum. Genet. 89, 432–437 (2011).
Teoh, Y. L. & Tay, Y. K. Generalized pustular psoriasis with a novel mutation of interleukin-36 receptor antagonist, responding to methotrexate. JAAD Case Rep. 1, 51–53 (2015).
Marrakchi, S. et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 365, 620–628 (2011).
Sullivan, G. P. et al. Identification of small-molecule elastase inhibitors as antagonists of IL-36 cytokine activation. FEBS Open Bio. 8, 751–763 (2018).
Sullivan, G. P. et al. Suppressing IL-36-driven inflammation using peptide pseudosubstrates for neutrophil proteases. Cell Death Dis. 9, 378 (2018).
Vigne, S. et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood 118, 5813–5823 (2011).
Buhl, A. L. & Wenzel, J. Interleukin-36 in infectious and inflammatory skin diseases. Front. Immunol. 10, 1162 (2019).
Boutet, M. A., Nerviani, A. & Pitzalis, C. IL-36, IL-37, and IL-38 cytokines in skin and joint inflammation: a comprehensive review of their therapeutic potential. Int. J. Mol. Sci. 20, e1257 (2019).
Ding, L., Wang, X., Hong, X., Lu, L. & Liu, D. IL-36 cytokines in autoimmunity and inflammatory disease. Oncotarget 9, 2895–2901 (2018).
Bassoy, E. Y., Towne, J. E. & Gabay, C. Regulation and function of interleukin-36 cytokines. Immunol. Rev. 281, 169–178 (2018).
Boutet, M. A. et al. Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin. Exp. Immunol. 184, 159–173 (2016).
Boutet, M. A. et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann. Rheum. Dis. 76, 1304–1312 (2017).
Ciccia, F. et al. Interleukin-36α axis is modulated in patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 181, 230–238 (2015).
Li, J. et al. New interleukins in psoriasis and psoriatic arthritis patients: the possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology 233, 37–46 (2017).
Van De Veerdonk, F. L. et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc. Natl Acad. Sci. USA 109, 3001–3005 (2012).
Mora, J. et al. Interleukin-38 is released from apoptotic cells to limit inflammatory macrophage responses. J. Mol. Cell Biol. 8, 426–438 (2016).
Dehghan, A. et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 123, 731–738 (2011).
Mercurio, L. et al. IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment. Cell Death Dis. 9, 1104 (2018).
Yang, N. et al. Elevated interleukin-38 level associates with clinical response to atorvastatin in patients with hyperlipidemia. Cell. Physiol. Biochem. 49, 653–661 (2018).
Chu, M. et al. Aberrant expression of novel cytokine IL-38 and regulatory T lymphocytes in childhood asthma. Molecules 21, e933 (2016).
Xu, F. et al. Interleukin 38 protects against lethal sepsis. J. Infect. Dis. 218, 1175–1184 (2018).
Rudloff, I. et al. Interleukin-38 exerts antiinflammatory functions and is associated with disease activity in systemic lupus erythematosus. Arthritis Rheumatol. 67, 3219–3225 (2015).
Sana, T. R., Debets, R., Timans, J. C., Bazan, J. F. & Kastelein, R. A. Computational identification, cloning, and characterization of IL-1R9, a novel interleukin-1 receptor-like gene encoded over an unusually large interval of human chromosome Xq22.2-q22.3. Genomics 69, 252–262 (2000).
Takenaka, S. I. et al. IL-38: A new factor in rheumatoid arthritis. Biochem. Biophys. Rep. 4, 386–391 (2015).
Lin, H. et al. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J. Biol. Chem. 276, 20597–20602 (2001).
Bensen, J. T., Dawson, P. A., Mychaleckyj, J. C. & Bowden, D. W. Identification of a novel human cytokine gene in the interleukin gene cluster on chromosome 2q12-14. J. Interferon Cytokine Res. 21, 899–904 (2001).
De Graaf, D. M. et al. Human IL-38 reduces joint inflammation in a mouse model of gouty arthritis [abstract]. Ann. Rheum. Dis. 77 (Suppl 2), 135 (2018).
Chu, M. et al. In vivo anti-inflammatory activities of novel cytokine IL-38 in Murphy Roths Large (MRL)/lpr mice. Immunobiology 222, 483–493 (2017).
Rossi-Semerano, L. et al. Tolerance and efficacy of off-label anti-interleukin-1 treatments in France: a nationwide survey. Orphanet. J. Rare Dis. 10, 19 (2015).
Vitale, A. et al. A snapshot on the on-label and off-label use of the interleukin-1 inhibitors in Italy among rheumatologists and pediatric rheumatologists: a nationwide multi-center retrospective observational study. Front. Pharmacol. 7, 380 (2016).
Vitale, A., Cantarini, L., Rigante, D., Bardelli, M. & Galeazzi, M. Anakinra treatment in patients with gout and type 2 diabetes. Clin. Rheumatol. 34, 981–984 (2015).
Abbate, A., Canada, J. M., Van Tassell, B. W., Wise, C. M. & Dinarello, C. A. Interleukin-1 blockade in rheumatoid arthritis and heart failure: a missed opportunity? Int. J. Cardiol. 171, e125–e126 (2014).
Ruscitti, P. et al. IL-1 inhibition improves insulin resistance and adipokines in rheumatoid arthritis patients with comorbid type 2 diabetes: an observational study. Medicine 98, e14587 (2019).
Ruscitti, P. et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, randomised, open, prospective, controlled, parallel-group trial. PLOS. Med. in the press (2019).
Economides, A. N. et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat. Med. 9, 47–52 (2003).
Kucuksahin, O. et al. Anti-interleukin-1 treatment in 26 patients with refractory familial Mediterranean fever. Mod. Rheumatol. 27, 350–355 (2017).
Haviv, R. & Hashkes, P. J. Canakinumab investigated for treating familial Mediterranean fever. Expert Opin. Biol. Ther. 16, 1425–1434 (2016).
Ozdogan, H. & Ugurlu, S. Canakinumab for the treatment of familial Mediterranean fever. Expert Rev. Clin. Immunol. 13, 393–404 (2017).
de Koning, H. D. et al. Sustained efficacy of the monoclonal anti-interleukin-1β antibody canakinumab in a 9-month trial in Schnitzler’s syndrome. Ann. Rheum. Dis. 72, 1634–1638 (2013).
de Koning, H. D. et al. The role of interleukin-1 beta in the pathophysiology of Schnitzler’s syndrome. Arthritis Res. Ther. 17, 187 (2015).
Krause, K. et al. Efficacy and safety of canakinumab in Schnitzler syndrome: a multicenter randomized placebo-controlled study. J. Allergy Clin. Immunol. 139, 1311–1320 (2017).
Alten, R. et al. Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskelet. Disord. 12, 153 (2011).
Solomon, D. H. et al. Relationship of interleukin-1beta blockade with incident gout and serum uric acid levels. Ann. Intern. Med. 169, 535–542 (2018).
Gul, A. et al. Interleukin-1β-regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of Behcet’s disease: an open-label pilot study. Ann. Rheum. Dis. 71, 563–566 (2012).
Cardiel, M. H. et al. A phase 2 randomized, double-blind study of AMG 108, a fully human monoclonal antibody to IL-1R, in patients with rheumatoid arthritis. Arthritis Res. Ther. 12, R192 (2010).
Lacy, S. E. et al. Generation and characterization of ABT-981, a dual variable domain immunoglobulin (DVD-IgTM) molecule that specifically and potently neutralizes both IL-1α and IL-1β. MAbs 7, 605–619 (2015).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380, 752–762 (2019).
Tak, P. P., Bacchi, M. & Bertolino, M. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. Eur. J. Drug Metab. Pharmacokinet. 31, 109–116 (2006).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03512314 (2019).
Striz, I. Cytokines of the IL-1 family: recognized targets in chronic inflammation underrated in organ transplantations. Clin. Sci. 131, 2241–2256 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02345928 (2017).
Towne, J. E. & Sims, J. E. IL-36 in psoriasis. Curr. Opin. Pharmacol. 12, 486–490 (2012).
Gay, N. J. & Keith, F. J. Drosophila Toll and IL-1 receptor. Nature 351, 355–356 (1991).
Heguy, A., Baldari, C. T., Macchia, G., Telford, J. L. & Melli, M. Amino acids conserved in interleukin-1 receptors (IL-1Rs) and the Drosophila Toll protein are essential for IL-1R signal transduction. J. Biol. Chem. 267, 2605–2609 (1992).
Opal, S. M. et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309, 1154–1162 (2013).
Dinarello, C. A. & Van Der Meer, J. W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 25, 469–484 (2013).
Hoffman, H. M. Rilonacept for the treatment of cryopyrin-associated periodic syndromes (CAPS). Expert Opin. Biol. Ther. 9, 519–531 (2009).
Petryna, O., Cush, J. J. & Efthimiou, P. IL-1 Trap rilonacept in refractory adult onset Still’s disease. Ann. Rheum. Dis. 71, 2056–2057 (2012).
Ruperto, N. et al. A phase II, multicenter, open-label study evaluating dosing and preliminary safety and efficacy of canakinumab in systemic juvenile idiopathic arthritis with active systemic features. Arthritis Rheum. 64, 557–567 (2012).
Kosloski, M. P. et al. Pharmacokinetics and tolerability of a dual variable domain immunoglobulin ABT-981 against IL-1α and IL-1β in healthy subjects and patients with osteoarthritis of the knee. J. Clin. Pharmacol. 56, 1582–1590 (2016).
Acknowledgements
The work of C.A.D. is supported by NIH Grant AI-15614. C.A.D. thanks P. Libby, A. Rubartelli, J.-M. Dayer, L. A. B. Joosten, M. Netea, M. Donath, T. Mandrup-Poulsen, D. B. Skouras, T. L. Jansen, M. Janssen, G. Cavalli, G. Kaplanski and D. Novick for helpful discussions and for providing information and feedback in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.A.D. serves as chair of the SAB of Olatec Therapeutics, LLC, which develops the NLRP3 inhibitor OLT1177 (Dapansutrile).
Additional information
Peer review information
Nature Reviews Rheumatology thanks F. Blanchard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol 15, 612–632 (2019). https://doi.org/10.1038/s41584-019-0277-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0277-8
This article is cited by
-
Neutrophil extracellular traps and neutrophilic dermatosis: an update review
Cell Death Discovery (2024)
-
Lactococcus lactis as an Interleukin Delivery System for Prophylaxis and Treatment of Inflammatory and Autoimmune Diseases
Probiotics and Antimicrobial Proteins (2024)
-
Involvement of inflammasomes in tumor microenvironment and tumor therapies
Journal of Hematology & Oncology (2023)
-
Pharmacologic inhibition of NLRP3 reduces the levels of α-synuclein and protects dopaminergic neurons in a model of Parkinson’s disease
Journal of Neuroinflammation (2023)
-
Strategies to therapeutically modulate cytokine action
Nature Reviews Drug Discovery (2023)