Abstract
Mammalian sperm cells must respond to cues originating from along the female reproductive tract and from the layers of the egg in order to complete their fertilization journey. Dynamic regulation of ion signalling is, therefore, essential for sperm cells to adapt to their constantly changing environment. Over the past 15 years, direct electrophysiological recordings together with genetically modified mouse models and human genetics have confirmed the importance of ion channels, including the principal Ca2+-selective plasma membrane ion channel CatSper, for sperm activity. Sperm ion channels and membrane receptors are attractive targets for both the development of contraceptives and infertility treatment drugs. Furthermore, in this era of assisted reproductive technologies, understanding the signalling processes implicated in defective sperm function, particularly those arising from genetic abnormalities, is of the utmost importance not only for the development of infertility treatments but also to assess the overall health of a patient and his children. Future studies to improve reproductive health care and overall health care as a function of the ability to reproduce should include identification and analyses of gene variants that underlie human infertility and research into fertility-related molecules.
Key points
-
Mammalian sperm cells undergo intracellular alkalinization during their fertilization journey as they encounter a drastic extracellular pH change in the female reproductive tract.
-
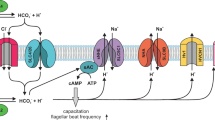
During capacitation, the sperm membrane potential hyperpolarizes, primarily via KSper activation and K+ efflux.
-
Increases in intracellular Ca2+ are required for inducing hyperactivated motility and acrosome reaction, two key physiological events essential for fertilization.
-
CatSper, the multi-subunit Ca2+ channel, is the predominant Ca2+ entry pathway in sperm cells and organizes into linear Ca2+ signalling nanodomains along the flagella.
-
CatSper-mediated Ca2+ signalling is integrated into other sperm capacitation signalling pathways including phosphorylation cascades.
-
Improved understanding of spermatozoan ion channels and transporters will help elucidate the delicate and dynamic regulation of Ca2+ homeostasis in sperm motility and fertility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hille, B. Ion channels of excitable membranes (2001).
Clapham, D. E. Calcium signaling. Cell 131, 1047–1058 (2007).
Bagur, R. & Hajnóczky, G. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell 66, 780–788 (2017).
Bessen, M., Fay, R. B. & Witman, G. B. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol. 86, 446–455 (1980).
Böhmer, M. et al. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 24, 2741–2752 (2005).
Wood, C. D., Nishigaki, T., Furuta, T., Baba, S. A. & Darszon, A. Real-time analysis of the role of Ca(2+) in flagellar movement and motility in single sea urchin sperm. J. Cell Biol. 169, 725–731 (2005).
Yanagimachi, R. et al. Chemical and physical guidance of fish spermatozoa into the egg through the micropyle. Biol. Reprod. 96, 780–799 (2017).
Suarez, S. S., Varosi, S. M. & Dai, X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc. Natl Acad. Sci. USA 90, 4660–4664 (1993).
Smith, E. F. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol. Biol. Cell 13, 3303–3313 (2002).
Mizuno, K. et al. A novel neuronal calcium sensor family protein, calaxin, is a potential Ca(2+)-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biol. Cell 101, 91–103 (2009).
Bannai, H., Yoshimura, M., Takahashi, K. & Shingyoji, C. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J. Cell Sci. 113, 831–839 (2000).
Tash, J. S. et al. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J. Cell Biol. 106, 1625–1633 (1988).
Kirichok, Y., Navarro, B. & Clapham, D. E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737–740 (2006).
Zeng, X. H., Navarro, B., Xia, X. M., Clapham, D. E. & Lingle, C. J. Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J. Gen. Physiol. 142, 305–313 (2013).
Ren, D. et al. A sperm ion channel required for sperm motility and male fertility. Nature 413, 603–609 (2001).
Carlson, A. E. et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl Acad. Sci. USA 100, 14864–14868 (2003).
Quill, T. A. et al. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl Acad. Sci. USA 100, 14869–14874 (2003).
Qi, H. et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl Acad. Sci. USA 104, 1219–1223 (2007).
Yanagimachi, R. The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fertil. 23, 193–196 (1970).
Suarez, S. S. & Ho, H. C. Hyperactivated motility in sperm. Reprod. Domest. Anim. 38, 119–124 (2003).
Pacey, A. A., Davies, N., Warren, M. A., Barratt, C. L. & Cooke, I. D. Hyperactivation may assist human spermatozoa to detach from intimate association with the endosalpinx. Hum. Reprod. 10, 2603–2609 (1995).
Ho, K., Wolff, C. A. & Suarez, S. S. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fertil. Dev. 21, 345–350 (2009).
Miki, K. & Clapham, D. E. Rheotaxis guides mammalian sperm. Curr. Biol. 23, 443–452 (2013).
Coy, P., Garcia-Vazquez, F. A., Visconti, P. E. & Aviles, M. Roles of the oviduct in mammalian fertilization. Reproduction 144, 649–660 (2012).
Hunter, R. H. Components of oviduct physiology in eutherian mammals. Biol. Rev. Camb. Philos. Soc. 87, 244–255 (2012).
Brenker, C. et al. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J. 31, 1654–1665 (2012).
Lishko, P. V. et al. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 74, 453–475 (2012).
Miller, M. R., Mansell, S. A., Meyers, S. A. & Lishko, P. V. Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium 58, 105–113 (2015).
Chung, J. J. et al. CatSperζ regulates the structural continuity of sperm Ca(2+) signaling domains and is required for normal fertility. eLife 6, e23082 (2017).
Chung, J. J. et al. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 157, 808–822 (2014).
Hwang, J. Y. et al. Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 177, 1480–1494.e19 (2019).
Miller, M. R. et al. Asymmetrically positioned flagellar control units regulate human sperm rotation. Cell Rep. 24, 2606–2613 (2018).
Kerns, K., Zigo, M., Drobnis, E. Z., Sutovsky, M. & Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 9, 2061 (2018).
Matamoros-Volante, A. & Trevino, C. L. Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J. Cell Sci. 133, jcs238816 (2020).
Matamoros-Volante, A. et al. Semi-automatized segmentation method using image-based flow cytometry to study sperm physiology: the case of capacitation-induced tyrosine phosphorylation. Mol. Hum. Reprod. 24, 64–73 (2018).
Lin, J. & Nicastro, D. Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360, eaar1968 (2018).
Zabeo, D., Croft, J. T. & Hoog, J. L. Axonemal doublet microtubules can split into two complete singlets in human sperm flagellum tips. FEBS Lett. 593, 892–902 (2019).
Zabeo, D. et al. A lumenal interrupted helix in human sperm tail microtubules. Sci. Rep. 8, 2727 (2018).
Bernardino, R. L., Carrageta, D. F., Sousa, M., Alves, M. G. & Oliveira, P. F. pH and male fertility: making sense on pH homeodynamics throughout the male reproductive tract. Cell Mol. Life Sci. 76, 3783–3800 (2019).
Levine, N. & Marsh, D. J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J. Physiol. 213, 557–570 (1971).
Wales, R. G., Wallace, J. C. & White, I. G. Composition of bull epididymal and testicular fluid. J. Reprod. Fertil. 12, 139–144 (1966).
Liu, Y., Wang, D. K. & Chen, L. M. The physiology of bicarbonate transporters in mammalian reproduction. Biol. Reprod. 86, 99 (2012).
Ng, K. Y. B., Mingels, R., Morgan, H., Macklon, N. & Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Hum. Reprod. Update 24, 15–34 (2018).
Breckenridge, M. A., Pederson, D. P. & Pommerenke, W. T. A pH study of human cervical secretions. Fertil. Steril. 1, 427–434 (1950).
Fox, C. A., Meldrum, S. J. & Watson, B. W. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J. Reprod. Fertil. 33, 69–75 (1973).
Owen, D. H. & Katz, D. F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26, 459–469 (2005).
Tampion, D. & Gibbons, R. A. Effect of pH on the swimming rate of bull spermatozoa. J. Reprod. Fertil. 5, 249–258 (1963).
Moghissi, K. S., Dabich, D., Levine, J. & Neuhaus, O. W. Mechanism of sperm migration. Fertil. Steril. 15, 15–23 (1964).
Orlowski, J. & Grinstein, S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflug. Arch. 447, 549–565 (2004).
Garcia, M. A. & Meizel, S. Regulation of intracellular pH in capacitated human spermatozoa by a Na+/H+ exchanger. Mol. Reprod. Dev. 52, 189–195 (1999).
Klanke, C. A. et al. Molecular cloning and physical and genetic mapping of a novel human Na+/H+ exchanger (NHE5/SLC9A5) to chromosome 16q22.1. Genomics 25, 615–622 (1995).
Goyal, S., Vanden Heuvel, G. & Aronson, P. S. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am. J. Physiol. Ren. Physiol. 284, F467–F473 (2003).
Wang, D., King, S. M., Quill, T. A., Doolittle, L. K. & Garbers, D. L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 5, 1117–1122 (2003).
Liu, T. et al. A novel testis-specific Na+/H+ exchanger is involved in sperm motility and fertility. Front. Biosci. 2, 566–581 (2010).
Chen, S. R. et al. Sodium-hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis. 7, e2152 (2016).
Oberheide, K., Puchkov, D. & Jentsch, T. J. Loss of the Na(+)/H(+) exchanger NHE8 causes male infertility in mice by disrupting acrosome formation. J. Biol. Chem. 292, 10845–10854 (2017).
Wang, D. et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc. Natl Acad. Sci. USA 104, 9325–9330 (2007).
Windler, F. et al. The solute carrier SLC9C1 is a Na(+)/H(+)-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat. Commun. 9, 2809 (2018).
Lishko, P. V., Botchkina, I. L., Fedorenko, A. & Kirichok, Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337 (2010).
Lee, S. Y., Letts, J. A. & Mackinnon, R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl Acad. Sci. USA 105, 7692–7695 (2008).
Tombola, F., Ulbrich, M. H. & Isacoff, E. Y. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58, 546–556 (2008).
Ramsey, I. S. et al. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17, 869–875 (2010).
Berger, T. K. et al. Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating. J. Physiol. 595, 1533–1546 (2017).
Navarro, B., Kirichok, Y. & Clapham, D. E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl Acad. Sci. USA 104, 7688–7692 (2007).
Brenker, C. et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. eLife 3, e01438 (2014).
Strunker, T. et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471, 382–386 (2011).
Lishko, P. V., Botchkina, I. L. & Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 471, 387–391 (2011).
Clausen, M. V., Hilbers, F. & Poulsen, H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 8, 371 (2017).
Huxley, A. F. & Stampfli, R. Direct determination of membrane resting potential and action potential in single myelinated nerve fibers. J. Physiol. 112, 476–495 (1951).
Santi, C. M. et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 584, 1041–1046 (2010).
Calzada, L. & Tellez, J. Defective function of membrane potential (psi) on sperm of infertile men. Arch. Androl. 38, 151–155 (1997).
Brown, S. G. et al. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 31, 1147–1157 (2016).
Baro Graf, C. et al. Membrane potential assessment by fluorimetry as a predictor tool of human sperm fertilizing capacity. Front. Cell Dev. Biol. 7, 383 (2019).
Molina, L. C. P. et al. Membrane potential determined by flow cytometry predicts fertilizing ability of human sperm. Front. Cell Dev. Biol. 7, 387 (2019).
Sanchez, G., Nguyen, A. N. T., Timmerberg, B., Tash, J. S. & Blanco, G. The Na,K-ATPase α4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol. Hum. Reprod. 12, 565–576 (2006).
Wagoner, K., Sanchez, G., Nguyen, A. N., Enders, G. C. & Blanco, G. Different expression and activity of the α1 and α4 isoforms of the Na,K-ATPase during rat male germ cell ontogeny. Reproduction 130, 627–641 (2005).
Jimenez, T. et al. Increased expression of the Na,K-ATPase alpha4 isoform enhances sperm motility in transgenic mice. Biol. Reprod. 84, 153–161 (2011).
McDermott, J., Sanchez, G., Nangia, A. K. & Blanco, G. Role of human Na,K-ATPase alpha 4 in sperm function, derived from studies in transgenic mice. Mol. Reprod. Dev. 82, 167–181 (2015).
Jimenez, T., Sanchez, G., Wertheimer, E. & Blanco, G. Activity of the Na,K-ATPase α4 isoform is important for membrane potential, intracellular Ca2+, and pH to maintain motility in rat spermatozoa. Reproduction 139, 835–845 (2010).
Blanco, G., Melton, R. J., Sanchez, G. & Mercer, R. W. Functional characterization of a testes-specific α-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry 38, 13661–13669 (1999).
James, P. F. et al. Identification of a specific role for the Na,K-ATPase α2 isoform as a regulator of calcium in the heart. Mol. Cell 3, 555–563 (1999).
Jimenez, T., McDermott, J. P., Sánchez, G. & Blanco, G. Na,K-ATPase α4 isoform is essential for sperm fertility. Proc. Natl Acad. Sci. USA 108, 644–649 (2011).
Cooper, T. G. et al. Mouse models of infertility due to swollen spermatozoa. Mol. Cell Endocrinol. 216, 55–63 (2004).
Zeng, X. H., Yang, C., Kim, S. T., Lingle, C. J. & Xia, X. M. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl Acad. Sci. USA 108, 5879–5884 (2011).
Yang, C., Zeng, X. H., Zhou, Y., Xia, X. M. & Lingle, C. J. LRRC52 (leucine-rich-repeat-containing protein 52), a testis-specific auxiliary subunit of the alkalization-activated Slo3 channel. Proc. Natl Acad. Sci. USA 108, 19419–19424 (2011).
Zeng, X. H., Yang, C., Xia, X. M., Liu, M. & Lingle, C. J. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proc. Natl Acad. Sci. USA 112, 2599–2604 (2015).
Mansell, S. A., Publicover, S. J., Barratt, C. L. & Wilson, S. M. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol. Hum. Reprod. 20, 392–408 (2014).
Mannowetz, N., Naidoo, N. M., Choo, S. A., Smith, J. F. & Lishko, P. V. Slo1 is the principal potassium channel of human spermatozoa. eLife 2, e01009 (2013).
Geng, Y. et al. A genetic variant of the sperm-specific SLO3 K(+) channel has altered pH and Ca(2+) sensitivities. J. Biol. Chem. 292, 8978–8987 (2017).
Wijerathne, T. D., Kim, J., Yang, D. & Lee, K. P. Intracellular calcium-dependent regulation of the sperm-specific calcium-activated potassium channel, hSlo3, by the BKCa activator LDD175. Korean J. Physiol. Pharmacol. 21, 241–249 (2017).
Chavez, J. C. et al. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J. Biol. Chem. 289, 32266–32275 (2014).
Brown, S. G., Publicover, S. J., Barratt, C. L. R. & Martins da Silva, S. J. Human sperm ion channel (dys)function: implications for fertilization. Hum. Reprod. Update 25, 758–776 (2019).
Lievano, A. et al. T-type Ca2+ channels and α1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett. 388, 150–154 (1996).
Xia, J. & Ren, D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol. Reprod. 80, 1092–1098 (2009).
Jin, J. et al. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol. Reprod. 77, 37–44 (2007).
Liu, J., Xia, J., Cho, K. H., Clapham, D. E. & Ren, D. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J. Biol. Chem. 282, 18945–18952 (2007).
Wang, H., Liu, J., Cho, K. H. & Ren, D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol. Reprod. 81, 539–544 (2009).
Chung, J. J., Navarro, B., Krapivinsky, G., Krapivinsky, L. & Clapham, D. E. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat. Commun. 2, 153 (2011).
Carlson, A. E. et al. Identical phenotypes of CatSper1 and CatSper2 null sperm. J. Biol. Chem. 280, 32238–32244 (2005).
Avenarius, M. R. et al. Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet. 84, 505–510 (2009).
Hildebrand, M. S. et al. Genetic male infertility and mutation of CATSPER ion channels. Eur. J. Hum. Genet. 18, 1178–1184 (2010).
Smith, J. F. et al. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc. Natl Acad. Sci. USA 110, 6823–6828 (2013).
Schiffer, C. et al. Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca(2+) signaling. EMBO J. 39, e102363 (2020).
Luo, T. et al. A novel copy number variation in CATSPER2 causes idiopathic male infertility with normal semen parameters. Hum. Reprod. 34, 414–423 (2019).
Sinha, A., Singh, V., Singh, S. & Yadav, S. Proteomic analyses reveal lower expression of TEX40 and ATP6V0A2 proteins related to calcium ion entry and acrosomal acidification in asthenozoospermic males. Life Sci. 218, 81–88 (2019).
Brown, S. G. et al. Homozygous in-frame deletion in CATSPERE in a man producing spermatozoa with loss of CatSper function and compromised fertilizing capacity. Hum. Reprod. 33, 1812–1816 (2018).
Williams, H. L. et al. Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa. Hum. Reprod. 30, 2737–2746 (2015).
San Agustin, J. T., Pazour, G. J. & Witman, G. B. Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol. Biol. Cell 26, 4358–4372 (2015).
Zhang, Y. et al. Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice. Dev. Biol. 432, 125–139 (2017).
Liu, H. et al. IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation. Biol. Reprod. 96, 993–1006 (2017).
Zhang, Y. et al. Sensorineural deafness and male infertility: a contiguous gene deletion syndrome. J. Med. Genet. 44, 233–240 (2007).
Avidan, N. et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur. J. Hum. Genet. 11, 497–502 (2003).
Sumigama, S. et al. Progesterone accelerates the completion of sperm capacitation and activates CatSper channel in spermatozoa from the rhesus macaque. Biol. Reprod. 93, 130 (2015).
Miller, M. R. et al. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352, 555–559 (2016).
Mannowetz, N., Miller, M. R. & Lishko, P. V. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl Acad. Sci. USA 114, 5743–5748 (2017).
Brenker, C. et al. Action of steroids and plant triterpenoids on CatSper Ca(2+) channels in human sperm. Proc. Natl Acad. Sci. USA 115, E344–E346 (2018).
Mannowetz, N., Mundt, N. & Lishko, P. V. Reply to Brenker et al.: The plant triterpenoid pristimerin inhibits calcium influx into human spermatozoa via CatSper. Proc. Natl Acad. Sci. USA 115, E347–E348 (2018).
Diao, R. et al. CCR6 is required for ligand-induced CatSper activation in human sperm. Oncotarget 8, 91445–91458 (2017).
Schiffer, C. et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 15, 758–765 (2014).
Tavares, R. S. et al. p,p′-DDE activates CatSper and compromises human sperm function at environmentally relevant concentrations. Hum. Reprod. 28, 3167–3177 (2013).
Zou, Q. X. et al. Diethylstilbestrol activates CatSper and disturbs progesterone actions in human spermatozoa. Hum. Reprod. 32, 290–298 (2017).
Bailey, J. L. Factors regulating sperm capacitation. Syst. Biol. Reprod. Med. 56, 334–348 (2010).
Jaiswal, B. S. & Conti, M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl Acad. Sci. USA 100, 10676–10681 (2003).
Xie, F. et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol. 296, 353–362 (2006).
Nolan, M. A. et al. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc. Natl Acad. Sci. USA 101, 13483–13488 (2004).
Xia, J., Reigada, D., Mitchell, C. H. & Ren, D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biol. Reprod. 77, 551–559 (2007).
Kobori, H., Miyazaki, S. & Kuwabara, Y. Characterization of intracellular Ca(2+) increase in response to progesterone and cyclic nucleotides in mouse spermatozoa. Biol. Reprod. 63, 113–120 (2000).
Carlson, A. E., Hille, B. & Babcock, D. F. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev. Biol. 312, 183–192 (2007).
Wang, T. et al. The Ca(2+) channel CatSper is not activated by cAMP/PKA signaling but directly affected by chemicals used to probe the action of cAMP and PKA. J. Biol. Chem. 295, 13181–13193 (2020).
Orta, G. et al. CatSper channels are regulated by protein kinase A. J. Biol. Chem. 293, 16830–16841 (2018).
Gadella, B. M. & Harrison, R. A. The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 127, 2407–2420 (2000).
Visconti, P. E. et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev. Biol. 214, 429–443 (1999).
Xia, J. & Ren, D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod. Biol. Endocrinol. 7, 119 (2009).
Visconti, P. E. et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: β-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem. 274, 3235–3242 (1999).
Osheroff, J. E. et al. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol. Hum. Reprod. 5, 1017–1026 (1999).
Harrison, R. A. Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol. Reprod. Dev. 67, 337–352 (2004).
Battistone, M. A. et al. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol. Hum. Reprod. 19, 570–580 (2013).
Wennemuth, G. et al. Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development 130, 1317–1326 (2003).
Visconti, P. E. et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein-tyrosine phosphorylation. Development 121, 1129–1137 (1995).
Salicioni, A. M. et al. Signalling pathways involved in sperm capacitation. Soc. Reprod. Fertil. Suppl. 65, 245–259 (2007).
Visconti, P. E. et al. Capacitation of mouse spermatozoa. II. Protein-tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 121, 1139–1150 (1995).
Alvau, A. et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 143, 2325–2333 (2016).
Craig, A. W. B., Zirngibl, R., Williams, K., Cole, L. A. & Greer, P. A. Mice devoid of Fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell. Biol. 21, 603–613 (2001).
Tateno, H. et al. Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc. Natl Acad. Sci. USA 110, 18543–18548 (2013).
Navarrete, F. A. et al. Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci. Rep. 6, 33589 (2016).
Miyata, H. et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science 350, 442–445 (2015).
Mundt, N., Spehr, M. & Lishko, P. V. TRPV4 is the temperature-sensitive ion channel of human sperm. eLife 7, e35853 (2018).
Bahat, A. et al. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat. Med. 9, 149 (2003).
Boryshpolets, S., Pérez-Cerezales, S. & Eisenbach, M. Behavioral mechanism of human sperm in thermotaxis: a role for hyperactivation. Hum. Reprod. 30, 884–892 (2015).
Aitken, R. J. & Nixon, B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 19, 785–793 (2013).
Hamano, K., Kawanishi, T., Mizuno, A., Suzuki, M. & Takagi, Y. Involvement of transient receptor potential vanilloid (TRPV) 4 in mouse sperm thermotaxis. J. Reprod. Dev. 62, 415–422 (2016).
Kumar, A. et al. TRPV4 is endogenously expressed in vertebrate spermatozoa and regulates intracellular calcium in human sperm. Biochem. Biophys. Res. Commun. 473, 781–788 (2016).
Bjorkgren, I. & Lishko, P. V. Purinergic signaling in testes revealed. J. Gen. Physiol. 148, 207–211 (2016).
Navarro, B., Miki, K. & Clapham, D. E. ATP-activated P2X2 current in mouse spermatozoa. Proc. Natl Acad. Sci. USA 108, 14342–14347 (2011).
King, B. F., Wildman, S. S., Ziganshina, L. E., Pintor, J. & Burnstock, G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br. J. Pharmacol. 121, 1445–1453 (1997).
Wildman, S. S., King, B. F. & Burnstock, G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br. J. Pharmacol. 123, 1214–1220 (1998).
Catterall, W. A., Goldin, A. L. & Waxman, S. G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 57, 397–409 (2005).
Westenbroek, R. E. & Babcock, D. F. Discrete regional distributions suggest diverse functional roles of calcium channel α1 subunits in sperm. Dev. Biol. 207, 457–469 (1999).
Wennemuth, G., Westenbroek, R. E., Xu, T., Hille, B. & Babcock, D. F. CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J. Biol. Chem. 275, 21210–21217 (2000).
Sakata, Y. et al. Ca(v)2.3 (α1E) Ca2+ channel participates in the control of sperm function. FEBS Lett. 516, 229–233 (2002).
Cohen, R. et al. Lipid modulation of calcium flux through CaV2.3 regulates acrosome exocytosis and fertilization. Dev. Cell 28, 310–321 (2014).
Kirichok, Y. & Lishko, P. V. Rediscovering sperm ion channels with the patch-clamp technique. Mol. Hum. Reprod. 17, 478–499 (2011).
Shen, P. S. et al. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167, 763–773.e11 (2016).
Cordido, A., Besada-Cerecedo, L. & Garcia-Gonzalez, M. A. The genetic and cellular basis of autosomal dominant polycystic kidney disease–a primer for clinicians. Front. Pediatr. 5, 279 (2017).
Yoder, B. K., Hou, X. & Guay-Woodford, L. M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 13, 2508–2516 (2002).
Su, Q. et al. Structure of the human PKD1-PKD2 complex. Science 361, eaat9819 (2018).
Kierszenbaum, A. L. Polycystins: what polycystic kidney disease tells us about sperm. Mol. Reprod. Dev. 67, 385–388 (2004).
Vora, N., Perrone, R. & Bianchi, D. W. Reproductive issues for adults with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 51, 307–318 (2008).
Li Vecchi, M., Cianfrone, P., Damiano, R. & Fuiano, G. Infertility in adults with polycystic kidney disease. Nephrol. Dial. Transpl. 18, 190–191 (2003).
Okada, H. et al. Assisted reproduction for infertile patients with 9+0 immotile spermatozoa associated with autosomal dominant polycystic kidney disease. Hum. Reprod. 14, 110–113 (1999).
Sutton, K. A., Jungnickel, M. K. & Florman, H. M. A polycystin-1 controls postcopulatory reproductive selection in mice. Proc. Natl Acad. Sci. USA 105, 8661–8666 (2008).
Hughes, J., Ward, C. J., Aspinwall, R., Butler, R. & Harris, P. C. Identification of a human homologue of the sea urchin receptor for egg jelly: a polycystic kidney disease-like protein. Hum. Mol. Genet. 8, 543–549 (1999).
Butscheid, Y. et al. Polycystic kidney disease and receptor for egg jelly is a plasma membrane protein of mouse sperm head. Mol. Reprod. Dev. 73, 350–360 (2006).
Linsdell, P. et al. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J. Gen. Physiol. 110, 355–364 (1997).
Illek, B., Yankaskas, J. R. & Machen, T. E. cAMP and genistein stimulate HCO3- conductance through CFTR in human airway epithelia. Am. J. Physiol. 272, L752–L761 (1997).
Chen, H., Ruan, Y. C., Xu, W. M., Chen, J. & Chan, H. C. Regulation of male fertility by CFTR and implications in male infertility. Hum. Reprod. Update 18, 703–713 (2012).
van der Ven, K., Messer, L., van der Ven, H., Jeyendran, R. S. & Ober, C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum. Reprod. 11, 513–517 (1996).
Xu, W. M. et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc. Natl Acad. Sci. USA 104, 9816–9821 (2007).
Snouwaert, J. N. et al. An animal model for cystic fibrosis made by gene targeting. Science 257, 1083–1088 (1992).
Li, C. Y. et al. CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum. Reprod. 25, 317–327 (2010).
Hernandez-Gonzalez, E. O. et al. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J. Biol. Chem. 282, 24397–24406 (2007).
Chen, W. Y. et al. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biol. Reprod. 80, 115–123 (2009).
Chavez, J. C. et al. Participation of the Cl−/HCO3− exchangers SLC26A3 and SLC26A6, the Cl− channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol. Reprod. 86, 1–14 (2012).
Hoglund, P. et al. Disruption of the SLC26A3-mediated anion transport is associated with male subfertility. Fertil. Steril. 85, 232–235 (2006).
Schweinfest, C. W. et al. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 281, 37962–37971 (2006).
Wang, Y. Y. et al. Loss of SLC9A3 decreases CFTR protein and causes obstructed azoospermia in mice. PLoS Genet. 13, e1006715 (2017).
Figueiras-Fierro, D. et al. Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm. J. Cell Physiol. 228, 590–601 (2013).
Rode, B. et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum. Mol. Genet. 21, 1287–1298 (2012).
Strehler, E. E. & Zacharias, D. A. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 81, 21–50 (2001).
Keeton, T. P., Burk, S. E. & Shull, G. E. Alternative splicing of exons encoding the calmodulin-binding domains and C termini of plasma membrane Ca(2+)-ATPase isoforms 1, 2, 3, and 4. J. Biol. Chem. 268, 2740–2748 (1993).
Okunade, G. W. et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 279, 33742–33750 (2004).
Schuh, K. et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 279, 28220–28226 (2004).
Prasad, V., Okunade, G. W., Miller, M. L. & Shull, G. E. Phenotypes of SERCA and PMCA knockout mice. Biochem. Biophys. Res. Commun. 322, 1192–1203 (2004).
Yamazaki, D. et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 9, e1003983 (2013).
Yamazaki, D. et al. The Mg2+ transporter CNNM4 regulates sperm Ca2+ homeostasis and is essential for reproduction. J. Cell Sci. 129, 1940–1949 (2016).
Yamazaki, D., Funato, Y., Miyata, H., Ikawa, M. & Miki, H. Complementary role of CNNM2 in sperm motility and Ca(2+) influx during capacitation. Biochem. Biophys. Res. Commun. 474, 441–446 (2016).
Long, J. E., Lee, M. S. & Blithe, D. L. Male contraceptive development: update on novel hormonal and nonhormonal methods. Clin. Chem. 65, 153–160 (2019).
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schioth, H. B. & Gloriam, D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 (2017).
Garcia, M. L. & Kaczorowski, G. J. Ion channels find a pathway for therapeutic success. Proc. Natl Acad. Sci. USA 113, 5472–5474 (2016).
McManus, O. B. HTS assays for developing the molecular pharmacology of ion channels. Curr. Opin. Pharmacol. 15, 91–96 (2014).
Rennhack, A. et al. A novel cross-species inhibitor to study the function of CatSper Ca(2+) channels in sperm. Br. J. Pharmacol. 175, 3144–3161 (2018).
Schaefer, M., Habenicht, U. F., Brautigam, M. & Gudermann, T. Steroidal sigma receptor ligands affect signaling pathways in human spermatozoa. Biol. Reprod. 63, 57–63 (2000).
Gruber, F. S., Johnston, Z. C., Barratt, C. L. & Andrews, P. D. A phenotypic screening platform utilising human spermatozoa identifies compounds with contraceptive activity. eLife 9, e51739 (2020).
Janes, J. et al. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc. Natl Acad. Sci. USA 115, 10750–10755 (2018).
Choy, J. T. & Eisenberg, M. L. Male infertility as a window to health. Fertil. Steril. 110, 810–814 (2018).
De Jonge, C. & Barratt, C. L. R. The present crisis in male reproductive health: an urgent need for a political, social, and research roadmap. Andrology 7, 762–768 (2019).
Sermondade, N. et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 19, 221–231 (2013).
Li, Y., Lin, H., Li, Y. & Cao, J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil. Steril. 95, 116–123 (2011).
Trottmann, M. et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur. Urol. 52, 355–367 (2007).
Jacobsen, R. et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ 321, 789–792 (2000).
Walsh, T. J., Croughan, M. S., Schembri, M., Chan, J. M. & Turek, P. J. Increased risk of testicular germ cell cancer among infertile men. Arch. Intern. Med. 169, 351–356 (2009).
Breuss, M. W. et al. Autism risk in offspring can be assessed through quantification of male sperm mosaicism. Nat. Med. 26, 143–150 (2020).
Leung, A. K., Henry, M. A. & Mehta, A. Gaps in male infertility health services research. Transl. Androl. Urol. 7, S303–S309 (2018).
Yu, J., Chen, Z., Ni, Y. & Li, Z. CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic review and meta-analysis. Hum. Reprod. 27, 25–35 (2012).
Tilley, A. E., Walters, M. S., Shaykhiev, R. & Crystal, R. G. Cilia dysfunction in lung disease. Annu. Rev. Physiol. 77, 379–406 (2015).
Inaba, K. & Mizuno, K. Sperm dysfunction and ciliopathy. Reprod. Med. Biol. 15, 77–94 (2016).
Serrano, C. J., Treviño, C. L., Felix, R. & Darszon, A. Voltage-dependent Ca(2+) channel subunit expression and immunolocalization in mouse spermatogenic cells and sperm. FEBS Lett. 462, 171–176 (1999).
Santi, C. M., Darszon, A. & Hernandez-Cruz, A. A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am. J. Physiol. 271, C1583–C1593 (1996).
Arnoult, C., Cardullo, R. A., Lemos, J. R. & Florman, H. M. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc. Natl Acad. Sci. USA 93, 13004–13009 (1996).
Meizel, S. The sperm, a neuron with a tail: ‘neuronal’ receptors in mammalian sperm. Biol. Rev. Camb. Philos. Soc. 79, 713–732 (2004).
Kurata, S., Hiradate, Y., Umezu, K., Hara, K. & Tanemura, K. Capacitation of mouse sperm is modulated by gamma-aminobutyric acid (GABA) concentration. J. Reprod. Dev. 65, 327–334 (2019).
Zeng, Y., Oberdorf, J. A. & Florman, H. M. pH regulation in mouse sperm: identification of Na(+)-, Cl(−)-, and HCO3(−)-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 173, 510–520 (1996).
Singh, J. P., Babcock, D. F. & Lardy, H. A. Increased calcium-ion influx is a component of capacitation of spermatozoa. Biochem. J. 172, 549–556 (1978).
Ruknudin, A. & Silver, I. A. Ca2+ uptake during capacitation of mouse spermatozoa and the effect of an anion transport inhibitor on Ca2+ uptake. Mol. Reprod. Dev. 26, 63–68 (1990).
Zhou, R., Shi, B., Chou, K. C., Oswalt, M. D. & Haug, A. Changes in intracellular calcium of porcine sperm during in vitro incubation with seminal plasma and a capacitating medium. Biochem. Biophys. Res. Commun. 172, 47–53 (1990).
Baldi, E. et al. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl. 12, 323–330 (1991).
Zeng, Y., Clark, E. N. & Florman, H. M. Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol. 171, 554–563 (1995).
Demarco, I. A. et al. Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J. Biol. Chem. 278, 7001–7009 (2003).
Naz, R. K. & Rajesh, P. B. Role of tyrosine phosphorylation in sperm capacitation / acrosome reaction. Reprod. Biol. Endocrinol. 2, 75 (2004).
Neill, J. M. & Olds-Clarke, P. A computer-assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete Res. 18, 121–140 (1987).
Cohen-Dayag, A., Tur-Kaspa, I., Dor, J., Mashiach, S. & Eisenbach, M. Sperm capacitation in humans is transient and correlates with chemotactic responsiveness to follicular factors. Proc. Natl Acad. Sci. USA 92, 11039–11043 (1995).
Bleil, J. D. & Wassarman, P. M. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol. 95, 317–324 (1983).
Hino, T. et al. The behavior and acrosomal status of mouse spermatozoa in vitro, and within the oviduct during fertilization after natural mating. Biol. Reprod. 95, 50 (2016).
La Spina, F. A. et al. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 411, 172–182 (2016).
Jin, M. et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl Acad. Sci. USA 108, 4892–4896 (2011).
Muro, Y. et al. Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biol. Reprod. 94, 80 (2016).
Sidhu, K. S. et al. A flow cytometric assay for global estimation of tyrosine phosphorylation associated with capacitation of spermatozoa from two marsupial species, the tammar wallaby (Macropus eugenii) and the brushtail possum (Trichosurus vulpecula). Reproduction 127, 95–103 (2004).
Zoppino, F. C., Halón, N. D., Bustos, M. A., Pavarotti, M. A. & Mayorga, L. S. Recording and sorting live human sperm undergoing acrosome reaction. Fertil. Steril. 97, 1309–1315 (2012).
Uhler, M. L., Leung, A., Chan, S. Y., Schmid, I. & Wang, C. Assessment of human sperm acrosome reaction by flow cytometry: validation and evaluation of the method by fluorescence-activated cell sorting. Fertil. Steril. 60, 1076–1081 (1993).
Escoffier, J. et al. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol. Reprod. 92, 121 (2015).
Seifert, R. et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 34, 379–392 (2015).
Marquez, B. & Suarez, S. S. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol. Reprod. 76, 660–665 (2007).
Loux, S. C. et al. CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol. Reprod. 89, 123 (2013).
Roldan, E. R. S. Assessments of sperm quality integrating morphology, swimming patterns, bioenergetics and cell signalling. Theriogenology 150, 388–395 (2020).
Chang, H. & Suarez, S. S. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol. Reprod. 86, 141–148 (2012).
Demott, R. P. & Suarez, S. S. Hyperactivated sperm progress in the mouse oviduct. Biol. Reprod. 46, 779–785 (1992).
Suarez, S. S. Sperm transport and motility in the mouse oviduct: observations in situ. Biol. Reprod. 36, 203–210 (1987).
Williams, M. et al. Sperm numbers and distribution within the human fallopian tube around ovulation. Hum. Reprod. 8, 2019–2026 (1993).
Suarez, S. S. Interactions of spermatozoa with the female reproductive tract: inspiration for assisted reproduction. Reprod. Fertil. Dev. 19, 103–110 (2007).
Ishikawa, Y., Usui, T., Yamashita, M., Kanemori, Y. & Baba, T. Surfing and swimming of ejaculated sperm in the mouse oviduct. Biol. Reprod. 94, 89 (2016).
Xu, W. M. et al. Defective CFTR-dependent CREB activation results in impaired spermatogenesis and azoospermia. PLoS ONE 6, e19120 (2011).
Miyata, H. et al. Genome engineering uncovers 54 evolutionarily conserved and testis-enriched genes that are not required for male fertility in mice. Proc. Natl Acad. Sci. USA 113, 7704–7710 (2016).
Suzuki, M., Mizuno, A., Kodaira, K. & Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668 (2003).
Wedenoja, S. et al. A missense mutation in SLC26A3 is associated with human male subfertility and impaired activation of CFTR. Sci. Rep. 7, 14208 (2017).
Acknowledgements
The authors thank Jae Yeon Hwang for valuable discussion and critical reading of the draft manuscript. This work was supported by start-up funds from Yale University School of Medicine, Grantham Foundation, and NIH (R01 HD 096745) to J.-J.C.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussion of content, and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks P. Lishko, M. Yeste, Y. Okamura, P. Visconti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- CatSper
-
Sperm-specific calcium channel.
- NHEs
-
Sodium–hydrogen exchangers.
- HV1
-
Proton channel.
- KSper
-
Native sperm-specific potassium current/channel.
- Na+/K+ ATPase
-
Sodium–potassium adenosine triphosphatase; also known as the sodium–potassium pump.
- SLO3
-
The mediator of KSper, which is also used as the name of the protein or channel expressed heterologously.
- Quadrilateral compartmentalization
-
The four linear Ca2+ signalling nanodomains.
- DSper
-
Depolarizing channel of sperm.
- TRPV4
-
Transient receptor potential cation channel subfamily V member 4.
- P2X2
-
P2X purinoceptor 2.
- Cav2.3
-
R type, voltage-dependent, calcium channel, α1E subunit.
- PKD
-
Polycystin, transient receptor potential cation channel, autosomal dominant polycystic kidney disease protein.
- PKDREJ
-
Polycystin family receptor for egg jelly.
- CFTR
-
Cystic fibrosis transmembrane conductance regulator.
- PMCA4
-
Plasma membrane calcium ATPase 4.
- CNNM4
-
Cyclin and CBS domain divalent metal cation transport mediator 4.
Rights and permissions
About this article
Cite this article
Wang, H., McGoldrick, L.L. & Chung, JJ. Sperm ion channels and transporters in male fertility and infertility. Nat Rev Urol 18, 46–66 (2021). https://doi.org/10.1038/s41585-020-00390-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-00390-9
This article is cited by
-
Flagellar beating forces of human spermatozoa with different motility behaviors
Reproductive Biology and Endocrinology (2024)
-
The Detection of CatSper1 and CatSper3 Expression in Men with Normozoospermia and Asthenoteratozoospermia and Its Association with Sperm Parameters, Fertilization Rate, Embryo Quality
Reproductive Sciences (2024)
-
Trends in semen parameters of infertile men in South Africa and Nigeria
Scientific Reports (2023)
-
3D structure and in situ arrangements of CatSper channel in the sperm flagellum
Nature Communications (2022)
-
Structure of a mammalian sperm cation channel complex
Nature (2021)