Abstract
Rice is an excellent source of protein, and has an adequate balance of amino acids with the exception of the essential amino acid lysine. By using a combined enhancement of lysine synthesis and suppression of its catabolism, we had produced two transgenic rice lines HFL1 and HFL2 (High Free Lysine) containing high concentration of free lysine. In this study, a 70-day rat feeding study was conducted to assess the nutritional value of two transgenic lines as compared with either their wild type (WT) or the WT rice supplemented with different concentrations of L-lysine. The results revealed that animal performance, including body weight, food intake, and food efficiency, was greater in the HFL groups than in the WT group. Moreover, the HFL diets had increased protein apparent digestibility, protein efficiency ratio, and lysine availability than the WT diet. Based on the linear relationship between dietary L-lysine concentrations and animal performance, it indicated that the biological indexes of the HFL groups were similar or better than that of the WT20 group, which was supplemented with L-lysine concentrations similar to those present in the HFL diets. Therefore, lysine-biofortified rice contributed to improved growth performance, food efficiency, and lysine availability in growing rats.
Similar content being viewed by others
Introduction
Transgenic strategies may improve the nutritional value of crops and ensure food security1. Transgenic crops with enhanced nutritional value may have improved nutrient bioavailability and/or lower levels of anti-nutritional factors2,3,4, such as high-methionine lupin5, lupin that expresses methionine-rich sunflower albumin6, low-phytate transgenic maize7, and tryptophan-enriched rice8.
As an essential amino acid (EAA), lysine cannot be synthesized by humans or farm animals and represents an indicator of other dietary EAAs9. Animal growth performance, carcass characteristics, and immune function are affected by lysine deficiency10,11,12,13,14,15,16. Supplementation with synthetic lysine enhances nitrogen retention and protein accretion and improves animal growth performance and immune function10, 17,18,19,20. In cereals, lysine is a limiting EAA. In an attempt to reduce the incidence of lysine deficiency, cereals such as maize21, sorghum22, and rice23,24,25 have been biofortified with the amino acid. Animal studies have shown that the bioefficacy of high-lysine transgenic maize is similar to that wild-type maize supplemented with L-lysine, and high-lysine transgenic maize is considered to be more nutritious than wild-type maize26,27,28. In pigs, lysine bioefficacy is dependent on the source of dietary lysine29. Altogether, these studies have shown that animal weight gain, protein digestibility, and amino acid bioavailability are associated with increased dietary lysine.
Rice represents an important source of energy and protein for approximately one-third of the world’s population30. Rice has an adequate balance of amino acids with the exception of lysine31. We have developed several types of high-lysine transgenic rice by overexpressing endogenous rice histone proteins enriched with lysine24 or heterogeneous lysine-rich proteins32. Furthermore, we have generated high-lysine transgenic rice by modifying lysine metabolism23, 25. The transgenic rice lines HFL1 and HFL2 were developed by enhancing lysine anabolism and reducing lysine catabolism25. These two HFL transgenic lines contain free lysine levels in seeds up to 25-fold over that of the wild type, but without the selectable marker gene. All target transgenes in both transgenic lines were integrated into the intragenic region of the rice genome, with no differences in major agronomic traits, including yield25. These two transgenic rice lines, which are currently undergoing nutritional and food safety assessments, could potentially alleviate malnutrition33.
The objective of this study was to evaluate the nutritional value of HFL transgenic rice. Transgenic rice and their wild-type counterpart were fed to growing rats. Animal growth performance, food efficiency, and protein and amino acid availability were analyzed and compared. Additionally, to evaluate whether lysine in transgenic rice is bioavailable to animals, another feeding trial was performed with wild-type rice supplemented with different dosages of L-lysine (Table 1).
Results
Grain composition and amino acid balance in HFL and WT rice
The composition of HFL and WT rice grains is shown in Table 2. The free lysine level dramatically increased in the two HFL lines, and total lysine content was higher in HFL1 (+24%) and HFL2 (+19%) than in WT rice. Additionally, the total protein content in transgenic rice seeds was also slightly higher, by 1.05% and 0.85% in HFL1 and HFL2, respectively, than that of WT rice. There were no significant differences in moisture, ash, fiber, lipid, or carbohydrate contents between the transgenic and WT rice flour. Furthermore, in rat feeding study, the two HFL diets showed higher lysine content than the non-supplemented WT diet (Fig. 1 and Supplementary Table S1) as well.
We calculated the amino acid scores (AAS) in HFL and WT diets (Supplementary Table S2) based on the protein reference pattern recommended for school-aged children and adolescents34. In WT rice, the first, second, and third limiting EAAs are lysine, threonine, and leucine, respectively35. In this study, the WT diet had a total lysine content of 1.94 mg/g (Fig. 1) and an AAS of 0.75 (Supplementary Table S2). HFL1 and HFL2 had a total lysine content of 2.40 and 2.32 mg/g, respectively (Fig. 1), and an AAS of 0.83 and 0.78, respectively (Supplementary Table S2). Lysine was the first limiting EAA in transgenic and WT rice. However, lysine AAS was significantly higher in the two transgenic diets than in the WT diet. There were no differences in threonine and leucine AAS between the transgenic and WT diets (Supplementary Table S2). Therefore, diets containing transgenic rice HFL1 and HFL2 had a better amino acid balance than diets containing WT rice.
Enhanced growth performance in HFL groups
During the 70-d feeding trial, the rats grew well, and no diseases or deaths were recorded. Body weight changes are presented in Fig. 2 and Supplementary Fig. S1. All rats, which had a similar initial body weight of ~50 g (Supplementary Table S2), grew similarly during the preliminary feeding stage (the first 7-d). Following this preliminary feeding stage, rats fed ad libitum either HFL transgenic rice diets or L-lysine-supplemented WT rice diets grew faster than those fed the non-supplemented WT rice diet. Interestingly, body weight increased with increasing lysine concentrations (from 1.94 mg/g in WT to 2.88 mg/g in WT40; Fig. 2 and Supplementary Table S3).
Compared with the WT group, the HFL groups had greater growth performance (Fig. 2). At the end of 70-d feeding trial, the final body weights of the HFL1 and HFL2 groups were 134.08 g/rat and 134.54 g/rat, respectively, which were 25.97% and 26.40% higher, respectively, than that of the WT group (Supplementary Table S3). The growth curve of the two HFL groups was quite similar to that of the WT20 group. The WT20 diet contained 20% more L-lysine than the WT diet and similar total lysine concentrations as the HFL diets (Fig. 1).
Increased food efficiency in rats fed with HFL rice diets
After one week of preliminary feeding, the food intake, body weight gain and food efficiency increased in either HFL or L-lysine-supplemented WT groups than in WT group. At the end of the experiment, the HFL groups had higher food intake and food efficiency than the WT group (Fig. 3 and Supplementary Table S3). Similarly, food intake, body weight gain and food efficiency were higher in the L-lysine- supplemented groups than in the WT group (Fig. 3). Food efficiency of the HFL1 and HFL2 diets was similar to that of the WT10 and WT20 diets, but lower than that of the WT40 diet.
Improved protein digestibility and nitrogen balance in HFL groups
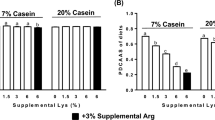
During the nitrogen-balance stage (6-d following the 7-d acclimation period), fecal and urine samples were collected separately and analyzed. There were no significant differences in total feces (dry weight, g/6 d/rat) or fecal nitrogen (g/6 d/rat) among the six groups (Supplementary Fig. S2). Therefore, fecal excretion was normal during the experimental stage. The HFL1 and HFL2 groups had higher total protein intake (Supplementary Fig. S2) and greater protein apparent digestibility than the WT group (Fig. 4a). The protein efficiency ratio (PER) of the HFL groups was higher than that of the WT group, but similar to that of the WT20 group (Fig. 4b).
Among the L-lysine-supplemented WT diets, their protein apparent digestibility reached highest in the case of WT40 while improvement was observed accordingly with increasing lysine content in the diet from 1.94 mg/g to 2.88 mg/g (Fig. 4a). In addition, the PER was also significantly enhanced in accordance with the increase in supplementary L-lysine in the diets between the WT10 and WT40 groups (Fig. 4b).
Improved availability of EAAs in growing rats fed HFL rice diets
The availability of most amino acids is presented in Fig. 5 and Supplementary Fig. S3. As expected, lysine availability was 90.69% and 89.85% for the HFL1 and HFL2 groups, respectively, which was higher than that of the WT or L-lysine-supplemented WT groups, especially of the WT group (85.57%; Fig. 5a). Among the L-lysine-supplemented groups, lysine availability was the highest in the WT20 group, but not in others including WT 40, implying that there is an optimum requirement of lysine level in the diet for animal feeding.
In addition to lysine, leucine and threonine are also limiting EAAs in rice. Even though the AAS of lysine was higher in the two HFL transgenic rice diets than in the WT diet, the AAS of leucine and threonine were similar between the transgenic HFL and WT diets. Interestingly, the availability of other three EAAs, including leucine, methionine and isoleucine, improved in HFL rice diets (Fig. 5b–d). There was no difference in the availability of other amino acids between transgenic HFL and WT diets (Supplementary Fig. S3).
Effect of HFL diets on viscera coefficient
At the end of the feeding experiment, the rats were anaesthetized and their main organs were collected and weighed. No abnormity was observed. The viscera coefficient of each group was calculated as the ratio between organ weight and body weight (Supplementary Table S4). There were no significant differences in viscera coefficient among the six groups. Liver and kidney coefficients of the HFL groups were lower than those of the WT group, but similar to those of the group consuming commercially available the standard rat diet (2018SX, Teklad Global, USA, containing 18% protein) (data no shown). Therefore, lysine had no effect on the health of the growing rats.
Enhanced levels of serum lysine and muscle nitrogen in growing rats fed HFL diets
At the end of the feeding experiment, the free lysine in serum and nitrogen in some organs of all groups were measured, and the data were presented in Fig. 6. The results shown that serum lysine content was increased significantly in the HFL groups and WT40 group, with no significant increase in WT10 and WT20 groups, compared with that of WT groups (Fig. 6a). Furthermore, nitrogen content in spine muscle was positively correlated with dietary lysine level (Fig. 6b), while moisture in muscle was not significantly different within different groups (Supplementary Fig. S4). But, there was similar nitrogen level in heart and liver tissues among the six groups, except it was greater in the WT40 group than other groups. (Fig. 6c,d) These data suggested that the serum lysine level and muscle nitrogen content could be improved in growing rats fed either high lysine HFL transgenic rice or wild type rice supplemented with crystalline L-lysine.
Curve fitting for diets differing in lysine content
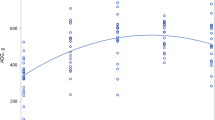
All diets in present study, which were derived from the same rice cultivar (same genetic background), differed in lysine content. Thus, we evaluated the relationship between lysine content in the diets and animal growth performance as well as biomarkers (Fig. 7). Animal growth performance parameters (e.g., body weight, food efficiency, protein apparent digestibility, PER, and lysine availability) were higher in WT10, WT20, or WT40 groups than in WT group. The data were subjected to regression analysis to assess the dietary lysine requirement of growing rats. We obtained a linear relationship between lysine concentration and feeding response (Fig. 7). Except for food intake, all other animal growth performance parameters had R 2 values more than 0.95 (P < 0.05) (Fig. 7).
Linear relationship between dietary lysine concentrations and growth biomarkers. The coefficient fitted the value of the linear function. Observed mean values are represented by blue (WT, WT10, WT20, and WT40) and red (HFL1 and HFL2) dots. Solid line represents the fitted dose-response curve. A–F represent the dose-response curve between lysine concentrations and body weight gain (a), food intake (b), food efficiency (c), protein efficiency ratio (d), protein apparent digestibility (e), and lysine availability (f).
Amino acid analyses revealed that lysine concentrations increased by 24% and 19% in HFL1 and HFL2 transgenic rice flour, respectively (Table 2). The biological indicators of rats fed the HFL1 and HFL2 diets might be similar to those of rats fed the WT20 diet, because all three diets had similar lysine concentrations. According to the regression line (Fig. 7e), the theoretical apparent digestibility values of the HFL1 and HFL2 groups were 71.21% and 69.69%, respectively (R 2 = 0.978). Interestingly, the actual apparent digestibility values of the HFL1 and HFL2 groups were 74.01% and 71.98%, respectively, which were slightly higher than the theoretical values. Similar results were also observed with other indexes, including body weight gained, food efficiency, protein efficiency ratio, and lysine availability (Fig. 7).
Discussion
The two HFL transgenic rice lines had higher lysine concentrations, total protein concentrations, and lysine scores than WT rice25 (Supplementary Table S2). The nutritional value of foods depends on the protein amount, protein quality, and amino acid balance36. In general, the quality or nutritional value of proteins is dependent on the capacity to replace the nitrogen that the organism inevitably loses during metabolic processes37. Therefore, dietary proteins must be evaluated in terms of amount, amino acid profile, and availability38. Our findings revealed that the availability of both proteins and amino acids was higher from HFL transgenic rice than from WT rice (Figs 4 and 5).
Several studies have evaluated the nutritional value of transgenic crops, such as rice39, 40, maize41, soybean42, and wheat43. In this study, growing rats fed HFL transgenic rice diets had similar growth performance parameters as those fed WT20 diet. The actual protein values and amino acid scores of the HFL groups were within the scope of the theoretical values obtained from curve fittings based on supplemented WT diets (Fig. 7). Therefore, lysine-biofortified rice improved the nutritional value of the crop without affecting other nutrients (Table 2).
Up to now, many studies had shown that the food intake increased in rats fed with diet containing additional lysine, and subsequently the body weight gain, feed efficiency, and growth responsiveness were also improved26,27,28. Indeed, feed intake is usually affected by dietary amino acids44, 45. The linkage between dietary lysine to appetite and feed intake had been reported by several groups46, 47. The results by Liao et al. showed that lysine is a substrate for generating body proteins, peptides, and non-peptide molecules in typical swine diets46. Nguyen et al. reported that the neuropeptide Y serves as an orexigenic factor48. But, these studies suggested further investigation to elucidate the linkage between dietary lysine to arginine ratio and regulation of appetite and food intake. Recently, Liao et al. reported that dietary supplementation of crystalline lysine can increase the muscle nitrogen retention and protein accretion, and improved the growth performance of animals46.
In this study, the serum lysine content and muscle nitrogen level in rats were also significantly increased in the HFL groups compared with that of WT group (Fig. 6). Both suggested that protein synthesis might be enhanced in rats fed with high lysine diets than those fed WT diet, in agreement with previous reports. Further, in our study, the nitrogen level in other organs (liver and heart) showed no significant difference between the HFL groups and WT group, in agreement with the results of Luo et al.49, with the exception that the WT40 group rat fed high lysine diet had higher nitrogen content in liver than those fed with WT or WT20 diet (Fig. 6c). This is probably because of liver is regarded as a site for primary pathway of lysine catabolism (saccharopine pathway), which contributes to the whole body lysine catabolism46, 50, 51, while supplemental L-lysine level in WT40 diet might be excessive to be digested in time in the growing rats.
The de novo synthesis of amino acids, especially lysine, by gut bacteria takes place in both the small and large intestines52. A number of studies have shown that dietary protein including protein source and amino acid composition can greatly influence the gut microbial community53. These may be due to decarboxylation of amino acids and peptides leading to the formation of a large array of amines, which are considered to be contributed by the clostridia, bifidobacteria and bacteroides54. However, there were also studies on lysine supplementation that indicated lysine as limiting amino acid in the basal diet, but it did not influence the microbial counts in feces and small intestine55. In our study, as the main objective was to evaluate the nutritive value of HFL rice on growing rats, we did not measure the gut microflora. It would be interesting to further elucidate the mechanism and function of additional lysine on animal growing.
The quality of food ingredients is estimated by digestibility, availability, and efficiency of amino acids and protein56. Among them, amino acid availability is an important factor because amino acids might interact with other components in the diet57. O’Quinn et al.26 reported that digestible lysine increased from 0.80% to 1.15% in young pigs fed high-lysine maize diets. Compared to wild-type lupin seeds, high-lysine and high-methionine transgenic lupin seeds contributed to greater weight gain and higher net protein efficiency and biological value in rats58. In this study, lysine availability increased in growing rats fed HFL diets (Fig. 5). Moreover, body weight and PER increased in the groups fed high-lysine transgenic rice diets (Figs 2 and 4). The lysine-biofortified transgenic rice not only contributes to a more cost-effective and sustainable food/feed, but also reduces nitrogen emission levels.
Due to ethical considerations, this study did not include a group of rats fed with diet without protein to provide quantitation of endogenous nitrogen from protein metabolism, thus the protein apparent digestibility was calculated instead of true digestibility. Boisen et al.59 reported that true digestibility of three raw Indica rice diets (7.5 to 10.4% protein) ranged between 68% and 78%. In this study, apparent digestibility was slightly lower (60% to 75%), possibly due to a lower protein content in milled rice than in non-milled rice. Lysine availability ranged between 85% and 90%, which was slightly higher than that reported from high-lysine corn (80%)60.
Dietary lysine may affect the metabolism of other nutrients61, 62, 63. Excessive dietary lysine has antagonistic effects on arginine in rats, chicks, guinea pigs, and dogs, but not in growing pigs or adult cats64, 65. Baker64 reported that excessive dietary lysine enhances arginine catabolism by inducing the synthesis of arginase in the kidneys. In pigs, excessive dietary lysine affects growth performance rates65. In addition, excessive lysine may affect calcium metabolism, protein methylation, and hormone synthesis in animals46, 62, 66. In fish, excessive lysine decreases protein and lysine deposition67. In this study, we evaluated the effects of lysine on the absorption of other amino acids. The results revealed that lysine had no obvious effects on arginine, threonine, valine, phenylalanine, or histidine absorption in the WT40 group (Fig. 5 and Supplementary Fig. S3).
The shape and slope of dose-response curves have been used to assess drug doses, relative biological effectiveness, dietary nutrient requirements67, 68, 69, 70. In this study, we obtained a linear relationship between lysine concentrations and weight gain, food efficiency, apparent digestibility, PER, and lysine availability (Fig. 7). However, food intake was not affected (P > 0.05) by dietary lysine concentrations71. The curve fitting results revealed that apparent digestibility, PER, and lysine availability of high-lysine transgenic rice HFL1 and HFL2 were slightly superior to the theoretical values (Fig. 7f). It might due to: (1) The HFL1 and HFL2 contain not only higher lysine content but also more other nutrients, such as total protein and leucine (Table 2 and Supplementary Table S1); (2) The increase level of lysine in biofortified rice gives much higher nutritive value than that in L-lysine-supplemented WT rice. Taken together, the present HFL transgenic rice lines had higher nutritional value not only than the non-transgenic wild type but also wild type rice supplemented with corresponding level of L-lysine (Figs 2–4, Table 2, and Supplementary Table S1). Therefore, our lysine biofortified rice could be further considered for either food or feed application. We are currently conducting food safety assessment of these transgenic rice.
Methods
Rice flour preparation
Two high-lysine pyramid transgenic rice lines HFL1 and HFL2 and the non-transgenic wild-type (WT) Wuxiangjing 9 (Oryza sativa L. spp. japonica) were simultaneously grown at the experimental fields of Yangzhou University (Yangzhou, China) under identical climatic conditions. Seeds were harvested, milled, and processed into flour for composition analyses. For nutrient analyses, three random samples were selected from each type of rice, and each sample was analyzed in triplicate.
Diet preparation
A total of six rice diets were prepared based on the American Institute of Nutrition AIN-93G formulation (Table 1) for feeding experiments72. Two diets, HFL1 and HFL2, were prepared with rice flour from HFL1 and HFL2, respectively. The WT diet contained rice flour from the non-transgenic control WT. The WT10, WT20, and WT40 diets were prepared from WT rice flour supplemented with different concentrations (0.235, 0.47 and 0.94 g/kg, respectively) of crystalline L-lysine (Sigma, USA). L-lysine-supplemented WT rice contained 10% (WT10), 20% (WT20), and 40% (WT40) more lysine than the WT rice flour. Accordingly, HFL1 and HFL2 transgenic rice lines contained about 24% and 19% more total lysine content, respectively, in grains compared with that of the WT rice (Table 2).
All the above six diets contained the same level of mineral, vitamin and fiber. The protein, amino acids and starch were solely derived from rice and lysine supplement, amounting up to 85% (by weight) of the diets (Table 1). The rice diets were vacuum-packed with polyethylene bags until use.
Animals and experimental design
Animal feeding experiments were carried out in an animal house of the the Chinese University of Hong Kong (CUHK; Hong Kong, China). The experimental procedure was approved by the Animal Experimentation Ethics Committee of CUHK (AEEC No. 14/131/MIS). All experiments were performed in accordance with the guidelines for the use of live animals. In this study, 48 weaned, male Sprague-Dawley (SD) rats (3 weeks of age, body weight at 50 g around) were divided into six groups (with eight rats per group) and fed the corresponding diets (Table 1). Initially, rats were housed individually in polycarbonate cages with stainless steel covers. The room was maintained at 22 ± 2 °C and 45 to 60% relative humidity with 12-h light/dark daily cycles. Following a seven-day acclimation period, rats were housed individually in stainless steel metabolic cages in the same environment, and fecal and urine samples were collected. After six days of nitrogen balance, the rats were transferred to the polycarbonate cages for the end of the experiment.
During the experiments, the rats had ad libitum access to feed and water. Body weight and food intake were recorded daily. Urine and fecal samples were stored at −80 °C. At the end of the experiment, the rats were anaesthetized with sodium barbital and blood was collected through cardiac puncture. Serum was collected from blood samples by centrifugation (3000 x g for 30 min at 4 °C), then stored at −70 °C until analysis. Muscle samples from Longissimus dorsi, liver, heart, kidneys, stomach, spleen, lung, and intestine were collected and weighed. All samples were stored at −70 °C until analysis.
Chemical analysis
Dry matter, ash, fiber, and total fat of rice flours were determined by AOAC methods73. Carbohydrate concentration was calculated using the following equation, carbohydrates (%) = 100% − (% protein + % fat + % moisture + % ash)73. Crude protein content was estimated by Kjeldahl74. Starch and amino acid content were determined as reported by methods we used previously25, 75. Moisture in grains and muscle were determined by standard methods70. The organ samples were freeze-dried and then ground for nitrogen analyses. The serum was deproteinized using an Ultrafree-MC 10,000 nominal molecular weight limit filter unit (Millipore, Bedford, MA, USA) at 5000 g, 4 °C for 30 min76, and the supernatant filtrate was analyzed for amino acids by HPLC analysis25.
Feces and urine, which were collected daily during six days of nitrogen balance, were pooled and stored at −80 °C77, 78. The samples were freeze-dried and ground for nitrogen and amino acid analyses. The dried urine samples were acidified with 1 mol/L HCl, and the dried fecal samples were hydrolyzed by HCl and subjected to HPLC analysis25.
Calculations
Amino acid score (AAS) was calculated according to WHO recommendations for school-aged children and adolescents34. The AAS of sulfur-containing amino acids (methionine and cysteine) was not determined because cysteine was added to the diets. AAS was calculated using the following equation, AAS = amino acid (mg) in 1 g of test protein/reference pattern. The reference pattern of each amino acid is presented in Supplementary Table S3. Food intake (g diet/rat/day) was determined from the average amount of diet consumed by each rat during 70 d. Food efficiency (%)79 was calculated as body weight gain (g)/food intake (g) × 100. Apparent protein digestibility of proteins was calculated from (IP - FP) × 100/IP, where IP and FP represent ingested proteins and fecal proteins by weight, respectively. Protein content was calculated using a nitrogen-to-protein conversion factor of 6.25. Protein efficiency ratio (PER) was determined as: PER = (final body weight - initial body weight)/protein intake80. Amino acid availability was determined by the method45, 81:
Statistical analysis
Results are presented as mean ± SD. Statistical comparisons were designed to determine whether the differences between the transgenic and WT groups were attributed to lysine content. Homogeneity of variance was determined by one-way analysis of variance (ANOVA) using SPSS 17.0 for windows (SPSS Inc., Chicago, IL, USA). Differences were considered significant at P < 0.05. Regression model and curve fitting were used to determine the optimal dietary lysine concentration for growing rats. The relationship between dietary lysine and biological indicators were subjected to regression analysis. Theoretical values were determined from curve fitting.
References
Fraiture, M. A. et al. Biotech rice: Current developments and future detection challenges in food and feed chain. Trends Food Sci. Technol. 52, 66–79, doi:10.1016/j.tifs.2016.03.011 (2016).
Galili, G. & Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 11, 211–222 (2013).
Clemente, A. et al. Eliminating anti-nutritional plant food proteins: the case of seed protease inhibitors in pea. Plos One 10, e0138039 (2015).
EFSA GMO Panel Working Group on Animal Feeding Trials. Safety and nutritional assessment of GM plants and derived food and feed: The role of animal feeding trials. Food Chem Toxicol. 46, S2–S70 (2008).
Ravindran, V., Tabe, L. M., Molvig, L., Higgins, T. J. & Bryden, W. L. Nutritional evaluation of transgenic high-methionine lupins (Lupinusangustifolius L) with broiler chickens. Int. J. Mol. Sci. 82, 280–285 (2002).
White, C. L. et al. Increased efficiency of wool growth and live weight gain in Merino sheep fed transgenic lupin seed containing sunflower albumin. J. Sci. Food Agr. 81, 147–154 (2001).
Spencer, J. D., Allee, G. L. & Sauber, T. E. Phosphorus bioavailability and digestibility of normal and genetically modified low-phytate corn for pigs. J. Anim. Sci 78, 675–681 (2000).
Saika, H. et al. Application of gene targeting to designed mutation breeding of high-tryptophan rice. Plant Physiol. 156, 1269–1277 (2011).
Cline, P. M., Tsai, T. C., Stelzleni, A. M., Dove, C. R. & Azain, M. Interaction of dietary energy and protein on growth performance, carcass characteristics and digestibility in finishing barrows when fed at a constant digestible lysine to metabolizable energy ratio. Livest. Sci. 184, 1–6 (2016).
Roy, N., Lapierre, H. & Bernier, J. F. Whole-body protein metabolism and plasma profiles of amino acids and hormones in growing barrows fed diets adequate or deficient in lysine. Can. J. Anim. Sci. 80, 585–595 (2000).
Takenaka, A., Oki, N., Takahashi, S. I. & Noguchi, T. Dietary restriction of single essential amino acids reduces plasma insulin-like growth factor-I (IGF-I) but does not affect plasma IGF-binding protein-1 in rats. J. Nutr. 130, 2910–2914 (2000).
Datta, D., Bhinge, A. & Chandran, V. Lysine: Is it worth more? Cytotechnology 36, 3–32 (2001).
Bidner, B. S., Ellis, M., Witte, D. P., Carr, S. N. & McKeith, F. K. Influence of dietary lysine level, pre-slaughter fasting, and rendementnapole genotype on fresh pork quality. Meat Sci. 68, 53–60 (2004).
Li, P., Yin, Y. L., Lia, D. F., Kim, S. W. & Wu, G. Y. Amino acids and immune function. Brit. J. Nutr. 98, 237–252 (2007).
Tang, M. Z. et al. Nutritional assessment of transgenic lysine-rich maize compared with conventional quality protein maize. J. Sci. Food Agric. 93, 1049–1054 (2013).
Tous, N. et al. Effect of reducing dietary protein and lysine on growth performance, carcass characteristics, intramuscular fat, and fatty acid profile of finishing barrows. J. Anim. Sci. 92, 129–140 (2014).
Salter, D. N., Montgomery, A. I., Hudson, A., Quelch, D. B. & Elliott, R. J. Lysine requirements and whole-body protein turnover in growing pigs. Brit. J. Nutr. 63, 503–513 (1990).
Shelton, N. W. et al. Effects of increasing dietary standardized ileal digestible lysine for gilts grown in a commercial finishing environment. J. Anim. Sci. 89, 3587–3595 (2011).
Fraga, A. L. et al. Lysine requirement of starting barrows from two genetic groups fed on low crude protein diets. Braz. Arch. Biol. Technol. 51, 49–56 (2008).
Faluyi, O. B., Agbede, J. O. & Adebayo, I. A. Growth performance and immunological response to Newcastle disease vaccinations of broiler chickens fed lysine supplemented diets. J. Vet. Intern. Med. 7, 77–84 (2015).
Azevedo, R. A. & Arruda, P. High-lysine maize: the key discoveries that have made it possible. Amino Acids 39, 979–989 (2010).
Zhao, Z. et al. Nutritionally improved transgenic sorghum. Plant Biotechnol. 2002 and beyond 413–416 (2003).
Long, X. H., Liu, Q. Q., Chan, M. L., Wang, Q. & Sun, S. S. M. Metabolic engineering and profiling of rice with increased lysine. Plant Biotechnol. J 11, 490–501 (2013).
Wong, H. W., Liu, Q. Q. & Sun, S. S. M. Biofortification of rice with lysine using endogenous histones. Plant Mol. Bio. 87, 235–248 (2015).
Yang, Q. Q. et al. Biofortification of rice with the essential amino acid lysine: Molecular characterization, nutritional evaluation, and field performance. J. Exp. Bot. 67, 4285–4296 (2016).
O’Quinn, P. R. et al. Nutritional value of a genetically improved high-lysine, high-oil corn for young pigs. J. Anim. Sci. 78, 2144–2149 (2000).
Taylor, M. L. et al. Broiler performances and carcass parameters of broiler fed diets containing lysine maize. J. Dairy Sci. 87, 315 (2004).
Lucas, D. M. et al. Broiler performance and carcass characteristics when fed diets containing lysine maize (LY038 or LY038 × MON 810), control, or conventional reference maize. Poultry Sci. 86, 2152–2161 (2007).
Colina, J. J., Miller, P. S., Lewis, A. J., Fischer, R. L. & Diedrichsen, R. M. Body composition, tissue deposition, and lysine utilization for protein deposition of barrows and gilts fed crystalline or protein-bound lysine. J. Anim. Sci. 94, 1972–1981 (2016).
Kusano, M. et al. Using metabolomic approaches to explore chemical diversity in rice. Mol. Plant 8, 58–67 (2015).
FAO/WHO. Energy and protein requirement. Report of a joint FAO/WHO ad hoc expert committee 52, Geneva: FAO (1973).
Liu, X. et al. Development of high-lysine rice via endosperm-specific expression of a foreign LYSINE RICH PROTEIN gene. BMC Plant Biol. 16, 147 (2016).
Wang, W. Y. & Galili, G. Transgenic high-lysine rice–a realistic solution to malnutrition? J. Exp. Bot. 67, 4009–4011 (2016).
World Health Organization/Food and Agriculture Organization/United Nations University. Protein and amino acid requirements in human nutrition report of a joint WHO/FAO/UNU expert consultation. WHO Technical Report Series No. 935. Geneva: WHO (2007).
FAO/WHO. Protein quality evaluation. Report of the joint FAO/WHO expert consultation. FAO Food and Nutrition, paper No. 51 Food and Agriculture Organization of the Untied Nations, Rome, Italy (1991).
Millward, D. J., Layman, D. K., Tomé, D. & Schaafsma, G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 87, 1576S–81S (2008).
Aguilar, E. G. et al. Evaluation of the nutritional quality of the grain protein of new Amaranths varieties. Plant Foods Hum. Nutr. 70, 21–26 (2015).
Gupta, R. B., Batey, I. L. & MacRITCHIE, F. Relationships between protein composition and functional properties of wheat flours. Cereal Chem. 69, 125–131 (1992).
Li, X. et al. Comparison of nutritional quality between chinese Indica rice with sck and cry1Ac genes and its non-transgenic counterpart. J. Food Sci. 72, S420–S424 (2007).
Gayen, D., Sarkar, S. N., Datta, S. K. & Datta, K. Comparative analysis of nutritional compositions of transgenic high iron rice with its non-transgenic counterpart. Food Chem. 138, 835–840 (2013).
Rayan, A. M. & Abbott, L. C. Compositional analysis of genetically modified corn events (NK603, MON88017 × MON810 and MON89034 × MON88017) compared to conventional corn. Food Chem. 176, 99–105 (2015).
Zhu, J. et al. Transcriptome response to glyphosate in sensitive and resistant soybean. J. Agric. Food Chem. 56, 6355–6363 (2008).
Baker, J. M. et al. Ametabolomic study of substantial equivalence of field-grown genetically modified wheat. Plant Biotechnol. J 4, 381–392 (2006).
Morley, J. E. & Flood, J. F. Evidence that nitric oxide modulates food intake in mice. Life Sci 49, 707–711 (1991).
Hughes, S. G. Effect of Supplemental Dietary Crystalline Amino Acids on the Feed Consumption of Juvenile Striped Bass, Morone saxatilis. J Appl aquac 7, 45–51 (1997).
Liao, S. F., Wang, T. J. & Regmi, N. Lysine nutrition in swine and the related monogastric animals: muscle protein biosynthesis and beyond. SpringerPlus 4, 147 (2015).
MacKenzie, D. S., VanPutte, C. M. & Leiner, K. A. Nutrient regulation of endocrine function in fish. Aquaculture. 161, 3–25 (1998).
Nguyen, M. V. et al. Feed intake and brain neuropeptide Y (NPY) and cholecystokinin (CCK) gene expression in juvenile cobia fed plant-based protein diets with different lysine to arginine ratios. Comp Biochem Phys A 165, 328–337 (2013).
Luo, Z. et al. Quantitative L-lysine requirement of juvenile grouper Epinephelus coioides. Aquac Nutr. 12, 165–172 (2006).
Papes, F., Kemper, E. L., Cord-neto, G., Langone, F. & Arruda, P. Lysine degradation through the saccharopine pathway in mammals: involvement of both bifunctional and monofunctional lysine-degrading enzymes in mouse. Biochem. J 344, 555–563 (1999).
Gatrell, S. K. et al. Tissue distribution of indices of lysine catabolism in growing swine. J Anim Sci. 91, 238–247 (2013).
Dai, Z., Wu, Z., Hang, S., Zhu, W. & Wu, G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol Hum Reprod. 21, 389–409 (2015).
Yang, Y. X., Dai, Z. L. & Zhu, W. Y. Important impacts of intestinal bacteria on utilization of dietary amino acids in pigs. Amino Acids. 46, 2489–2501 (2014).
Scott, K. P., Gratz, S. W., Sheridan, P. O., Flint, H. J. & Duncan, S. H. The influence of diet on the gut microbiota. Pharmacol Res. 69, 52–60 (2013).
Muhl, A. & Liebert, F. Growth and parameters of microflora in intestinal and faecal samples of piglets due to application of a phytogenic feed additive. J Anim Physiol An N. 91, 411–418 (2007).
Levesque, C. L., Moehn, S., Pencharz, P. B. & Ball, R. O. Review of advances in metabolic bioavailability of amino acids. Livest. Sci. 133, 4–9 (2010).
Friedman, M. Nutritional value of proteins from different food sources.A Review. J. Agr. Food Chem. 44, 6–29 (1996).
Molvig, L. et al. Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinusangustifolius L.) expressing a sunflower seed albumin gene. Pro. Natl. Acad. Sci. USA 94, 8393–8398 (1997).
Boisen, S., Duldulao, J. B. A., Mendoza, E. M. T. & Juliano, B. O. Comparative protein digestibility in growing rats of cooked rice and protein properties of indica and japonica milled rice. J. Cereal Sci. 33, 183–191 (2001).
Klein, R. G., Beeson, W. M., Cline, T. R. & Mertz, E. T. Lysine availability of opaoue-2 corn for rats. J. Anim. Sci. 35, 551–555 (1972).
Jarowski, C. I. & Pytelewski, R. Utility of fasting essential amino acid plasma levels in formulation of nutritionally adequate diets III: Lowering of rat serum cholesterol levels by lysine supplementation. J. Pharm. Sci. 64, 690–691 (1975).
Civitelli, R. Kinney International Award for Nutrition and Metabolism. Dietary L-lysine and calcium metabolism in humans: background. Nutr. 9, 299–300 (1992).
Liaudet, L. et al. Effect of L-lysine on nitric oxide overproduction in endotoxic shock. Brit. J. Pharmacol. 122, 742–748 (1997).
Baker, D. H. Lysine, arginine, and related amino acids: an introduction to the 6th amino acid assessment workshop. J. Nutr. 137, 1599S–1601S (2007).
Fascetti, A. J., Maggs, D. J., Kanchuk, M. L., Clarke, H. E. & Rogers, Q. R. Excess dietary lysine does not cause lysine-arginine antagonism in adult cats. J. Nutr. 134, 2042S–2045S (2004).
Wu, S. C. & Zhang, Y. Minireview: Role of protein methylation and demethylation in nuclear hormone signaling. Mol. Endocrinol. 23, 1323–1334 (2009).
Farhat, K. M. A. Dietary l-lysine requirement of fingerling stinging catfish, Heteropneustesfossilis (Bloch) for optimizing growth, feed conversion, protein and lysine deposition. Aquac. Res. 44, 523–533 (2013).
Borota, D. et al. Post-study caffeine administration enhances memory consolidation in humans. Nat. Neurosci. 17, 201–203 (2014).
Nemechek, J. E. et al. Evaluation of standardized ileal digestible lysine requirement of nursery pigs from seven to fourteen kilograms. J. Anim. Sci. 90, 4380–4390 (2012).
Vier, C. M. et al. Determining the standardized ileal digestible lysine requirement of 6.8 to 15.9 kg pigs. J. Anim. Sci. 94, 191 (2016).
Kumari, K. N. R. et al. Effect of supplementation of crystalline lysine on the performance of WL layers in tropics during summer. Trop. Anim. Health Prod. 48, 705–710 (2016).
Reeves, P. G., Nielsen, F. H. & Fahey, G. C. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123, 1939–1951 (1993).
AOAC International. Official methods of analysis of AOAC International.18th ed. AOAC Int., Gaithersburg, MD (2005).
Zhu, L. J., Liu, Q. Q., Sang, Y. J., Gu, M. H. & Shi, Y. C. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 120, 94–100 (2010).
Zhu, L. J., Liu, Q. Q., Wilson, J. D., Gu, M. H. & Shi, Y. C. Digestibility and physicochemical properties of rice (Oryza sativa L.) flours and starches differing in amylose content. Carbohydr. Polym. 86, 1751–1759 (2011).
Sunde, J., Kiessling, A., Higgs, D. & Opstvedt, J. Evaluation of feed protein quality by measuring plasma free amino acids in Atlantic salmon (Salmo salar L.) after dorsal aorta cannulation. Aquac. Nutr. 9, 351–360 (2003).
Wolber, F. M., McGrath, M., Jackson, F., Wylie, K. & Broomfield, A. Cysteic acid in dietary keratin is metabolized to glutathione and liver taurine in a rat model of human digestion. Nutrients 8, 104 (2016).
Bertinato, J. et al. L-Lysine supplementation does not affect the bioavailability of copper or iron in rats. J. Trace Elem. Med. Bio. 38, 194–200 (2016).
Wang, Y. P. et al. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 84, 341–347 (2009).
Hoseini, S. M., DaraniK, K. & Mozafari, M. R. Nutritional and medical applications of spirulina microalgae. Mini. Rev. Med. Chem. 13, 1231–1237 (2013).
Sun, M. J., Mu, T. H., Zhang, M. & Arogundade, L. A. Nutritional assessment and effects of heat processing on digestibility of Chinese sweet potato protein. J. Food Compos. Anal. 26, 104–110 (2012).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31471459), Ministry of Agriculture of China (grants 2016ZX08001006 and 2009ZX08009-001B), and Jiangsu PAPD and Qinglan Projects to Q.Q.L., the Bill and Melinda Gates Foundation (Grand Challenges in Global Health initiative, USA), University Grants Committee of the Hong Kong Special Administrative Region (Project No. AoE/B-07/99), the State Key Laboratory of Agrobiotechnology (CUHK), the Lo KweeSeong Foundation, and the Lee Hysan Foundation to S.S.M.S.
Author information
Authors and Affiliations
Contributions
Q.Q.L., S.S.M.S. and M.H.G. conceived and designed the experiments, Q.Q.Y., P.K.S., C.Q.Z., and W.S.M. performed the experiments; Q.Q.Y. and P.K.S. analyzed the data. Q.Q.Y. and Q.Q.L. wrote the paper. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, QQ., Suen, P.K., Zhang, CQ. et al. Improved growth performance, food efficiency, and lysine availability in growing rats fed with lysine-biofortified rice. Sci Rep 7, 1389 (2017). https://doi.org/10.1038/s41598-017-01555-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01555-0
This article is cited by
-
Black beans (Glycine max (L.) Merrill) included in a multi-grain rice reduce total cholesterol and enhance antioxidant capacity in high-fat diet-induced obese mice
Food Science and Biotechnology (2024)
-
Low-protein diets supplemented with methionine and lysine alter the gut microbiota composition and improve the immune status of growing lambs
Applied Microbiology and Biotechnology (2021)
-
Improving nutritional quality of rice for human health
Theoretical and Applied Genetics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.