Abstract
The demand to discover new materials is scientifically as well as industrially a continuously present topic, covering all different fields of application. The recent scientific work on thin film materials has shown, that especially for nitride-based protective coatings, computationally-driven understanding and modelling serves as a reliable trend-giver and can be used for target-oriented experiments. In this study, semi-automated density functional theory (DFT) calculations were used, to sweep across transition metal diborides in order to characterize their structure, phase stability and mechanical properties. We show that early transition metal diborides (TiB2, VB2, etc.) tend to be chemically more stable in the AlB2 structure type, whereas late transition metal diborides (WB2, ReB2, etc.) are preferably stabilized in the W2B5−x structure type. Closely related, we could prove that point defects such as vacancies significantly influence the phase stability and even can reverse the preference for the AlB2 or W2B5−x structure. Furthermore, investigations on the brittle-ductile behavior of the various diborides reveal, that the metastable structures are more ductile than their stable counterparts (WB2, TcB2, etc.). To design thin film materials, e.g. ternary or layered systems, this study is important for application oriented coating development to focus experimental studies on the most perspective systems.
Similar content being viewed by others
Introduction
Designing new materials is a highly relevant topic covering many different aspects, like discovering materials with entirely new properties (e.g., carbon-nanotubes), improving existing materials in use, (e.g., reduce costs or weight), or increasing biocompatibility or environmental sustainability. These examples are just a small fraction of the never-ending quest fueled by modern industry. The increasing computational power opens new fruitful approaches for materials design1. Trial and error can be supported (thus focused) or even replaced by fundamental and sophisticated knowledge-based methods using semi-automated density functional theory calculations to scan across entire material classes2. Nitride-based materials, as prototypes of protective thin films for cutting and milling tool applications, have been intensively studied from their binary systems (e.g., TiN, AlN, CrN)3,4,5 up to their ternary6,7 or even multinary systems8,9. For example, TiN and Ti-Al-N are two highly prominent representatives present widely used in industry (e.g., tooling, microelectronics, decorative purpose). Additionally, considerable research activities concentrate on further application-oriented improvement of these materials using different architectural designs, which will combine their beneficial properties or create entirely new features10.
A rather new and extremely promising class of materials–with the potential to be used for many different applications ranging from superconductivity to wear- and corrosion-resistance–are borides11,12. Contrary to nitrides, only little is known about borides and more specifically about diborides, with the chemical formula XB2 (where X stands for transition metals (TM)). While there are several experimental and theoretical studies on binary diborides13,14, multinary diborides are rather unexplored15,16,17. Importantly, ReB2 has been theoretically predicted to be the most incompressible material exceeding the properties of diamond suggesting the use in (iron alloys) cutting applications were traditional materials (e.g., diamond) cannot be used due to the formation of carbides18,19. A huge drawback, when consindering TM-diborides for hard coating applications is the pronounced brittle behaviour of this material class. Hence, it is of great importance to classify their ductility and increase the toughness.
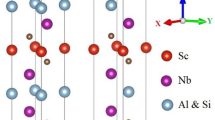
Many diborides tend to crystallize in two different hexagonal structures. While early transition metal diborides crystallize in the so-called AlB2 structure type (α, space group 191 - P6/mmm)2, late transition metal diborides (e.g., Tungstendiboride) prefer a W2B5−x based structure (ω, space group 194 - P63/mmc)20 - see Fig. 1. The AlB2 α-structure consists of a hexagonal shaped unit cell with an alternating stacking of covalently bonded graphite-like boron hexagons and metal layers. The closely related W2B5−x ω-structure type consists of hexagonal unit cell with alternating flat and puckered boron hexagons between the metal layers. Regarding the synthesis via physical vapor deposition, it is a well known fact, that metastable structures can be captured via this synthesis route21,22, which is often achieved by the implementation of point defects such as vacancies23,24. This circumstance of depositing a metastable structure can lead to extraordinary and positive effects (e.g., AlN, which shows significantly higher mechanical strength and elastic constants for its metastable face centered cubic structure than its thermodynamically stable hexagonal close packed wurtzite-type structure) but includes also the fact that upon providing the necessary activation energy, the metastable structure will transform in its thermodynamically stable modification (which is well known and investigated for many of the metastable Al2O3 polymorphs that transform to corundum-type Al2O3). Here, materials science allows for at least two helpful scenarios: 1) decreasing the difference in Gibbs free energy between metastable and stable state (which decreases the driving force for the phase transformation) and 2) increasing the Gibbs free energy of the peak separating the metastable and stable state (which increases the necessary activation energy to reach the stable state.) Picking up the concept of ZrO2 based ceramics, where alloying of certain elements (e.g., Mg, Y, Ce)25 leads to stabilization of high temperature modifications at room temperature, the two hexagonal diboride phases draw a great starting point for the exploration of ternary diboride materials. The combination of these phase stabilizing routes for the preferred α-type structure are schematically depicted in Fig. 1. To use such concepts for designing new materials (not necessarily limited only to thin films), it is essential to know and understand the fundamental properties (e.g., energy of formation (E f ), lattice constants, etc.) of the building blocks. Furthermore, such a knowledge database allows to set-up target-oriented experiments aiming on fulfilling various demands.
In this study, we use density functional theory (DFT) to obtain basic properties of transition metal diborides (TMB2) in their AlB2 and W2B5−x structures (α-TMB2 and ω-TMB2). In addition to perfect structures we also investigate the impact of vacancies on the energy of formation (which characterizes the chemical stability) as materials prepared by physical vapor deposition techniques are known for their high density of point defects such as vacancies. Since mechanical properties are of central importance for protective coatings, we calculated the elastic constants (bulk and shear modulus, poisson ratio, and the cauchy pressure) of all these transition metal diborides (3 to 5d elements excluding Hg) in their α and ω crystal structure.
Results and Discussion
Ground state properties
The energy of formation E f , which is a fundamental indicator (for chemical stability) of a solid matter, quantifies the energy gain (for negative E f values) upon forming a compound out of the individual elements, calculated as:
where, E tot is the total energy of the compound (here, TMB2), E i the energy of the individual elemental constituent i in its stable crystalline configuration, and n i denotes the number of atoms for the different species i. Figure 2 shows the energy of formation for all 28 TMB2 materials calculated, spanning the whole range of 3d, 4d and 5d (excluding Hg) transition metals, in the two different but relevant hexagonal structures, α- and ω-type.
Energies of formation (E f ) per atom for all TMB2 investigated (3d, 4d, and 5d transition metal elements, excluding Hg). The green and red hexagons represent the α- (AlB2 prototype) and ω- (W2B5−x Prototype) type structures, respectively. Please not, that some diborides have very similar E f in the two structure types and therefore overlapping symbols (e.g. Cu or Ta).
With increasing atomic number (within each period), the E f values become less negative and even positive (i.e., chemically instable) for TMB2 with Ni, Cu, Zn (3d elements), Pd, Ag, Cd (4d elements), Os, Ir, Pt, Au (5d elements). RuB2 and RhB2 (4d elements) are special border candidates as E f is negative for their ω-structure but positive for their α-structure. The increasing chemical stability (i.e., more negative E f values) with decreasing atomic mass (within the individual periods) underlines the increased reactivity of atoms with fewer valence electrons. Scandium and Yttrium slightly deviate from this tendency, which is comparable to the results obtained for nitrides26.
The data also show that the early TMB2 prefer the α-structure, which is in good agreement with earlier reports16, and the largest difference in E f between the α and ω structure of chemical stable TMB2 is obtained for TiB2, ZrB2, and HfB2, respectively. These results agree with the density of states (DOS), which show a pseudogap between the bonding and antibonding (TM-d and B-p) states. Within each period, the occupied bonding states change, to partially filled anti-bonding states with increasing atomic number. The decreasing N(E F ) (number of states at the fermi level (E F ) - corresponding to the position of the fermi level) reflects the chemical stability27 and is in excellent agreement with literature reports28,29,30. Hence, in the case of α-structured TiB2, ZrB2, and HfB2 the fermi level can be found in the pseudogap (lowest N(E F ) - showing highest chemical stability), whereas for ScB2 and YB2 as well as VB2, NbB2, and TaB2 the fermi level can be found in the bonding or antibonding states, respectively (reduced chemical stability). Furthermore, this circumstance also reflects the trend in melting temperatures (and the directionality of the bonding - covalent bonding character) for the individual diborides31,32.
As expected from the presence of puckered B-planes in the W2B5−x structures, all ω-TMB2 exhibit a higher equilibrium volume (and therefore lower mass density, which is not shown here) as compared to their corresponding α-TMB2 relatives, see Fig. 3. The equilibrium volume decreases with the atomic radii (in each period), but only up to that transition metal where E f becomes positive, please compare Figs 2 and 3. For higher atomic radii of the TM, the euquilibrium volume of the TMB2 increases again.
Equilibrium volumes for all TMB2 obtained from fitting the Birch-Murnaghan equation. Green and red hexagons denote to euqilibrium volumes for α- and ω type, respectively. The elements showing positive E f (see Fig. 2) are faded - starting at Ni, Ru, and Os for 3d, 4d, and 5d elements, respectively.
In respect to the lattice parameters (shown in Fig. 4a,b) a and c, and their ratio (c/a), the trends are consistent for both structure types. For elements from the 3d period, lattice parameter a stays fairly constant whereas lattice parameter c draws a clear decrease. Therefore, the trend for the c/a-ratio is dominated by the change in c with increasing atomic number. Elements from the fourth period show a decrease in lattice constants a and c until MoB2 for the α-structure and TcB2 for the ω-structure. Hence, the c/a-ratio for the α-structure decreases to the point where the compounds energetically prefer the ω-structure. For the 5d elements both, the a and c lattice parameter, slightly decrease with increasing atomic number. α-VB2 as well as α-CrB2 even reveal a cubic-like c/a-ratio of ~1. Farenholtz et al.33 stated, that the highly covalent B-B bonds are strong compared to TM-B or TM-TM bonds, which explains the small changes in lattice parameter a, with increasing atomic number (especially for the α-type). On the contrary, lattice parameter c is more dominated by the different atomic radii of the specific TM. Furthermore, stable α-TMB2 are only formed with respect to strained B-B bonds (increased/decreased bonding length caused by the present TMs), which would be crucial for smaller TM-atoms as Cr or larger than Zr. The trends for the ω-type is a rather controversial discussed topic in literature and difficult to relate to experimental data. Several experimental and computational studies have been conducted treating the off-stoichiometry of this crystal structure (W2B5−x-prototype)34,35. Nevertheless, assuming perfect structures, the observed trends follow similar behavior as obtained for the α-type, which leads to the conclusion, that the presence of the puckered boron planes compensate the strain introduced by other TM-atoms such as Mo or W.
Mechanical properties
Outstanding mechanical properties, e.g. super- or ultra-hardness, strongly correlate to bonding types and hence strength. Fitting the total energy data obtained for variable volumes, with the Birch-Murnaghan equation of state, gives the bulk moduli quantifying the materials’ resistance to isotropic pressure, and therefore represents a good guide for mechanical properties. The data (see Appendix S.1 and S.2) exhibit a maximum for each period as a function of the atomic number, which is at α-CrB2 (B = 287 GPa), α-MoB2 (B = 307 GPa), and ω-ReB2 (B = 333 GPa), for the 3d, 4d, and 5d elements, respectively. Also within our study, ReB2 exhibits the highest bulk modulus among all TMB2 studied. This is in excellent agreement with literature, stating that ReB2 is one of the hardest material available, approaching or even exceeding the excellent properties of diamond18,19. Comparing the maxima with the equilibrium volumes, their lowest volumes are at slightly higher atomic numbers, but within the chemically stable regions. As these ceramic-like TMB2 materials exhibit a mixture of metallic, covalent, and ionic bonds, the bulk moduli maxima (which are within the chemically stable region, as mentioned) are an indication of stronger bonds leading also to smaller interatomic distances (represented by the specific volume). Generally, the α-TMB2 exhibit higher bulk moduli than their ω-TMB2 counterparts, corresponding to their smaller specific volumes, only MnB2, TcB2, and WB2, slightly deviate, and FeB2 strongly deviates from this trend.
The entire elastic tensor was calculated for all TMB2 compounds in both structures, α and ω. These allow evaluating semi-empirical criteria for the ductile behavior. In this study, we use three different criteria: the Cauchy pressure (being C12–C44), the Pugh criteria (G/B ratio) as well as Frantsevich criteria on the Poisson ratio. After Pettifor et al.36, a positive Cauchy pressure indicates a metallic bonding character, hence a ductile behavior. Consequently, a negative Cauchy pressure indicates a brittle behavior due to the directional bonding character (hence more covalent contribution). Frantsevich37,38 and Pugh39 introduced additional criteria to classify a brittle or ductile behavior, by using the Poisson ratio (ν) and the G/B ratio, respectively. A ductile behavior is obtained for ν > 0.26 (Frantsevich criterion) or for G/B < 0.57 (Pugh criterion).
After these three criteria, presented in Fig. 5a (Frantsevich vs. Pugh criterion) and Fig. 5b (Pettifor vs. Pugh criterion), the most ductile ω-TMB2 compounds are ZnB2, PdB2, NiB2, and AuB2, but these are actually chemically not stable (positive E f and therefore not plotted in Fig. 5). All other ω-TMB2 compounds are at the minimum of at least one criterion or are classified as brittle. Please note that within Fig. 5a,b not all data points are shown, namely ω type ScB2, YB2, RuB2, as well as RhB2, suggesting either extreme brittle or non-stable structures (more positive E f as compared to their α type counterpart). The classical stable α-TMB2, which exhibit a more negative E f value than their ω-TMB2 relatives - ScB2, YB2, TiB2, ZrB2, HfB2, NbB2 - are classified as brittle after all three criteria. Contrary to that, there are many chemically stable α-TMB2 compounds, like MnB2, FeB2, CoB2 (3d), and TcB2 (4d), and WB2 (5d), which are classified as ductile after all three criteria. Also α-MoB2 as well as α-TaB2 can be added to this list as it is ductile after Frantsevich and Pugh and only slightly brittle after Pettifor. These transition metal diborides actually prefer the ω-structure, but are believed to be stabilized in their α-structure by introducing point defects such as vacancies (see following sections). Here, the energetically barrier is very small for α-TaB2, see Fig. 2.
Pugh criterion (G/B-ratio) as a dependence of the Poisson ratio (Frantsevich criterion, (a)) and the Cauchy pressure (C12–C44), Pettifor criterion, (b). The green and red hexagons correspond to α- and ω-structure, respectively. (*ReB2 in a,b) was taken from literature50. Please note, that some diborides have very similar values and are therefore overlapping.
Recalling Fig. 2, we see that the chemical stability (increases with decreased N(E F )) is reflected in the brittle/ductile-behavior of the individual compounds. Liu et al. recently showed by studying differnt structure types for WB2, that α-structured WB2 reveals smaller number of bonds accompanied by increased bond lengths. Therefore, α-WB2 is predicted to obtain a decreased hardness compared to its ω-structured polymorph. This is in good agreement to our results stating that α-WB2 shows a rather metallic bonding character than in its ω-structure, hence being in the highly ductile regime after all criteria.
Influence of vacancies on the phase stability of TMB2
Because PVD promotes the formation of many point defects such as vacancies–and these mutually influence not just the preferred structure28, mechanical properties40, thermal properties5, or magnetic properties41, for example–the impact of TM as well as B vacancies on the phase stabilities of α-TMB2 and ω-TMB2 is studied. To optimize the supercell size, which should be large enough (to allow the calculation of low vacancy concentrations and to minimize their mutual interaction), supercells with 3 × 3 × 3 (81 lattice sites) and 3 × 3 × 1 (with 108 lattice sites) were used for α-TMB2 and ω-TMB2, respectively. Vacancies in these structures were created by removing either one boron or one TM atom, hence ~3.7 at.% TM and ~1.9 at.% B vacancies in α-TMB2, and ~2.8 at.% TM and ~1.5 at.% B vacancies in ω-TMB2.
For the ω-TMB2 structure, the effect of B vacancies is studied for the flat and the puckered B plane individually. The energies of formation for such defected structures (\({{\rm{E}}}_{f}^{vac}\)), also calculated using Equation 1, are used to obtain the difference in E f (ΔE f = \({{\rm{E}}}_{f}^{vac}\) − \({{\rm{E}}}_{f}^{perf}\)), presented in Fig. 6. Thus, if ΔE f is positive, the formation of vacancies is energetically not preferred. Many of the α-TMB2 compounds actually prefer the formation of vacancies, such as CrB2, MnB2, FeB2, CoB2 (3d), and MoB2, TcB2 (4d), and TaB2, WB2 (5d). Whereas CrB2 and NbB2 only prefer the formation of B vacancies, the other mentioned α-TMB2 compounds prefer the formation of B as well as TM vacancies. Comparing Fig. 6a with Fig. 2 clearly shows that those α-TMB2 compounds, which are metastable with respect to ω-TMB2 (e.g. MnB2, MoB2, WB2), favour the formation of vacancies, whereas those α-TMB2 compounds, which are stable with respect to ω-TMB2 do not want to have vacancies (e.g. TiB2, ZrB2, HfB2). Although ΔE f is also negative for NiB2 (3d), and RuB2, RhB2, PdB2 (4d), and OsB2, IrB2, PtB2, AuB2 (5d), these compounds still exhibit positive \({{\rm{E}}}_{f}^{vac}\) values, and are thus still chemically instable.
(a) Energies of formation per vacancy for all TMB2 crystallizing in α structured cells. The yellow hatched bars indicate the energies of formation of a randomly taken boron vacancy. The blue bars represent the values for a randomly taken metal vacancy. (b) Energies of formation per vacancy for all TMB2 crystallizing in ω. The yellow and red hatched bars indicate the energies of formation of a randomly taken boron vacancy on the flat and puckered planes. The blue bars represent the values for a randomly taken metal vacancy.
The majority of the TMB2 compounds are chemically destabilized by vacancies in their ω-structure. Although ΔE f is negative for CuB2, CdB2, OsB2, and AuB2, these compounds are still chemically unstable with \({{\rm{E}}}_{f}^{vac}\) being positive. ScB2, TiB2 (3d), and YB2, ZrB2 (4d), are interesting compounds, as the formation of B-vacancies is only preferred at the puckerd planes, whereas for MnB2, FeB2 (3d), TcB2, RuB2, (4d), and WB2 (5d), the formation of B-vacancies is only preferred at the flat planes.
If we consider those TMB2 compounds that exhibit a metastale α-TMB2 (with respect to ω-TMB2) without defects, the preference for ω-TMB2 or α can alter when introducing vacancies. In this respect, we only concentrate on those TMB2 compounds that are chemically stable (i.e., negative E f values), even when introducing defects (i.e., still negative \({{\rm{E}}}_{f}^{vac}\) values), which are CrB2, MnB2, FeB2, CoB2 (3d), and MoB2, TcB2(4d), and TaB2, WB2 (5d). As for these compounds ΔE f is always more negative for the α- than for the ω-structure, the two structures come closer in E f with introducing vacancies. For TaB2, already the 3.7 at.% TM and 1.9 at.% B vacancies considered in this study, the α-structure overrules the ω-structure. For 2.8 at.% TM vacancies or 1.4 at.% B vacancies, ω-WB2 is still slightly preferred over the α-WB2 relative, but the difference significantly decreases from ~25 meV/at (no vacancies) to ~21 meV/at. Thus, we propose that the defects induced by PVD processes are responsible for the preferred formation of α-WB233,34 rather than the chemically more stable ω-WB2, if no defects are considered.
Designing ternary TM(I)1−xTM(II) x B2 thin film materials
Several experimental studies on WB2 report the formation of the α-structure when deposited as thin film via physical vapor deposition16,42,43. Taking into account the results presented before, where α-WB2 is located in the ductile regime (for all criteria), this compound is the ideal candidate for designing ternary borides. Furthermore, finding a good alloying element – with respect to matching lattice constants (equilibrium volume), high bulk modulus, similar tendency for vacancies, and lower E f - TaB2 points out as an excellent candidate for stabilizing the structure with low cost on ductility. Figure 7, shows the vacancy impact on the α- and ω-structure in the ternary W1−xTa x B2−z system (see Fig. 7a) and the impact of shottky (stoichiometric) defects on the binary systems (see Fig. 7b,c). Clearly, the data for the α-structure reveals the favor in stabilizing the structure via vacancies decreasing with increasing Ta content. Contrary to low Ta contents, where the structure strongly desires metal defects, the structure even gets slightly destabilized at high Ta contents for metal vacancies. The small discrepancy to the data presented in Fig. 6 is based on the uncertainty (in the range of 1 to 2 meV/at) of the calculations and the use of different SQS supercells. However, B vacancies stabilize in the full compositional range the α structure (also negative E f for all compositions investigated). Especially for α-TaB2, this is in good agreement to experimental studies showing a slight boron deficiency44. For α-WB2, no experimental studies regarding the chemical composition have been conducted so far.
Impact of metal and boron (flat (open symbols) and puckered (half filled symbols) vacancies on the stability of α- and ω-structured W1−xTa x B2−z depending on the Ta/(Ta + W) ratio (a). Impact of vacancy concentration (shottky defects) on E f of the two discussed structures for WB2 (b) and TaB2 (c). The green and dark red symbols correspond to the α- and ω-structure, respectively.
For the ω-structure the trends for increasing Ta contents remain, whereas the different vacancy types destabilize the structure. Exceptions here are boron vacancies (flat layer) at high W contents where a (insignificant) gain in energy can be obtained. Considering the results for shottky defects (see Fig. 7b,c) introduced in the α- and ω-structure for the binaries, it can be clearly seen, that the α-structure is more stable at ≥7.5 at.% vacancies and in the range of 0 > x ≥ 22.5 at.% for WB2 and TaB2, respectively. In respect to the concept shown in Fig. 1, the data suggests to start experimental work on W1−xTa x B2 thin films. These films should be strongly stabilized due to alloying (low Ta content) and the implementation of vacancies. Furthermore, the material should show a rather ductile behavior compared to “classical” diborides like TiB2. Preliminary experimental studies on single-phased DC magnetron sputtered α-W1−xTa x B2 (with x = 0, 0.07, 0.14, 0.26) thin films showed, that by annealing in vacuum the decomposition of the α-phase is postponed with increasing Ta content from 800–1000 °C (α-WB2) to 1200–1400 °C (α-W0.74Ta0.26B2). Additionally, micromechanical bending tests of cantilevers revealed a small decrease in fracture toughness for increasing Ta content from ~\(3.7\,MPa\sqrt{m}\) (α-WB2) to ~\(3.0\,MPa\sqrt{m}\) (α-W0.74Ta0.26B2).
Conclusion
Summarizing the results, we have confirmed the tendency of early TMB2 compounds preferring the α-structure due to their lower E f values compared to the ω-structure. TMB2 compounds with higher atomic numbers in their period than VB2 (3d), NbB2 (4d), and HfB2 (5d) reveal more chemical stability in the ω-structure.
The equilibrium volume of the individual elements in the α-structure is throughout smaller compared to their ω-structured counterpart due to the absence of the puckered boron planes. Moreover, it decreases with atomic radii up to that transition metal where E f becomes positive. Regarding the bulk moduli, the opposite trend compared to the equilibrium volume is shown reaching its maxima for α-CrB2 (3d), α-MoB2 (4d), and α-ReB2 (5d). Based on our studies on point defects, we can conclude, that diborides where the α-structure is chemically more stable than the ω-structure are destabilized when introducing metal or boron vacancies. Furthermore, ScB2, TiB2, YB2, and ZrB2 prefer the formation of vacancies on the puckered boron plane when considering the ω-structure which underlines their strong tendency to crystallize in the α-structure.
After applying three different criteria (Frantsevich, Pugh, and Pettifor) on the mechanical properties obtained from the calculation of the full elastic tensor, α-MnB2, α-FeB2, α-CoB2, α-TcB2, and α-WB2 are classified as ductile diborides. Hence, these are all TMB2 in the α-structure, where the ω-structure shows more chemical stability due to the more negative E f value.
Based on our calculations we propose α-W1−xTa x B2−z as a promising ternary material system, providing excellent mechanical strength as well as ductility. WB2 only provides excellent ductility data (according to the three ductility criteria) when stabilized in the metastable α-structure (AlB2-type). With the addition of vacancies (which are typical for physical vapor deposited materials) the α-structure becomes energetically preferred over the ω-structure (W2B5−x type). TaB2 exhibits almost the same energy of formation for the α-as well as ω-structure, and again significantly better ductility data for the α-structure. Only the Pettifor criterion indicates α-TaB2 as brittle whereas the other two criteria (Pugh and Frantsevich) indicate α-TaB2 as ductile. Thus, the addition of Ta is predicted to further promote the stabilization of α-WB2 structure with relatively low costs in ductility.
Methods
Applying density functional theory (DFT) coded in VASP (Vienna Ab Initio Simulation Package)45,46 using the projector augmented waves method within the generalized gradient approximation (PAW-PBE)47, structure and stability of transition metal diborides (TMB2) were studied. To avoid human errors, the calculations were semi-automated prepared and analyzed for all TM-Borides using python- and bash-scripting, respectively. 28 transition metals were considered, including all 3d, 4d and 5d elements except for Mercury (In case of Re the calculation of elastic constants for the α- and ω-structure exceeded the chosen time limit for computational effort as the compound was studied in detail in literature18,19). Therefore, all energy cutoffs and k-point meshes, where carefully chosen to ensure energy convergence of a few meV/at. The lattice parameter, the equilibrium volume and bulk moduli where fitted using a Birch-Murnaghan fit. To calculate the single-crystal elastic constants the method suggested by R. Yu et al.48 was obtained. Furthermore, the formation energy of single vacancies where studied using the Alloy-Theoretic Automated Toolkit49 where in the case of α-structure 3 × 3 × 3 supercells containing 81 Atoms and 3 × 3 × 1 supercells containing 108 atoms for the ω-structure where created. For the α-structure one - either metal or boron site - was eliminated which corresponds to a vacancy concentration of ~3.7 at.% and ~1.9 at.%, respectively. However, concerning the ω-structure, the two different boron sites (flat and puckered) as well as the metal site where taken into account interrelated to ~1.4 at.% boron vacancies and ~2.8 at.% metal vacancies.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Rodgers, J. R. & Cebon, D. Materials informatics. MRS Bull. 31, 975–980 (2006).
Ivanovskii, A. L. Mechanical and electronic properties of diborides of transition 3d–5d metals from first principles: Toward search of novel ultra-incompressible and superhard materials. Prog. Mater Sci. 57, 184–228 (2012).
Mayrhofer, P. H., Tischler, G. & Mitterer, C. Microstructure and mechanical/thermal properties of Cr-N coatings deposited by reactive unbalanced magnetron sputtering. Surf. Coat. Technol. 142–144, 78–84 (2001).
Mitterer, C., Mayrhofer, P. H. & Musil, J. Thermal stability of PVD hard coatings. Vacuum 71, 279–284 (2003).
Moraes, V. et al. Thermal conductivity and mechanical properties of AlN-based thin films. J. Appl. Phys. 119 (2016).
Mayrhofer, P. H. et al. Self-organized nanostructures in the Ti-Al-N system. Appl. Phys. Lett. 83, 2049–2051 (2003).
Willmann, H. et al. Thermal stability of Al-Cr-N hard coatings. Scr. Mater. 54, 1847–1851 (2006).
Chen, L., Holec, D., Du, Y. & Mayrhofer, P. H. Influence of zr on structure, mechanical and thermal properties of Ti-Al-N. Thin Solid Films 519, 5503–5510 (2011).
Mayrhofer, P. H., Hovsepian, P. E., Mitterer, C. & Münz, W.-D. Calorimetric evidence for frictional self-adaptation of TiAlN/VN superlattice coatings. Surf. Coat. Technol. 177–178, 341–347 (2004).
Hahn, R. et al. Superlattice effect for enhanced fracture toughness of hard coatings. Scr. Mater. 124, 67–70 (2016).
Mitterer, C. Borides in thin film technology. J. Solid State Chem. 133, 279–291 (1997).
Kayhan, M. et al. Neutron diffraction and observation of superconductivity for tungsten borides, WB and W2B4. Solid State Sci. 14, 1656–1659 (2012).
Mayrhofer, P. H., Mitterer, C., Wen, J. G., Greene, J. E. & Petrov, I. Self-organized nanocolumnar structure in superhard TiB2 thin films. Appl. Phys. Lett. 86, 1–3 (2005).
Nedfors, N. et al. Superhard NbB2−x thin films deposited by dc magnetron sputtering. Surf. Coat. Technol. 257, 295–300 (2014).
Euchner, H. & Mayrhofer, P. H. Designing thin film materials - ternary borides from first principles. Thin Solid Films 583, 46–49 (2015).
Euchner, H. et al. Solid solution hardening of vacancy stabilized Ti x W1−xB2. Acta Mater. 101, 55–61 (2015).
Alling, B., Högberg, H., Armiento, R., Rosen, J. & Hultman, L. A theoretical investigation of mixing thermodynamics, age-hardening potential, and electronic structure of ternary M1 1−xM2 x B2 alloys with AlB2 type structure. Sci. Rep. 5, 9888 (2015).
Chung, H.-Y. et al. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 316, 436–439 (2007).
Feng, S. et al. Ab initio study on structural, electronic properties, and hardness of re-doped W2B5. Solid State Commun. 245, 60–64 (2016).
Feng, S. et al. Theoretical investigations of physical stability, electronic properties and hardness of transition-metal tungsten borides WB x (x = 2.5, 3). Chem. Phys. Lett. 635, 205–209 (2015).
Rachbauer, R. et al. Decomposition pathways in age hardening of Ti-Al-N films. J. Appl. Phys. 110 (2011).
Euchner, H. & Mayrhofer, P. H. Vacancy-dependent stability of cubic and wurtzite Ti1−xAl x N. Surf. Coat. Technol. 275, 214–218 (2015).
Klimashin, F. F., Koutná, N., Euchner, H., Holec, D. & Mayrhofer, P. H. The impact of nitrogen content and vacancies on structure and mechanical properties of Mo–N thin films. J. Appl. Phys. 120, 185301 (2016).
Perry, A. J. On the existence of point defects in physical vapor deposited films of TiN, ZrN, and HfN. J. Vac. Sci. Technol. A 6, 2140–2148 (1988).
Christel, P., Meunier, A., Heller, M., Torre, J. P. & Peille, C. N. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. 23, 45–61 (1989).
Gregoire, J. M., Kirby, S. D., Turk, M. E. & van Dover, R. B. Structural, electronic and optical properties of (Sc,Y)N solid solutions. Thin Solid Films 517, 1607–1609 (2009).
Ma, L. et al. Phase stability, anisotropic elastic properties and electronic structures of C15-type Laves phases ZrM2 (M = Cr, Mo and W) from first-principles calculationss. Philos. Mag. 97, 2406–2424 (2017).
Dahlqvist, M., Jansson, U. & Rosen, J. Influence of boron vacancies on phase stability, bonding and structure of MB2 (M = Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W) with AlB2 type structure. J. Phys. Condens. Matter 27 (2015).
Vajeeston, P., Ravindran, P., Ravi, C. & Asokamani, R. Electronic structure, bonding, and ground-state properties of AlB2-type transition-metal diborides. Phys. Rev. B Condens. Matter 63, 045115 (2001).
Liu, D., Duan, Y. & Bao, W. Correlation between electronic structure, mechanical properties and phase stability in intermetallic compounds. Ceram. Int. (2018).
Ravindran, P. & Asokamani, R. Correlation between electronic structure, mechanical properties and phase stability in intermetallic compounds. Bull. Matter. Sci. 20, 10185 (1997).
Wang, X.-B., Tian, D.-C. & Wang, L.-L. The electronic structure and chemical stability of the AlB2-type transition-metal diborides. J. Phys. Condens. Matter 6 (1999).
Fahrenholtz, W. G., Hilmas, G. E., Talmy, I. G. & Zaykoski, J. A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 90, 1347–1364 (2007).
Frotscher, M. et al. M2B5 or M2B4? A Reinvestigation of the Mo/B and W/B System. Anorg. Allg. Chem. 633, 2626–2630 (2007).
Otani, S., Ohashi, H. & Ishizawa, Y. Solid solution ranges of zirconium diboride with other refractory diborides: HfB2, TiB2, TaB2, NbB2, VB2 and CrB2. J. Alloys. Compd. 221, L8–L10 (1995).
Pettifor, D. G. Theoretical predictions of structure and related properties of intermetallics. Mater. Sci. Technol. 8, 345–349 (1992).
Ali, M. A., Hadi, M. A., Hossain, M. M., Naqib, S. H. & Islam, A. K. M. A. Theoretical investigation of structural, elastic, and electronic properties of ternary boride MoAlB. Phys. Status Solidi (2017).
Mir, S. H. et al. Static and dynamical properties of heavy actinide monopnictides of lutetium. Sci. Rep. 6, 29309 (2016).
Pugh, S. F. XCII. relations between the elastic moduli and the plastic properties of polycrystalline pure metals. The London, Edinburgh, Dublin Philos. Mag. J. Sci. 45, 823–843 (1954).
Sammalkorpi, M., Krasheninnikov, A., Kuronen, A., Nordlund, K. & Kaski, K. Mechanical properties of carbon nanotubes with vacancies and related defects. Phys. Rev. B Condens. Matter 70, 245416 (2004).
Venkatesan, M., Fitzgerald, C. B. & Coey, J. M. D. Thin films: unexpected magnetism in a dielectric oxide. Nat. 430, 630 (2004).
Woods, H. P., Wawner, F. E. Jr. & Fox, B. G. Tungsten diboride: preparation and structure. Science 151, 75 (1966).
Jiang, C. et al. Preparation and characterization of superhard AlB2-type WB2 nanocomposite coatings. Phys. Status Solidi 210, 1221–1227 (2013).
Motojima, S. & Sugiyama, K. Chemical vapour deposition of tantalum diboride. J. Mater. Sci. 14, 2859–2864 (1979).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Yu, R., Zhu, J. & Ye, H. Q. Calculations of single-crystal elastic constants made simple. Comput. Phys. Commun. 181, 671–675 (2010).
van de Walle, A., Asta, M. & Ceder, G. The alloy theoretic automated toolkit: A user guide. CALPHAD 26, 539–553 (2002).
Hao, X. et al. Low-compressibility and hard materials ReB2 and WB2: Prediction from first-principles study. Phys. Rev. B Condens. Matter 74, 224112 (2006).
Acknowledgements
The computational results presented have been achieved using the Vienna Scientific Cluster (VSC). The financial support by the Austrian Federal Ministry of Economy, Family and Youth and the National Foundation for Research, Technology and Development is gratefully acknowledged. We also thank for the financial support of Plansee Composite Materials GmbH and Oerlikon Balzers, Oerlikon Surface Solutions AG. The authors state that the project is funded within the framework of the Christian Doppler Laboratory for Application Oriented Coating Developement which is partly supported by the Austrian Federal Ministry of Economy, Family and Youth and the National Foundation for Research, Technology and Development. The conducted research was carried out within this framework including the cooperation between the Laboratory (located at TU Wien) and Oerlikon Surface Solutions AG as well as Plansee Composite Materials GmbH. The authors acknowledge the TU Wien University Library for financial support through its Open Access Funding Program.
Author information
Authors and Affiliations
Contributions
V.M., D.H. and P.H.M. conceived the present idea. V.M. and F.C. performed the calculations. V.M., H.R. and P.H.M. analyzed the data and shaped the study, especially creating all figures and text. All authors (V.M., H.R., C.F., P.P., H.B., D.H. and P.H.M.) discussed the results, provided critical feedback and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing Interests
V.M., R.H., C.F., D.H. and P.H.M. are funded by the Christian Doppler Laboratory. P.P. and H.B. are employees of the cooperating companies Plansee Composite Materials GmbH and Oerlikon Surface Solutions AG, respectively.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moraes, V., Riedl, H., Fuger, C. et al. Ab initio inspired design of ternary boride thin films. Sci Rep 8, 9288 (2018). https://doi.org/10.1038/s41598-018-27426-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27426-w
This article is cited by
-
Ceramic transition metal diboride superlattices with improved ductility and fracture toughness screened by ab initio calculations
Scientific Reports (2023)
-
Effect of zirconium doping on the mechanical properties of \(W_{1-x}Zr_{x}B_2\) on the basis of first-principles calculations and magnetron sputtered films
Archives of Civil and Mechanical Engineering (2022)
-
Tuning structure and mechanical properties of Ta-C coatings by N-alloying and vacancy population
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.