Abstract
Socioeconomic and climatic factors are modifying fire regimes with an increase of fire frequency and extension. Unfortunately, the effects of recurrent fires on biological processes that ultimately affect the genetic diversity of animal populations are mostly unknown. We examined genetic patterns of diversity in the wall lizard Podarcis guadarramae in northern Portugal, one of the European regions with the highest percentage of burnt land. This species is a small saxicolous lizard as it inhabits natural outcrops and artificial stone walls, likely in recurrent-fire landscapes. We genotyped nine microsatellites from ten populations selected according to a gradient in fire recurrence, and compared genetic diversity indexes and demographic patterns among them. At the population level, we hypothesize that a high level of mortality and population bottlenecks are expected to reduce genetic heterozygosity in sampled localities affected by recurrent fires. Alternatively, genetic signatures are expected to be absent whether fire did not cause high mortality. Regardless of levels of mortality, we expect a gain in genetic diversity whether recurrent fires facilitate lizard dispersal and migration due to the increased quality of the habitat for wall lizards. At the regional level, we examine whether a recurrent fire regime may disrupt the spatial structure of populations. Our results showed an increase in genetic diversity in recurrently burnt populations, and a decline in longer-unburnt populations. We did not detect bottleneck effects in repeatedly-burnt populations. High genetic diversity in recurrent fire populations suggests a high dispersion rate between adjacent metapopulations and perhaps immigration from outside the fire boundary. At the regional level, lizard populations show low differentiation and weak genetic structure, suggesting no effects of fire. This study confirms field-based censuses showing that recurrent-fire regimes give ecological opportunities to wall lizards that benefit from habitat openness.
Similar content being viewed by others
Introduction
Wildfires have played a determining role in shaping the evolution and function of many ecosystems around the world1,2. More than a local ecological disturbance, fire is a global ecosystem process2, with approximately one third of the land mass experiencing intensive burning3. In the last decades, fire-prone regions as the western Mediterranean are experiencing a major shift in the fire regime with an increase of fire frequency and extension4. The main driver of this shift is socioeconomic, i.e. increased amount of fuel due to rural abandonment4,5, in parallel to a global climate warming6,7. Shifts in particular fire-regimes can have devastating impacts on the sustainability of many ecosystem components8.
Fire shapes community composition for many taxonomic groups especially due to its major effect on the habitat structure9. Understanding the emergent genetic patterns of diversity resulting from habitat disturbance is critical in predicting how species will adapt to and persist in changing environments. However, the role of fire-regime as a driver of the patterns and distribution of genetic diversity is poorly understood10,11. Due to their mobility, the response of animals to fire is complex12, and the lack of information about the genetic patterns resulting from responses of organisms to fire is especially relevant13. To understand this issue, one must consider that the demographic impact of fire may depend on particular life history traits, such as dispersal ability, environmental requirements14, and resistance to fire15. The complexity builds up when both mortality and recruitment interact to determine the consequences of fire on within-population genetic diversity11. On the one hand, high mortality translates into population bottleneck, thereby reducing effective population sizes and producing a random loss of genetic diversity16. On the other hand, post-fire population recovery can stem from either survivors or through colonizers from adjacent unburnt areas17, which may also have an impact on the distribution patterns of genetic diversity. The loss of allelic diversity will be low if there is either a high survival rate18 or if recruitment comes from multiple sources19,20. Post-fire population dynamics, such as genetic drift and/or migration, can affect genetic differentiation between populations21,22. Conversely, if post-fire migration is facilitated between populations, increased gene flow can reduce genetic differentiation23,24. This is the case in open-habitat or early colonizer species, which increase their local density and genetic variation in recently burnt sites19,20. In contrast, genetic diversity in long-unburnt specialists may decrease due to habitat fragmentation patterns imposed by fire25.
In this study, we have investigated the effects of a recurrent fire regime on the genetic diversity and spatial structure of the wall lizard Podarcis guadarramae26 in northern Portugal. Wildfire is one of the most important agents of landscape change in Portugal27. The study area, in particular, is one of the European regions with the highest percentage of burnt land due to a combination of socioeconomic (continuous surface of highly flammable eucalyptus and pine forests) and climate (high rainfall rate episodes followed by dry periods) factors. Podarcis guadarramae is a small-size saxicolous wall lizard28 that uses rocks, trunks, and bare ground for thermoregulation, foraging and shelter29. In northern Portugal, this lizard has a patchy distribution with populations located in natural open rocky outcrops and artificial stone walls surrounding agriculture fields26. The forested and bush matrix isolates lizard populations and hampers inter-population contact due to the low dispersion ability of this species through vegetated areas. Wildfire reduces vegetation cover, increases the extent of open outcrops, and allows lizard dispersion among patches with favourable (open) habitats. These life-history traits can explain the positive population responses of P. guadarramae to fire observed in northern Portugal30,31. However, the demographic processes (e.g. mortality, survival, migration) underlying the positive response of wall lizards to fire are unknown. For this reason, we have collected lizard samples in ten populations selected according to a gradient of disturbance level (i.e. number of fires and time since the last fire). The objective of this study was to examine how recurrent fire regimes affect: i) at the population level, the genetic diversity of each lizard population sampled, and ii) at the regional level, the geographic/genetic structure of the sampled P. guadarramae populations. Emergent genetic patterns of diversity can help to understand demographic processes linked to fire recurrence and habitat use. Based on P. guadarramae life-history traits, we have addressed the following hypotheses.
At the population level, if fire causes high mortality rate, wall lizard populations located on rocky outcrops that have experienced higher number of fires are expected to exhibit genetic signatures consistent with recent demographic bottlenecks and low genetic diversity. Alternatively, such genetic signatures are expected to be absent (i.e. no diversity loss) when fire does not cause severe mortality. Moreover, if recurrent fires improve habitat quality and facilitate lizard dispersal and migration, genetic diversity gains are expected regardless of lizard mortality caused by fire.
At the regional level, we hypothesize that fire can shape population structure due to the expected fire impacts over the genetic diversity and ultimately over population differentiation. Accordingly, we expect fire to disrupt the spatial structure of populations by decreasing the naturally expected correlation between genetic and geographic distances.
Results
From the initial battery of nine microsatellite loci genotyped, one (Ph17) showed presence of null alleles in seven out of 10 populations (Supplementary material Table S1) and for this reason it was excluded from further analyses. Five out of the remaining eight loci showed evidence for the presence of null alleles and/or stuttering (Supplementary material Table S1); however, these patterns affected only a minority of populations. Despite the existence of null alleles, no deviations from either HWE or LE were found in the eight loci, and therefore we opted not to exclude any other loci.
Mean number of alleles per locus was 7.73 (±0.67 s.d.; range 5–19 alleles). Overall, the expected heterozygosity (HE) of the eight loci showed moderate values, ranging from 0.663 to 0.732 (average 0.694 ± 0.019), while the observed heterozygosity averaged 0.647 (±0.026). The allelic richness (AR) per population ranged from 5.922 to 7.763, whereas the mean number of alleles (NA) per population varied from 6.125 to 8.625 (Table 1). Four of the populations sampled had private alleles, especially those populations subjected to recurrent fire-regimes (Table 1). AR, NA and HE diversity indices showed a general pattern of increasing diversity values from unburnt towards burnt populations, with significant differences detected for three out of five pairs, suggesting that fire changed the pattern of genetic diversity. When pools of populations with the similar condition (unburnt vs. repeatedly burnt) were compared, a clear difference was reported for NA and PA (Table 1). Similarly, there was a trend of increasing FIS towards the repeatedly burnt populations detected for all pairs, although pairwise differences within each pair was only significant for one population (Table 1).
All genetic diversity measures (AR, NA, HE)) and FIS were positively correlated with the number of fires that occurred at each population (Fig. 1A for AR, and Supplementary material Fig. S2 for remaining metrics). Moreover, these measures were negatively correlated with the time since the last fire occurred at each population (Fig. 1B for AR, and Supplementary material Fig. S3 for the rest of the metrics).
Almost all population pairs showed significant, although low, allele-frequency-based differentiation (Supplementary material Table S4). Significant pairwise FST values were low (0.015–0.088). With exception of Moledo pair, all remaining locations showed no significant differentiation regarding the pairs of populations. AMOVA results revealed that all levels of genetic differentiation were significant (Table 2). Most of the genetic variation was found within populations (96.2%); only a small but significant percentage was attributed to the variation among the five population pairs (2.17%), and to between pairs of populations within each pair (1.63%), which corroborates the fact that almost all pairwise FST values were significant.
The Mantel test showed a significant correlation between genetic distance (Supplementary material Table S5) and log-transformed geographical distance (rM = 0.547; P < 0.0001; 999 permutations; Fig. 2B), meaning that the observed differences were most likely a result of spatial structure and isolation by distance and not fire history. This result can be visualized in the PCoA (the two coordinate axes together explained 71.98% of the total genetic variation) since it mirrors the paired organized spatial location of sampled populations (Fig. 2).
Patterns of genetic variation plotted with a Principal Coordinate Analysis based on Slatkin’s linearized FST (FST/(1 − FST)) genetic distance between all the ten populations sampled (A), and relationship between FST (FST/(1 − FST)) genetic distance and log-transformed geographic distances (in km) between populations (B).
Bottleneck test results were not robust to changes in the assumptions regarding mutation models (Table 3). M-ratio values were generally high across populations, suggesting low general evidence for bottlenecks. Nevertheless, there were five localities (notably including both unburnt and burnt populations) in which M-ratios fall below the highest Mc threshold which corresponds to the assumptions of the strict SMM (which is unlikely to fit most microsatellites) and 4Neµ = 5 (see Supplementary material Table S6). Only two unburnt populations (Leonte and Póvoa de Lanhoso) showed M-ratios lower than the Mc values calculated relaxing the assumptions of the strict SMM, but these values are still quite high and hence not robust to small changes in model assumptions. With respect to the heterozygosity excess test, results ranged from significant heterozygosity excess in all populations (when assuming the IAM) to no evidence of bottleneck in any population (when assuming the SMM). When fitting the TPM, the evidence varied along the proportion of single step mutations included in the model. Assuming the default parameters incorporated in BOTTLENECK (70% of mutations fitting the SMM, with a variance of 30%), only two unburnt localities showed significant heterozygote excess, as suggested by the Wilcoxon test. In summary, comparing the various tests, only the unburnt population located at Leonte showed consistent evidence for the occurrence of a bottleneck. Even though results are not robust to changes in the mutation model, it is clear that the evidence is not larger for bottlenecks in repeatedly burnt, when compared to unburnt, localities.
Discussion
Our study design (a gradient of recurrent-fire histories) provided the opportunity to perform tests to enlighten which genetic patterns and processes are driven by the occurrence of recurrent wildfires, and to extend the knowledge in this research field that is currently lacking information11,13. We demonstrated emergent genetic patterns of diversity resulting from demographic responses to fire by the wall lizard P. guadarramae at population level (i. e. genetic diversity indexes), but not at regional level (i. e. relationship between genetic and geographic distances). Due to its environmental requirements of open outcrops32, P. guadarramae benefits from recurrent burned landscapes dominated by granitic rocks where lizard populations achieve high effective population sizes30,33. The positive response of P. guadarramae to fire in northern Portugal30,31 showed that recurrent-burnt landscapes give ecological opportunities to this wall lizard that seem to be related with the increase of genetic variability in the repeatedly burnt populations.
Wildfires alter the patterns of genetic diversity in P. guadarramae
Population recovery following major disturbances such as wildfires is likely a reflection of two non-mutually exclusive biological processes: i) abundance of survivors; and ii) post-fire recruitment (e.g. local reproduction or immigration)13,16,17. Some studies have documented a short-term post-fire decline of fire-sensitive species34, although studies on vertebrates have shown that fires rarely cause complete mortality and that residual populations may remain in situ in burnt areas16,18, specifically cliff- and rock-specialist species for which habitat is not destroyed by fire34. Biological processes, such as mortality, survival, and post-fire recolonization can affect genetic diversity in contrasting ways:11 reductions in heterozygosity and allelic richness resulting from population bottlenecks22; increases in genetic diversity and reduced regional genetic differentiation resulting from high immigration rates19,20; and intermediate values of unchanged genetic diversity when mortality is low or is compensated by immigration18.
Analyses of genetic diversity helped us to unravel how recurrent fires affect the population dynamics of P. guadarramae. Our results clearly showed that fires altered the genetic diversity of wall lizards. Our first expectation was that fires could alter patterns of genetic diversity in burnt populations by decreasing it through successive bottlenecks and founder effects. These results have been found in populations of butterflies25 and lizards23 and have been further attributed to bottlenecks in several taxa such as mammals35, birds22, and lizards36. Consequently, burnt populations would become more differentiated from unburnt populations by means of genetic drift. However, our results did not meet this expectation. Interestingly, we obtained the opposite pattern: significantly increasing allelic diversity with number of fires, and conversely, a significant decreasing of allelic diversity with time since the last fire. This trend was valid for the overall correlation between fire and genetic diversity parameters, and also, in general, to local pairwise comparisons.
We suggest that the pattern of higher genetic diversity (mean number of alleles, allelic richness, private alleles, expected heterozygosity) found in repeatedly burnt populations of P. guadarramae can be explained by a combination of both high survival rate, mobility, immigration, and a post-fire increase in the carrying capacity of disturbed habitats.
The likelihood of immediate survival of an individual during a fire will be influenced by the severity of the fire, its distance to potential refuges and behavioural mechanisms the organisms may use to avoid direct flames and heat16,37. Refuges enhance immediate survival during a fire event but can also facilitate the post-fire persistence of individuals and populations within the burnt landscape by providing resources in the short- (food, shelter) or long-term (resident habitat) from a series of disjunct isolated populations to a metapopulation, and to a patchy population linked by frequent movements16,17,38,39.
The response of P. guadarramae to fire has been demonstrated in several demographic studies in the region30,31. Physiological experiments40,41 and habitat preference studies have highlighted that this lizard copes very well with post-fire habitat conditions. These studies and our personal observations in recently burnt spots support the increased dispersal ability of individuals in post-fire habitats that brings new diversity (and increase allelic richness and heterozygosity). Increased dispersal activity in recently burnt habitats was the cause of increased genetic diversity in the agamid Amphibolurus norrisi in Australia42. Schrey, Ashton, et al. (2011) also found increased genetic diversity in burnt areas, in one of the examined lizard species. They tested a metapopulation source/sink model based on the habitat preferences of each species, by examining the direction of gene flow (i.e. immigration into or out of preferred habitat). The burnt area indeed received influx of individuals from open-habitat species, increasing genetic diversity. In other taxonomic groups with higher dispersal capability, a post-fire increase in genetic diversity was also reported; e.g. the mountain brushtail possum Trichosurus cunnunghami43, and the Gran Canaria chaffinch Fringilla teydea polatzeki18. A combination of life history traits (e.g. dispersal abilities), and habitat characteristics (fire-adapted vegetation, use of unaffected refuges by fire) could have contributed to increase genetic diversity.
Our results suggest that refuges are preponderant both in the survival rate of wall lizards as well as for colonizers. Podarcis guadarramae is a highly saxicolous lizard and it is associated with rocky habitats28,44. Likewise, its preference for these habitats with a naturally high number of crevices and burrows, where the lizard could take refuge during the fire events, may allow a considerable level of survival among animals (see a similar result for the saxicolous gecko Tarentola mauritanica34), allowing the retention of a large fraction of the pre-fire genetic diversity. This fact is in line with the lack of evidence for severe bottlenecks in burnt populations.
Moreover, given that fire clears vegetation and exposes previously unsuitable rocky outcrops, unoccupied habitats represent a new ecological opportunity for wall lizards. This not only supports an increase in the local effective population size, but also allows the dispersal of individuals between metapopulations, and potentially, recruitment and settlement of migrants from outside the affected area. Both mobility and migration can positively increase genetic diversity at the population level. Our results shown an increase in FIS in recently burnt localities, which may be in line with Wahlund effects caused by recent migrants from different gene pools45. We acknowledge that this hypothesis cannot be corroborated by the absence of pre-fire genetic data nor movement data. However, the negative correlation between genetic diversity and the time since the last fire was indirect evidence of the mobility/migration lizard pattern in burnt populations. Indeed, long-unburnt localities showed reduced genetic diversity of lizard populations likely by vegetation re-growth that reduces lizard mobility and isolates small populations in small rocky spots. The incorporation of knowledge of both direct, in particular the demographic and mobility processes13, and indirect effects (shift in habitat structure) of fire is important to understand the underlying processes behind the patterns of genetic diversity found11.
Wildfires did not alter the patterns of geographic differentiation in P. guadarramae
The observed general increments of genetic differentiation according to geographic distance (isolation by distance) and not to fire history, suggested limited gene flow among all P. guadarramae populations. This result configures a classical metapopulation scenario, especially when individuals show low dispersal ability, like the lizard case46, and matches findings from closely related species (P. bocagei and P. carbonelli) also inhabiting western Iberia, in which, similarly low or slightly higher levels of population differentiation were reported across their distribution areas, yet with a clear correspondence to geography (Pinho, Harris, & Ferrand47; Pinho, Kaliontzopoulou, Harris, & Ferrand48).
Reptiles show strong succession responses to fire49 that are driven by habitat structure changes and are a product of species life history (Santos et al.34; Smith50). Thus, as general rule, the open-habitat dwelling species are favoured by post-fire habitat conditions, then, while the ecological succession is recovering, forest-species increase in abundance whereas open-habitat species tend to decrease (Santos et al.34; Valentine, Reaveley, Johnson, Fisher, & Wilson51). In our study areas (i.e. locations), these metapopulations could be spatially arranged according to the distribution of rocky and agricultural (walls) areas given its saxicolous ecology28.
Concluding remarks
Due to their particular life-history traits (small size, low mobility, ectothermy, low reproductive recruitment), reptiles are important organisms to examine in their responses to fire at different complementary approach levels, i. e. demographic, genetic, ecologic. Given the saxicolous preferences of P. guadarramae, all the population trends suggest that this lizard benefits from recurrent fires. Microhabitat preference and post-fire dispersal seem to be traits that help this lizard to resist fire and persist afterwards. Mobility is an attribute that makes fauna a challenging group to examine their responses to fire12. However, data on this trait needs to be integrated with other biological traits to capture all relevant information in predicting how reptile populations respond to recurrent disturbances50. Integrative approaches are necessary to forecast biodiversity patterns in future scenarios of shifts in fire regime.
Methods
Study species: the wall lizard Podarcis guadarramae
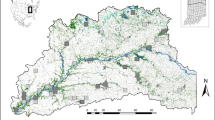
Wall lizards of the genus Podarcis (Squamata: Lacertidae) are among the most conspicuous, abundant and widely distributed reptiles in Europe and North Africa. Podarcis guadarramae occurs in northern Portugal, north-western Spain, and Central Iberian Mountains in Spain (Catarina Pinho, Ferrand, & Harris52; Fig. 3). The populations that are the object of this study correspond to the north-western subspecies, P. g. lusitanicus. It is a saxicolous and small (50–70 mm adult snout-to-vent length) diurnal lacertid lizard28 with Atlantic ecological affinities53. Its depressed body shape and flattened skull facilitate entrance into narrow, irregular crevices. Individuals aggregate around favourable open areas with rock crevices, artificial walls, and isolated big blocks33.
Fire regime, site selection and experimental design
Portugal is currently one of the European regions with the highest percentage of burnt land54,55,56. Fire frequency and intensity have increased remarkably since the 1960s57,58, due to a combination of socioeconomic (i.e. rural abandonment and traditional practices (Moreira et al., 2001); plantations of fire-prone tree species such as eucalypt and pines (Fernandes et al.59; Moreira et al., 2001) and environmental factors (hot, dry summers and cool, wet winters; Nunes et al., 2005). Coincident with P. guadarramae distribution, the northern part of the country has the highest fire frequency and size (Nunes et al., 2005; Pereira et al., 2006) due to the dominance of fire-prone tree species (maritime pine, Pinus pinaster; and Eucalyptus spp.) and shrubs (genus Erica, Calluna, Ulex and Cytisus; Carmo, Moreira, Casimiro, & Vaz60; Scotto et al.61).
The sampling area (latitudinal range: 41°05′ to 42°15′ N, and longitudinal range: −8°90′ to −7°76′ W) was located in the transition between the Mediterranean and Eurosiberian biogeographic zones close to the Atlantic coast62 with a transitional climate from Atlantic (NW) to Mediterranean (NE). Average annual precipitation ranges from approximately 1200 mm at the coast to 2800 mm at the upper elevations in the interior, mostly falling between October and April and with 0–2 rainless months. The average annual temperature is 14.5 °C, mean maximum and minimum temperatures are respectively 25 °C in July and 4 °C in January63. Native forests have become very fragmented, and the characteristic species are Quercus robur, Q. pyrenaica, Acer pseudoplatanus, Pinus pinaster, Pyrus cordata, and Ilex aquifolium. The most common type of vegetation are fire-prone scrub communities composed by genus Erica, Calluna, Ulex and Cytisus64.
To test how the repeated fire regime that characterizes the study area affected genetic variation in the wall lizard P. guadarramae, we selected ten locations based on a digital national cartography of burnt areas from 1975 to 2013 (http://www.icnf.pt/portal/florestas/dfci/inc/info-geo). These locations followed a gradient of recurrent fires and time-since-fire periods. Burnt locations had 5.8 fires on average (range 2–8 fires) during the period 1975–2013 (Supplementary material Table S7. Moreover, locations were selected following a paired-population sampling scheme, each pair composed with locations with contrasting fire histories (from unburnt to repeatedly burnt). Pairs of locations were located on average at 4.9 km distance between each (range 2.0–12.5 km per location; Fig. 3). This sampling design avoids spatial pseudo-replication in fire regimes, and minimizes the potential effect of spatial structuring to mask the effect of recurrent fires on the genetic diversity.
Sampling was conducted between July-October 2014. Except one recurrently burnt population, at least 20 individuals were sampled from each population. Individuals were captured using a noose or by hand. Sampling syntopic individuals was avoided as well as sampling juveniles. The tail tip of each individual was collected and stored in 96% ethanol. All individuals were then released back to their collection site. The experiments were performed in accordance with general guidelines and regulations of live vertebrates. The experimental protocol was approved by the Instituto da Conservação da Natureza e das Florestas (ICNF) from the Portuguese Government that provided the permit for sampling lizards in northern Portugal (number 578/2014/CAPT).
Laboratory procedures
DNA was extracted from tail muscle of 201 P. guadarramae lizards using the EasySpin Genomic DNA Tissue Kit (Citomed, Portugal) following the manufacturer’s instructions. Samples were genotyped at nine microsatellite loci chosen based on further multiplex optimization and reports of scoring errors by Ribeiro65; Supplementary material Table S8) which modifies those reported in Agostini et al.66. PCR products were then separated by size on an automatic sequencer ABI 3130xl Genetic Analyser (Applied Biosystems, U.S.A.) with size standard GS500 LIZ (Applied Biosystems, U.S.A.). Allele scoring was performed with GeneMapper v4.1 (Applied Biosystems, U.S.A.) and checked manually.
Microsatellite data analyses
The final data set included 201 samples genotyped for nine microsatellite loci. No individual or locus was excluded based on missing data. We used MICRO-CHECKER v2.2.367 to test for the presence of null alleles or scoring errors. We used ARLEQUIN v3.568 to test for departure from Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium (LD). Bonferroni corrections were applied due to multiple tests69. With the final data set, we performed three types of analyses: i) population genetics; ii) population differentiation; and iii) bottleneck tests.
Analysis of genetic diversity
Using Arlequin, we calculated allele frequencies, expected (HE) and observed (HO) heterozygosities, mean number of alleles (NA), and number of private alleles (PA) for each population. We also calculated allelic richness (AR; calculated using a rarefaction methodology considering the lowest number of individuals genotyped in a locality sensu El Mousadik & Petit70) and inbreeding coefficient FIS71 with FSTAT v2.9.3.272.
Differences in genetic diversity indexes (NA, PA, HE, and FIS) between each population pair, and between burnt and unburnt populations as a whole were explored using a permutation approach with 999 permutations. These analyses were performed with Python 2.7 scripts (available from the authors upon request).
We further explored the relationship between genetic diversity indices (NA, AR, HE, FIS) and fire history variables (number of fires, time since the last fire) by using simple linear regression in STATISTICA v8.073. Due to the independence of each fire event, these analyses disregard the paired sampling design and focus on an overall comparison of fire-history and genetic diversity among all ten populations sampled.
Analysis of population differentiation
To test for genetic differentiation among all populations pairs, pairwise FST values were calculated. A hierarchical population structure was evaluated through an analysis of molecular variance (AMOVA) grouping by pairs of location in order to test the null hypothesis that genetic variation was not associated with spatial structure. Both analyses were performed in ARLEQUIN using 10,000 permutations.
Geographic distances between populations were calculated from the pairwise Euclidean distance according to the latitude and longitude of each location with Geographic Distance Matrix Generator, version 1.2.3 (Ersts, American Museum of Natural History, Center for Biodiversity and Conservation, http://biodiversityinformatics.amnh.org/open_source/gdmg). We tested for isolation by distance by regressing log transformed geographic distances (in km) against Slatkin’s linearized FST (FST/(1 − FST)). Statistical significance was examined by Mantel test with 999 permutations in PASSaGE74. A Principal Coordinate Analysis (PCoA) was computed in GenAlex 6.575,76 to visualize patterns of genetic differentiation among populations.
Bottleneck tests
Bottlenecks (such as those expected to derive from the occurrence of wildfires) can be detected by taking into account patterns of microsatellite allele size distribution and/or frequency data77,78,79. To evaluate the hypothesis that wildfires caused a significant reduction in population sizes in lizard populations, we employed two commonly used methods to detect population bottlenecks: Garza and Williamson’s (2001) M- ratio, and a test for heterozygosity excess developed by Cornuet & Luikart77. This test is based on the principle that, when populations experience a sudden decrease in population size, the number of alleles decreases faster than heterozygosity. Hence, a transient excess in heterozygosity can be detected in bottlenecked populations when compared to mutation-drift equilibrium expectations. We used the software BOTTLENECK v. 1.280 to evaluate such heterozygosity excess. Because the expected patterns of variation vary extensively depending on the mutation model assumed, the test of the robustness of the inferences was performed under the infinite allele model (IAM, Kimura & Crow81), the strict stepwise mutation model (SMM, Ohta & Kimura82), and under a wide range of two-phase mutation model (TPM, Di Rienzo et al.83) parameters. BOTTLENECK provided several tools to compare hypothesis; we chose the Wilcoxon’s test, since it has been shown to be the most powerful, especially for data sets with a small number of loci such as in our case80.
The M-ratio test quantifies gaps in the distribution of microsatellite allele sizes. Under a bottleneck, the number of alleles k is expected to decrease faster than allele range r. Values of M = k/r lower than expected given equilibrium expectations may thus be indicative of a bottleneck. We used the R package strataG84 to perform the calculation of M and the script “Critical_M” (obtained at https://swfsc.noaa.gov/textblock.aspx?Division=FED&id=3298) was used to determine the “Critical M” (Mc) value. This program calculates the M-ratio from simulated data sets under equilibrium for a given set of relevant parameter values and returns the value representing the fifth percentile of this distribution. We calculated Mc based on the sample size and the number of loci specific to our study. We also used a range of reasonable parameter values for the pre-bottleneck population mutation rate (from 5 to 20) and for the microsatellite mutation model parameters, including both the strict SMM as well as allowing for larger size mutations in varying proportions (see Supplementary material for details).
Both types of tests rely up to a great extent on the mutation model assumed for microsatellite loci. Except in the case of the IAM, the mutation models typically used for microsatellite data do not tolerate the existence of alleles resulting from insertions and deletions in the flanking regions. An observation of allele sizes in our case revealed the existence of a few alleles not compatible with strict repeat-number mutations. Therefore, to accommodate our data to the models under which these hypotheses were tested, we performed the analyses described above in a reduced data set resulting from the removal of the loci that, in each population, deviated from the expected repeat number pattern. This resulted in data sets containing six loci in all localities, except Santo Tirso – burnt, where five loci were retained.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Bond, W. J. & Keeley, J. E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends in Ecology and Evolution 20, 387–394 (2005).
Bowman, D. M. J. S. et al. Fire in the Earth System. Science (80-.). 324, 481–484 (2009).
Chuvieco, E., Giglio, L. & Justice, C. Global characterization of fire activity: Toward defining fire regimes from Earth observation data. Glob. Chang. Biol. 14, 1488–1502 (2008).
Pausas, J. G. & Fernández-Muñoz, S. Fire regime changes in the Western Mediterranean Basin: from fuel-limited to drought-driven fire regime. Clim. Change 110, 215–226 (2012).
Chergui, B., Fahd, S., Santos, X. & Pausas, J. G. Socioeconomic Factors Drive Fire-Regime Variability in the Mediterranean Basin. Ecosystems 21, 619–628 (2018).
Batllori, E., Parisien, M.-A., Krawchuk, M. A. & Moritz, M. A. Climate change-induced shifts in fire for Mediterranean ecosystems. Glob. Ecol. Biogeogr. 22, 1118–1129 (2013).
Bedia, J., Herrera, S., Camia, A., Moreno, J. M. & Gutiérrez, J. M. Forest fire danger projections in the Mediterranean using ENSEMBLES regional climate change scenarios. Clim. Change 122, 185–199 (2014).
Pausas, J. G. & Keeley, J. E. Abrupt Climate-Independent Fire Regime Changes. Ecosystems 17, 1109–1120 (2014).
Swan, M., Christie, F., Sitters, H., York, A. & Di Stefano, J. Predicting faunal fire responses in heterogeneous landscapes: the role of habitat structure. Ecol. Appl. 25, 2293–2305 (2015).
Hughes, A. R., Inouye, B. D., Johnson, M. T. J., Underwood, N. & Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 11, 609–623 (2008).
Banks, S. C. et al. How does ecological disturbance influence genetic diversity? Trends Ecol. Evol. 28, 670–679 (2013).
Pausas, J. G. & Parr, C. L. Towards an understanding of the evolutionary role of fire in animals. Evol. Ecol. 32, 113–125 (2018).
Steinitz, O., Shohami, D., Ben-Shlomo, R. & Nathan, R. Genetic consequences of fire to natural populations. Isr. J. Ecol. Evol. 58, 205–220 (2012).
Izhaki, I. The Impact of Fire on Vertebrates in the Mediterranean Basin: An Overview. Israel Journal of Ecology and Evolution 58, 221–233, Available at: https://www.tandfonline.com/doi/abs/10.1560/IJEE.58.2-3.221?needAccess=true&journalCode=tiee20. (2012).
Budde, K. B. et al. Increased fire frequency promotes stronger spatial genetic structure and natural selection at regional and local scales in Pinus halepensis Mill. Ann. Bot. 119, 1061–1072 (2017).
Banks, S. C. et al. Starting points for small mammal population recovery after wildfire: recolonisation or residual populations? Oikos 120, 26–37 (2011).
Robinson, N. M. et al. REVIEW: Refuges for fauna in fire-prone landscapes: their ecological function and importance. J. Appl. Ecol. 50, 1321–1329 (2013).
Suárez, N. M., Betancor, E., Fregel, R., Rodríguez, F. & Pestano, J. Genetic signature of a severe forest fire on the endangered Gran Canaria blue chaffinch (Fringilla teydea polatzeki). Conserv. Genet. 13, 499–507 (2012).
Pereoglou, F. et al. Landscape genetics of an early successional specialist in a disturbance-prone environment. Mol. Ecol. 22, 1267–1281 (2013).
Schrey, A. W. et al. Broad-scale latitudinal patterns of genetic diversity among native European and introduced house sparrow (Passer domesticus) populations. Mol. Ecol. 20, 1133–1143 (2011).
Templeton, A. R., Robertson, R. J., Brisson, J. & Strasburg, J. Disrupting evolutionary processes: the effect of habitat fragmentation on collared lizards in the Missouri Ozarks. Proc. Natl. Acad. Sci. USA 98, 5426–32 (2001).
Brown, S. M., Harrisson, K. A., Clarke, R. H., Bennett, A. F. & Sunnucks, P. Limited Population Structure, Genetic Drift and Bottlenecks Characterise an Endangered Bird Species in a Dynamic, Fire-Prone Ecosystem. PLoS One 8, e59732 (2013).
Schrey, A. W., Ashton, K. G., Heath, S., McCoy, E. D. & Mushinsky, H. R. Fire Alters Patterns of Genetic Diversity Among 3 Lizard Species in Florida Scrub Habitat. J. Hered. 102, 399–408 (2011).
Pierson, J. C., Allendorf, F. W., Drapeau, P. & Schwartz, M. K. Breed Locally, Disperse Globally: Fine-Scale Genetic Structure Despite Landscape-Scale Panmixia in a Fire-Specialist. PLoS One 8, e67248 (2013).
Fauvelot, C., Cleary, D. F. R. & Menken, S. B. J. Short-term impact of disturbance on genetic diversity and structure of Indonesian populations of the butterfly Drupadia theda in East Kalimantan. Mol. Ecol. 15, 2069–2081 (2006).
Geniez, P., Sá-Sousa, P., Guillaume, C. P., Cluchier, A. & Crochet, P. A. Systematics of the Podarcis hispanicus complex (Sauria, Lacertidae) III: Valid nomina of the western and central Iberian forms. Zootaxa 3794, 1–51 (2014).
Silva, J. S., Vaz, P., Moreira, F., Catry, F. & Rego, F. C. Wildfires as a major driver of landscape dynamics in three fire-prone areas of Portugal. Landsc. Urban Plan. 101, 349–358 (2011).
Sá-Sousa, P. A predictive distribution model for the Iberian wall lizard (Podarcis hispanicus) in Portugal. Herpetol. J. 10, 1–11 (2000).
Carretero, M. A. An integrated Assessment of a group with complex systematics: the Iberomaghrebian lizard genus Podarcis (Squamata, Lacertidae). Integr. Zool. 3, 247–266 (2008).
Ferreira, D., Mateus, C. & Santos, X. Responses of reptiles to fire in transition zones are mediated by bioregion affinity of species. Biodivers. Conserv. 25, 1543–1557 (2016).
Ferreira, D., Brito, J. C. & Santos, X. Long–interval monitoring reveals changes in the structure of a reptile community in a biogeographic transition zone. Basic Appl. Herpetol. https://doi.org/10.11160/bah.85 (2018).
Ferreira, D., Žagar, A. & Santos, X. Uncovering the rules of (Reptile) species coexistence in transition zones between bioregions. Salamandra 53, 193–200 (2017).
Diego-Rasilla, F. J. & Pérez-Mellado, V. Home range and habitat selection by Podarcis hispanica (Squamata, Lacertidae) in Western Spain. Folia Zool. 52, 87–98 (2003).
Santos, X., Badiane, A. & Matos, C. Contrasts in short- and long-term responses of Mediterranean reptile species to fire and habitat structure. Oecologia 180 (2016).
Collevatti, R. G., Leite, K. C. E., Miranda, G. H. Bde & Rodrigues, F. H. G. Evidence of high inbreeding in a population of the endangered giant anteater, Myrmecophaga tridactyla (Myrmecophagidae), from Emas National Park, Brazil. Genet. Mol. Biol. 30, 112–120 (2007).
Ujvari, B., Dowton, M. & Madsen, T. Population genetic structure, gene flow and sex-biased dispersal in frillneck lizards (Chlamydosaurus kingii). Mol. Ecol. 17, 3557–3564 (2008).
Friend, G. R. Impact of fire on small vertebrates in mallee woodlands and heathlands of temperate Australia: A review. Biol. Conserv. 65, 99–114 (1993).
Templeton, A. R., Brazeal, H. & Neuwald, J. L. The transition from isolated patches to a metapopulation in the eastern collared lizard in response to prescribed fires. Ecology 92, 1736–1747 (2011).
Driscoll, D. A., Whitehead, C. A. & Lazzari, J. Spatial dynamics of the knob-tailed gecko Nephrurus stellatus in a fragmented agricultural landscape. Landsc. Ecol. 27, 829–841 (2012).
Ferreira, C. C., Santos, X. & Carretero, M. A. Does ecophysiology mediate reptile responses to fire regimes? Evidence from Iberian lizards. PeerJ 4, e2107 (2016).
Sannolo, M., Barroso, F. M. & Carretero, M. A. Physiological differences in preferred temperatures and evaporative water loss rates in two sympatric lacertid species. Zoology 126, 58–64 (2018).
Smith, A. L., Bull, C. M., Gardner, M. G. & Driscoll, D. A. Life history influences how fire affects genetic diversity in two lizard species. Mol. Ecol. 23, 2428–2441 (2014).
Banks, S. C., Blyton, M. D. J., Blair, D., McBurney, L. & Lindenmayer, D. B. Adaptive responses and disruptive effects: How major wildfire influences kinship-based social interactions in a forest marsupial. Mol. Ecol. 21, 673–684 (2012).
Gomes, V., Carretero, M. A. & Kaliontzopoulou, A. The relevance of morphology for habitat use and locomotion in two species of wall lizards. Acta Oecologica 70, 87–95 (2016).
De Meeûs, T. Revisiting F IS, F ST, Wahlund Effects, and Null Alleles. J. Hered. 109, 446–456 (2017).
Pannell, J. R. & Charlesworth, B. Effects of metapopulation processes on measures of genetic diversity. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 355, 1851–64 (2000).
Pinho, C., Harris, D. J. & Ferrand, N. Contrasting patterns of population subdivision and historical demography in three western Mediterranean lizard species inferred from mitochondrial DNA variation. Mol. Ecol. 16, 1191–1205 (2007).
Pinho, C., Kaliontzopoulou, A., Harris, D. J. & Ferrand, N. Recent evolutionary history of the Iberian endemic lizards Podarcis bocagei (Seoane, 1884) and Podarcis carbonelli Pérez-Mellado, 1981 (Squamata: Lacertidae) revealed by allozyme and microsatellite markers. Zool. J. Linn. Soc. 162, 184–200 (2011).
Smith, A. L., Michael Bull, C. & Driscoll, D. A. Successional specialization in a reptile community cautions against widespread planned burning and complete fire suppression. J. Appl. Ecol. 50, n/a–n/a (2013).
Smith, A. L. Successional changes in trophic interactions support a mechanistic model of post-fire population dynamics. Oecologia 186, 129–139 (2018).
Valentine, L. E., Reaveley, A., Johnson, B., Fisher, R. & Wilson, B. A. Burning in Banksia Woodlands: How Does the Fire-Free Period Influence Reptile Communities? PLoS One 7, e34448 (2012).
Pinho, C., Ferrand, N. & Harris, D. J. Reexamination of the Iberian and North African Podarcis (Squamata: Lacertidae) phylogeny based on increased mitochondrial DNA sequencing. Mol. Phylogenet. Evol. 38, 266–273 (2006).
Caeiro-Dias, G. et al. Lack of congruence of genetic and niche divergence in Podarcis hispanicus complex. Journal of Zoological Systematics and Evolutionary Research https://doi.org/10.1111/jzs.12219 (2018).
Nunes, M. C. S. et al. Land Cover Type and Fire in Portugal: Do Fires Burn Land Cover Selectively? Landsc. Ecol. 20, 661–673 (2005).
Catry, F. X. et al. Effects of fire on tree survival and regeneration in a Mediterranean ecosystem. For. Ecol. Manage. 234, S197 (2006).
Oliveira, S. L. J., Pereira, J. M. C. & Carreiras, J. M. B. Fire frequency analysis in Portugal (19752005), using Landsat-based burnt area maps. International Journal of Wildland Fire 21, 48–60 (2012).
Moreira, F., Rego, F. C., Ferreira, P. G. & Temporal (1958–1995) pattern of change in a cultural landscape of northwestern Portugal: implications for fire occurrence. Landsc. Ecol. 16, 557–567 (2001).
Pereira, J. M. C., Carreiras, J. M. B., Silva, J. M. N. & Vasconcelos, M. J. In Incêndios florestais em Portugal: Caracterização, impactos e prevenção (eds Pereira, J. S., PereiraJ.M.C., Rego, F. C., Silva, J. M. N. & Silva, T. P.) 133–161 (ISA Press 2006).
Fernandes, P. M. et al. Prescribed burning in southern Europe: developing fire management in a dynamic landscape. Front. Ecol. Environ. 11, e4–e14 (2013).
Carmo, M., Moreira, F., Casimiro, P. & Vaz, P. Land use and topography influences on wildfire occurrence in northern Portugal. Landsc. Urban Plan. 100, 169–176 (2011).
Scotto, M. G. et al. Area burned in Portugal over recent decades: an extreme value analysis. Int. J. Wildl. Fire 23, 812 (2014).
Metzger, M. J., Bunce, R. G. H., Jongman, R. H. G., Mücher, C. A. & Watkins, J. W. A climatic stratification of the environment of Europe. Glob. Ecol. Biogeogr. 14, 549–563 (2005).
Ribeiro, O., Lautensach, H. & Daveau, S. Geografia de Portugal. (Edições João Sá da Costa 1987).
Costa, J., Aguiar, C., Capelo, J., Lousã, M. & Neto, C. Biogeografia de Portugal continental. Quercetea 1, 5–56 (1998).
Ribeiro, O. A landscape genetic perspective on the spatial dynamics of hybridization between two species of wall lizards. Master thesis. (Porto University 2014).
Agostini, C. et al. Permanent Genetic Resources added to Molecular Ecology Resources Database 1 April 2013-31 May 2013. Mol. Ecol. Resour. 13, 966–968 (2013).
Van Oosterhout, C., Hutchinson, W. F., Wills, D. P. M. & Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 4, 535–538 (2004).
Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567 (2010).
Rice, W. R. Analyzing Tables of Statistical Tests. Evolution (N. Y). 43, 223 (1989).
El Mousadik, A. & Petit, R. J. High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor. Appl. Genet. 92, 832–839 (1996).
Weir, B. S., Cockerham, C. C. & Estimating, F. -Statistics for the analysis of population structure. Evolution (N. Y). 38, 1358–1370 (1984).
Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 86, 485–486 (1995).
Statsoft. STATISTICA, version 8.0. (2007).
Rosenberg, M. S. & Anderson, C. D. PASSaGE: Pattern Analysis, Spatial Statistics and Geographic Exegesis. Version 2. Methods Ecol. Evol. 2, 229–232 (2011).
Peakall, R. O. D. & Smouse, P. E. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295 (2006).
Peakall, R. & Smouse, P. E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28, 2537–2539 (2012).
Cornuet, J. M. & Luikart, G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144, 2001–14 (1996).
Luikart, G. & Cornuet, J.-M. Empirical Evaluation of a Test for Identifying Recently Bottlenecked Populations from Allele Frequency Data. Conserv. Biol. 12, 228–237 (2008).
Garza, J. C. & Williamson, E. G. Detection of reduction in population size using data from microsatellite loci. Mol. Ecol. 10, 305–318 (2001).
Piry, S., Luikart, G. & Cornuet, J.-M. BOTTLENECK: a computer program for detecting recent reductions in the effective size using allele frequency data. J. Hered. 90, 502–503 (1999).
Kimura, M. & Crow, J. F. The numer of alleles that can be maintained. Genetics 49, 725–738 (1964).
Ohta, T. & Kimura, M. A model of mutation appropriate to estimate the number of electrophoretically detectable alleles in a finite population. Genet. Res. 22, 201–4 (1973).
Di Rienzo, A. et al. Mutational processes of simple-sequence repeat loci in human populations. Proc. Natl. Acad. Sci. USA 91, 3166–70 (1994).
Archer, F. I., Adams, P. E. & Schneiders, B. B. stratag: An r package for manipulating, summarizing and analysing population genetic data. Mol. Ecol. Resour. 17, 5–11 (2017).
Acknowledgements
We want to thank Antigoni Kalionzopoulou for fieldwork assistance, and Peneda-Gerês National Park staff for their logistic support and for providing us valuable information, especially Henrique Carvalho and Armando Loureiro. We further thank members of the Biodeserts and Functional Biodiversity groups in CIBIO for valuable suggestions regarding this article. The British Herpetological Society provided DF a grant from the Grant Student Scheme to support fieldwork. Laboratory costs were partially covered by FCT, Fundação para a Ciência e a Tecnologia (PTDC/BIA-BIC/2903/2012; IF/01597/2014/CP1256/CT0009) and FEDER funds through the Operational Programme for Competitiveness Factors - COMPETE (FCOMP-01-0124-FEDER-028276). This work was also financed by the project PTDC/BIA-EVL/30288/2017 - NORTE -01-0145-FEDER-30288 co-funded by NORTE2020 through Portugal 2020 and FEDER and FCT funds. CP and JCB are supported by FCT contracts (IF/01597/2014 and IF/00459/2013, respectively). XS was supported by Fundação para a Ciência e Tecnologia, FCT (SFRH⁄BPD⁄73176/2010).
Author information
Authors and Affiliations
Contributions
D.F. and X.S. designed research and conducted lizard sampling. D.F. and C.P. analysed data. D.F., C.P., J.C.B. and X.S. contributed to write the paper and discuss main results.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferreira, D., Pinho, C., Brito, J.C. et al. Increase of genetic diversity indicates ecological opportunities in recurrent-fire landscapes for wall lizards. Sci Rep 9, 5383 (2019). https://doi.org/10.1038/s41598-019-41729-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41729-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.