Abstract
Indium tin oxide (ITO) is one of the most widely used transparent conductors in optoelectronic device applications. We investigated the optical properties of ITO thin films at high temperatures up to 800 °C using spectroscopic ellipsometry. As temperature increases, amorphous ITO thin films undergo a phase transition at ~ 200 °C and develop polycrystalline phases with increased optical gap energies. The optical gap energies of both polycrystalline and epitaxial ITO thin films decrease with increasing temperature due to electron–phonon interactions. Depending on the background oxygen partial pressure, however, we observed that the optical gap energies exhibit reversible changes, implying that the oxidation and reduction processes occur vigorously due to the low oxidation and reduction potential energies of the ITO thin films at high temperatures. This result suggests that the electronic structure of ITO thin films strongly depends on temperature and oxygen partial pressure while they remain optically transparent, i.e., optical gap energies > 3.6 eV.
Similar content being viewed by others
Introduction
Tin-doped indium oxide or indium tin oxide (ITO) is a transparent conductor, which is widely used in modern optoelectronic devices such as thin-film transistors, resistive switching memories, and solar cells1,2,3,4,5. However, it is mostly unexplored if its high electrical conductivity and optical transparency will remain intact at extreme conditions such as high temperature or pressure. The question concerning the high-temperature property of materials is particularly important as modern technology evolves to device applications and operations in harsh environments above 500 °C6,7,8. Since the bandgap energies of semiconductors usually decrease with increasing temperature9,10, we may expect that ITO will also lose its optical transparency at high temperatures. Indeed, indium oxide (In2O3) single crystals have been reported to show a more significant reduction of its optical gap at high temperatures than semiconductor crystals such as Si and GaAs11. This somewhat pessimistic result is understood by a strong interaction between electrons and lattice vibrations (i.e., electron–phonon interaction), affecting the bandgap energy of In2O312. However, it is essential to note that high concentrations of doped Sn ions and oxygen vacancies play a crucial role in the electronic band structure of ITO and its optical properties. For example, the optical gap energy of ITO is larger than that of undoped In2O3 because of the existence of free electron carriers13, according to the Burstein-Moss effect14, and the temperature dependence of free carrier concentration is nearly constant15. Hence, investigating the high-temperature optical properties of ITO requires systematic measurements and understanding of the role of dopants and defects as well as electron–phonon interactions.
In this paper, we report the high-temperature optical properties of ITO thin films up to 800 °C. We studied ITO thin films of amorphous (a-ITO), polycrystalline (poly-ITO), and epitaxial (epi-ITO) phases rather than ITO bulk crystals because these thin-film forms are the ones used in various optoelectronic device applications. While a-ITO thin films show an abrupt increase of optical gap energies due to crystallization at ~ 200 °C, the optical gap energies of poly-ITO and epi-ITO thin films decrease gradually as temperature increases. Nevertheless, the optical gap energies (Eg) remain higher than the visible photon energies (i.e., Eg > 3 eV), meaning that their optical transparency is unaffected. We also found that background oxygen partial pressure at high temperatures alters the optical gap energies of ITO thin films reversibly by oxidation and reduction processes. Our results demonstrate that an unintentional oxygen annealing effect at high temperatures can result in different optical and transport properties of ITO thin films.
Results and discussion
Amorphous and polycrystalline ITO thin films
We observed that a-ITO thin films undergo a phase transition in the annealing process and develop a polycrystalline phase with increased Eg at high temperatures. Figure 1a shows X-ray diffraction (XRD) θ–2θ scans of both as-grown a-ITO and poly-ITO, which are ITO thin films on glass substrates before and after post-annealing at 500 °C, respectively. While the a-ITO thin film shows no visible peak, which verifies that the thin film is amorphous, clear diffraction peaks appear in the annealed sample. The diffraction peaks of the poly-ITO thin film match well with the cubic bixbyite ITO structure (ICSD #50848). Note that poly-ITO thin films exhibit a larger Eg (i.e., 4.25 ± 0.01 eV) than a-ITO thin films (i.e., 3.85 ± 0.01 eV) at room temperature, as shown in Fig. 1b,c. The relation between the complex dielectric function (\(\tilde{\varepsilon } = \tilde{n}^{2} = \varepsilon_{1} + i\varepsilon_{2}\)), and the optical conductivity (\(\sigma_{1}\)) is \(\sigma_{1} = \frac{{\varepsilon_{2} \omega }}{4\pi }\) , where ω is the photon energy. The spectral shapes of \(\varepsilon_{1}\) and \(\sigma_{1}\) and the change of Eg are consistent with each other according to the Kramers–Kronig relation. We extrapolated the spectra below 1.2 eV using the Drude model16 to match with the dc conductivities (i.e., \(\sigma_{1}\) at 0 eV) of these samples. The estimated plasma frequencies, where the dielectric constant crosses zero, increase from 0.7 ± 0.1 eV (a-ITO) to 0.8 ± 0.1 eV (poly-ITO). Since the plasma frequency (ωp) is proportional to the carrier concentration (ne) (\(\omega_{{\text{p}}}^{2} = 4\pi n_{e} e^{2} /m\), where e and m are the elementary charge and the effective mass of electron, respectively), the enhanced Eg in poly-ITO thin films is presumably due to increased free carriers (i.e., the Burstein-Moss effect14) and reduced disorder17.
Structural and optical characterization of a-ITO and poly-ITO thin films. (a) XRD θ–2θ scans of a-ITO (black) and poly-ITO (red) thin films. According to the diffraction indices of poly-ITO, it is a cubic bixbyite structure (ICSD #50848). (b) Dielectric constant (ε1) and (c) optical conductivity (σ1) spectra of a-ITO (black) and poly-ITO (red) thin films measured at room temperature. The grey dashed lines are the spectra from Ref.33 for comparison. The dots at zero photon energy are from dc-conductivity measurements. The solid thin lines between 0 and 1.2 eV are interpolated using the Drude model.
To determine high-temperature Eg of a-ITO and poly-ITO thin films, we calculated the absorption coefficient (α) from the extinction coefficient (k) using α = 4πk/λ, where λ is the wavelength of the photon. Since the direct Eg has the relation: α ≈ (ω – Eg)1/2 (Ref.14), Figure 2a,b shows α2 vs. photon energy plots of a-ITO and poly-ITO thin films above room temperature, respectively. Eg is estimated by linear extrapolation at each temperature. Figure 2c shows Eg as a function of temperature for both a-ITO and poly-ITO thin films. Note that Eg of a-ITO thin films shows an abrupt change above 200 °C, where polycrystalline ITO starts forming, as reported in Refs.18,19. On the other hand, Eg of poly-ITO thin films decreases gradually with increasing temperature, as also shown in Fig. 2c. We suggest that strong electron–phonon interaction of poly-ITO thin films is responsible for the temperature dependence of Eg. According to O’Donnell’s model10, Eg at absolute temperature T is described by
where S is a dimensionless coupling constant, \(\langle\hbar \omega_{ph}\rangle\) is the average phonon energy, and kB is the Boltzmann constant. Using the average phonon energy of In2O3 (38.6 meV)11, we can obtain Eg(0) = 4.40 ± 0.04 eV and S = 4.2 ± 0.5, as shown in Fig. 2c (red line). Note that the coupling constant S is smaller than that of undoped In2O3 (S = 8.24)11, implying that lattice imperfections such as substitutional Sn ions, oxygen vacancies, and grain boundaries, at which phonons are scattered20, suppress the electron–phonon interactions in poly-ITO thin films.
High-temperature optical properties of a-ITO and poly-ITO thin films. Absorption coefficient squared (α2) vs. photon energy plots for (a) a-ITO and (b) poly-ITO thin films obtained from ellipsometry spectra at high temperatures up to 500 °C and 600 °C, respectively. The black lines are the linear extrapolation of α2 spectra at 25 °C and 500 °C (600 °C) for the a-ITO (poly-ITO) thin film. (c) Optical gap energies of a-ITO and poly-ITO thin films as a function of temperature. The solid red line is a curve fit from O’Donnell’s model10.
Epitaxial ITO thin films
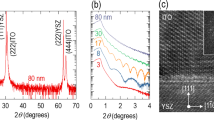
To explore intrinsic high-temperature optical properties, we measured epi-ITO thin films prepared in two different annealing conditions, i.e., p(O2) = 10 mTorr (O2-annealed) and p(O2) < 1.0 × 10–6 Torr (vacuum-annealed). Figure 3a,b shows XRD θ-2θ scans and reciprocal space mappings, respectively. Both epi-ITO thin films are coherently grown without any notable secondary phases. It is noteworthy that these samples show identical in-plane (10.25 ± 0.01 Å) and out-of-plane lattice constants (10.07 ± 0.01 Å) regardless of the annealing conditions. The epi-ITO thin films are under tensile strain (~ 1.3%) due to the lattice mismatch with YSZ substrates21. However, the room-temperature optical spectra of epi-ITO thin films strongly depend on the annealing conditions presumably due to different concentrations of oxygen vacancies. It was found that the carrier concentration of In2O3 thin films decreased as increasing the O2 pressure during growth22,23. DFT calculations suggested that the oxygen vacancies are shallow donors in In2O3, contributing to the carrier concentration24,25. Figure 3c shows ε1 and \(\sigma_{1}\) spectra of epi-ITO thin films, respectively. The vacuum-annealed epi-ITO thin film shows both increased ωp (0.8 ± 0.1 eV) and Eg (i.e., 4.26 ± 0.03 eV) compared to the O2-annealed epi-ITO thin film (ωp = 0.5 ± 0.1 eV and Eg = 4.00 ± 0.05 eV). This observation is consistent with the Burstein–Moss effect14 since larger free carrier concentrations result in higher Eg values. Therefore, the annealing conditions influence the number of oxygen vacancies, which are electrons donors13, and increase (decrease) the carrier concentrations by reducing (oxidizing) epi-ITO thin films and change their optical properties without any noticeable difference in the crystal structure.
Structural and optical characterization of epi-ITO thin films. (a) XRD θ-2θ scans of epi-ITO thin films annealed in O2 (green) and vacuum (blue) conditions. The asterisks (*) and the indices denote the peaks from the YSZ substrate and cubic bixbyite ITO thin films, respectively. (b) X-ray reciprocal space maps near the 204-reflection of YSZ. Both samples show clear 408-reflections from epi-ITO thin films. (c) ε1 and (d) σ1 spectra of vacuum-annealed and O2-annealed epi-ITO thin films at room temperature. The dots at zero photon energy are from dc-conductivity measurements. The solid thin lines between 0 and 1.2 eV are interpolated using the Drude model.
We measured Eg of epi-ITO thin films at high temperatures while maintaining the same environmental conditions as the post-annealing process. Figure 4a,b shows α2 vs. photon energy plots for vacuum-annealed and O2-annealed epi-ITO thin films, respectively. Figure 4c shows Eg of epi-ITO thin films as a function of temperature obtained from the spectra. Note that both samples show systematically decreasing Eg with increasing temperature. Based on the average phonon energy of In2O3 (38.6 meV)11, we find Eg(0) = 4.33 ± 0.01 eV and S = 3.0 ± 0.1 for the vacuum-annealed epi-ITO thin film and Eg(0) = 4.10 ± 0.02 eV and S = 3.6 ± 0.3 for the O2-annealed epi-ITO thin film. Interestingly, the O2-annealed epi-ITO shows an almost constant Eg above 600 °C. We speculate that these steady Eg values above 600 °C are related to the phase transition of In2O3 from a cubic bixbyite structure to a rhombohedral corundum structure26 because the Eg of corundum phase (3.8 eV)27 is higher than bixbyite phase (2.7 eV)11 at room temperature. However, it should be checked by high-temperature structural characterizations in future studies.
High-temperature optical properties of epi-ITO thin films. α2 vs. photon energy plots for (a) vacuum-annealed and (b) O2-annealed epi-ITO thin films obtained from ellipsometry spectra at high temperatures up to 800 °C. The black lines show the linear extrapolation of α2 spectra at 25 °C and 800 °C. (c) Optical gap energies of vacuum-annealed and O2-annealed epi-ITO thin films as a function of temperature. The blue and green lines are curve fits of vacuum-annealed and O2-annealed epi-ITO thin films, respectively, using O’Donnell’s model10.
Oxidation and reduction of ITO thin films at high temperatures
We observed that reversible oxidation and reduction processes affect Eg of epi-ITO thin films at high temperatures. Figure 5 shows temperature-dependent Eg of vacuum-annealed or O2-annealed epi-ITO thin films measured under the conditions of vacuum (p(O2) < 1.0 × 10–6 Torr) or oxygen (p(O2) = 10 mTorr). When the vacuum-annealed epi-ITO thin film is placed under the vacuum environment (Fig. 5a), its Eg shows identical temperature-dependence during the heating and cooling cycles. However, when the sample is under the oxygen environment (Fig. 5b), its Eg decreases significantly at around 300 °C during the heating process, and its Eg becomes about 0.23 eV smaller than the initial Eg after cooling down to room temperature. This observation indicates that exposure to high temperatures above 300 °C oxidizes epi-ITO thin films and reduces free electron carriers.
Optical gap energies of epi-ITO thin films during oxidation and reduction processes. The vacuum-annealed epi-ITO thin film is exposed to (a) vacuum and (b) p(O2) = 10 mTorr during the thermal cycle. The O2-annealed epi-ITO thin film is exposed to (c) vacuum and (d) p(O2) = 10 mTorr during the thermal cycle. The red and blue arrows indicate the directions of heating and cooling processes, respectively.
In contrast, when the O2-annealed epi-ITO thin film is located under the vacuum condition, its Eg at room temperature increases about 0.24 eV after the heating and cooling cycle (Fig. 5c) whereas its Eg does not change after the thermal cycling under the O2 environment (Fig. 5d). The increased Eg under the vacuum condition reveals that oxygen vacancies and free-electron carriers are generated at high temperatures. Therefore, we can see that the background oxygen partial pressure at high temperatures affect the number of oxygen vacancies, which alters Eg of epi-ITO thin films. Note that the change of Eg is reversible (compare Fig. 5b with Fig. 5c), meaning that the oxidation and reduction potential energies of the ITO thin films are so low that oxidation and reduction processes occur vigorously at high temperatures.
Conclusion
We have investigated the optical properties of ITO thin films at high temperatures using SE. The a-ITO thin films undergo a phase transition from amorphous to polycrystalline above 200 °C, and its Eg increases during the transition due to increased free carriers and reduced disorder. The poly-ITO and epi-ITO thin films exhibit a decrease in Eg with increasing temperature due to electron–phonon interactions. By monitoring Eg of epi-ITO thin films, we have observed that the oxidation and reduction processes occur reversibly at high temperatures depending on background oxygen partial pressure. All ITO thin films exhibit Eg higher than 3.5 eV up to 800 °C despite various changes in their electronic structures, suggesting that ITO thin films possess robust optical transparency for high-temperature device applications.
Methods
We synthesized a-ITO and poly-ITO thin films grown on glass substrates. The a-ITO thin films were grown by using the dc-magnetron sputtering technique with a mixture gas of Ar and O2. The as-grown a-ITO thin films were crystallized into poly-ITO thin films after annealing at 500 °C under O2 environment (p(O2) = 10 mTorr). We fabricated the epi-ITO thin films on yttria-stabilized zirconia (YSZ) Y:ZrO2 (001) substrates using pulsed laser deposition (PLD). The PLD conditions were the oxygen partial pressure (p(O2)) of 10 mTorr, and the laser (KrF excimer laser (λ = 248 nm)) influence of 1.5 J/cm2 with the repetition rate of 10 Hz. The substrate temperature was 600 °C to grow high crystalline epi-ITO thin film28. After the epi-ITO thin film growth, the samples were annealed under vacuum (p(O2) < 1.0 × 10–6 Torr) and O2 (p(O2) = 10 mTorr) environments, respectively, to control oxygen vacancies inside the thin films. Table S1 summarizes the growth condition of various ITO thin films in this study. We performed optical spectroscopic measurements with photon energies from 1.2 to 6.0 eV using a spectroscopic ellipsometer (Woollam M-2000) equipped with a custom-built vacuum chamber29 with an incident angle of 65° at high temperatures. We measured a-ITO (poly-ITO) thin films at p(O2) = 10 mTorr up to 500 °C (600 °C) before the glass substrate is deformed30. For epi-ITO thin films, we measured up to 800 °C under vacuum (p(O2) < 1.0 × 10–6 Torr) and oxygen (p(O2) = 10 mTorr) environments. We extracted the complex refractive index, \(\tilde{n} = n + ik\), where n and k are the refractive index and the extinction coefficient, respectively, from the measured spectroscopic ellipsometry parameters, Ψ and Δ, which are the amplitude ratio and phase difference31, using numerical interaction process based on the Fresnel equation32. (See the Supplementary material for details.) We used a single slab model, i.e., air/ITO/YSZ, without any correction of surface roughness.
References
Qian, K., Han, X., Li, H., Chen, T. & Lee, P. S. Uncovering the indium filament revolution in transparent bipolar ITO/SiOx/ITO resistive switching memories. ACS Appl. Mater. Interfaces 12, 4579–4585 (2020).
Lin, Y. et al. 17% Efficient organic solar cells based on liquid exfoliated WS2 as a replacement for PEDOT:PSS. Adv. Mater. 31, 1902965 (2019).
Liu, Z. et al. Flexible indium-tin-oxide homojunction thin-film transistors with two in-plane gates on cellulose-nanofiber-soaked papers. Adv. Electron. Mater. 5, 1900235 (2019).
Kim, J.-H. et al. Flexible ITO films with atomically flat surfaces for high performance flexible perovskite solar cells. Nanoscale 10, 20587–20598 (2018).
Delahoy, A. E. & Guo, S. In Handbook of Photovoltaic Science and Engineering (eds Luque, A. & Hegedus, S.) 716–796 (Wiley, New York, 2011).
Watson, J. & Castro, G. A review of high-temperature electronics technology and applications. J. Mater. Sci. Mater. Electron. 26, 9226–9235 (2015).
Medina, D. F. In Handbook of Cosmic Hazards and Planetary Defense (eds Pelton, J. N. & Allahdadi, F.) 295–314 (Springer International Publishing, Cham, 2015).
National Research Council Materials for High-Temperature Semiconductor Devices (The National Academies Press, 1995).
Varshni, Y. P. Temperature dependence of the energy gap in semiconductors. Physica 34, 149–154 (1967).
O’Donnell, K. P. & Chen, X. Temperature dependence of semiconductor band gaps. Appl. Phys. Lett. 58, 2924–2926 (1991).
Irmscher, K. et al. On the nature and temperature dependence of the fundamental band gap of In2O3. Phys. Status Solidi A 211, 54–58 (2014).
Morris, A. J. & Monserrat, B. Optical absorption driven by dynamical symmetry breaking in indium oxide. Phys. Rev. B 98, 161203 (2018).
Fan, J. C. C. & Goodenough, J. B. X-ray photoemission spectroscopy studies of Sn-doped indium-oxide films. J. Appl. Phys. 48, 3524–3531 (1977).
Hamberg, I. & Granqvist, C. G. Evaporated Sn-doped In2O3 films: Basic optical properties and applications to energy-efficient windows. J. Appl. Phys. 60, R123–R160 (1986).
Müller, H. K. Electrical and optical properties of sputtered In2O3 films. I. Electrical properties and intrinsic absorption. Phys. Status Solidi B 27, 723–731 (1968).
Ginley, D. S., Hosono, H. & Paine, D. C. Handbook of Transparent Conductors (Springer, New York, 2010).
Madan, A. & Shaw, M. P. In The Physics and Applications of Amorphous Semiconductors (eds Madan, A. & Shaw, M. P.) 1–10 (Academic Press, London, 1988).
Kim, J.-W. et al. Atomistic aspects of carrier concentration variation in post-annealed indium tin oxide films. J. Phys. D Appl. Phys. 48, 395307 (2015).
Paine, D. C. et al. A study of low temperature crystallization of amorphous thin film indium–tin–oxide. J. Appl. Phys. 85, 8445–8450 (1999).
Ziman, J. M. Electrons and Phonons: The Theory of Transport Phenomena in Solids (Oxford University Press, Oxford, 2001).
Taga, N. et al. Electrical properties of heteroepitaxial grown tin-doped indium oxide films. J. Appl. Phys. 80, 978–984 (1996).
Nistor, M., Gherendi, F. & Perrière, J. Degenerate and non-degenerate In2O3 thin films by pulsed electron beam deposition. Mater. Sci. Semicond. Process. 88, 45–50 (2018).
Nistor, M., Seiler, W., Hebert, C., Matei, E. & Perrière, J. Effects of substrate and ambient gas on epitaxial growth indium oxide thin films. Appl. Surf. Sci. 307, 455–460 (2014).
Chatratin, I. et al. Role of point defects in the electrical and optical properties of In2O3. Phys. Rev. Mater. 3, 074604 (2019).
Buckeridge, J. et al. Deep vs shallow nature of oxygen vacancies and consequent n-type carrier concentrations in transparent conducting oxides. Phys. Rev. Mater. 2, 054604 (2018).
Jiang, X. B., Jiang, M. & Zhao, M. Thermodynamic understanding of phase transitions of In2O3 nanocrystals. Chem. Phys. Lett. 563, 76–79 (2013).
Fujita, S. & Kaneko, K. Epitaxial growth of corundum-structured wide band gap III-oxide semiconductor thin films. J. Cryst. Growth 401, 588–592 (2014).
Ohta, H. et al. Highly electrically conductive indium–tin–oxide thin films epitaxially grown on yttria-stabilized zirconia (100) by pulsed-laser deposition. Appl. Phys. Lett. 76, 2740–2742 (2000).
Gruenewald, J. H., Nichols, J. & Seo, S. S. A. Pulsed laser deposition with simultaneous in situ real-time monitoring of optical spectroscopic ellipsometry and reflection high-energy electron diffraction. Rev. Sci. Instrum. 84, 043902 (2013).
Guerette, M. & Huang, L. In-situ Raman and Brillouin light scattering study of the international simple glass in response to temperature and pressure. J. Non-Cryst. Solids 411, 101–105 (2015).
Azzam, R. M. A. & Bashara, N. M. Ellipsometry and Polarized Light (North-Holland, Amsterdam, 1987).
Maulana, L. Z. et al. Inexpensive home-made single wavelength ellipsometer (λ = 633 nm) for measuring the optical constant of nanostructured materials. IOP Conf. Ser. Mater. Sci. Eng. 202, 012031 (2017).
König, T. A. F. et al. Electrically tunable plasmonic behavior of nanocube-polymer nanomaterials induced by a redox-active electrochromic polymer. ACS Nano 8, 6182–6192 (2014).
Acknowledgements
We acknowledge the support of NASA Kentucky under NASA award number NNX15AR69H (for high-temperature optical measurements) and a National Science Foundation grant DMR-1454200 (for thin-film synthesis and characterization). J.K. acknowledges the support from the Global Ph.D. Fellowship Program (2015H1A2A1034200) through the NRF Korea funded by the Ministry of Education.
Author information
Authors and Affiliations
Contributions
J.K. and J.G.C. synthesized the samples. J.K. carried out the X-ray diffraction and optical spectroscopic ellipsometry measurements. S.S., M.S. and S.P. provided technical assistance and scientific advice. J.K. and A.S. analyzed the experimental data, wrote the manuscript, and all authors reviewed it. A.S. conceived the project and supervised its progress for this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J., Shrestha, S., Souri, M. et al. High-temperature optical properties of indium tin oxide thin-films. Sci Rep 10, 12486 (2020). https://doi.org/10.1038/s41598-020-69463-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69463-4
This article is cited by
-
Influence of annealing temperature on structural, electrical, and optical properties of 80 nm thick indium-doped tin oxide on borofloat glass
Journal of Materials Science: Materials in Electronics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.