Abstract
Plants represent a safe and cost-effective platform for producing high-value proteins with pharmaceutical properties; however, the ability to accumulate these in commercially viable quantities is challenging. Ideal crops to serve as biofactories would include low-input, fast-growing, high-biomass species such as sugarcane. The objective of this study was to develop an efficient expression system to enable large-scale production of high-value recombinant proteins in sugarcane culms. Bovine lysozyme (BvLz) is a potent broad-spectrum antimicrobial enzyme used in the food, cosmetics and agricultural industries. Here, we report a novel strategy to achieve high-level expression of recombinant proteins using a combinatorial stacked promoter system. We demonstrate this by co-expressing BvLz under the control of multiple constitutive and culm-regulated promoters on separate expression vectors and combinatorial plant transformation. BvLz accumulation reached 1.4% of total soluble protein (TSP) (10.0 mg BvLz/kg culm mass) in stacked multiple promoter:BvLz lines, compared to 0.07% of TSP (0.56 mg/kg) in single promoter:BvLz lines. BvLz accumulation was further boosted to 11.5% of TSP (82.5 mg/kg) through event stacking by re-transforming the stacked promoter:BvLz lines with additional BvLz expression vectors. The protein accumulation achieved with the combinatorial promoter stacking expression system was stable in multiple vegetative propagations, demonstrating the feasibility of using sugarcane as a biofactory for producing high-value proteins and bioproducts.
Similar content being viewed by others
Introduction
Recombinant proteins are currently being produced in cultured cell-based systems in mammals, microbes (bacteria and yeast), insects and plants, as well as in transgenic animals (reviewed by Demain and Vaishnav)1. Transgenic plants constitute an attractive system for expression and production of a variety of proteins and biomolecules due to their efficient eukaryotic protein synthesis, high scalability, relatively low production costs and environmental footprint2,3,4. However, selecting suitable hosts and expression vectors are key considerations since protein accumulation is determined by expression levels.
Important factors to consider when selecting a plant-based production platform include biomass yield per hectare, recombinant protein yield per unit biomass, ease of transformation, scalability and safety5. Sugarcane (Saccharum spp. hybrids), a key feedstock in the expanding bioeconomy as a sugar and bioenergy crop6, is an ideal platform for recombinant protein production for several reasons: (1) It is a relatively fast growing tropical grass with the highly efficient C4 photosynthetic pathway, conferring high biomass production capacity with yields of up to 41.3 tons of biomass (harvested dry mass) per hectare per annum7,8; (2) it is highly efficient in utilizing radiation, water and nutrients to produce a large biomass and hence a higher recombinant protein yield; (3) it is readily amenable to genetic engineering, with established transformation and tissue regeneration techniques9,10; and (4) it has a low risk of out-crossing recombinant genes due to its primarily vegetative means of propagation; natural reproductive propagation in many temperate and subtropical regions is rare due to its photoperiod sensitivity.
Sugarcane was used as biofactory for the production of new biomolecules such as bioplastics11,12,13,14,15, alternative sugars (sorbitol and isomaltulose)16,17,18, and recombinant proteins including the human cytokine granulocyte macrophage colony stimulating factor GM-CSF19, canecystatins (cysteine protease inhibitors) CaneCP-1, CaneCP-2 and CaneCP-320,21,22, and the cellulolytic enzymes, endoglucanase and cellobiohydrolases I and II23,24. Accumulation levels of these recombinant proteins ranged from 0.02 to 2.0% of total soluble protein (TSP) in leaves. However, very few attempts have so far been made to express recombinant proteins in sugarcane culms (reporter proteins)25, which constitute the largest fraction of harvestable biomass and would be an ideal platform for production of bulk proteins.
Bovine lysozyme (BvLz) is more important industrially than other lysozymes because of its potent broad-spectrum antimicrobial activity26,27, especially against Gram-negative bacteria and fungi at concentrations as low as 25 ppm, its sixfold higher chitinase activity than that of chicken lysozyme28, and its thermal stability and resistance to proteolysis29. BvLz, unlike other enzymes, possesses biochemical properties that make it suitable for protein extraction and purification, such as stability over a broad pH range, thermal stability, resistance to proteolysis and convenient quantification assays30,31.
In this study, we demonstrate the feasibility of developing sugarcane as an expression platform for production and purification of recombinant proteins at high levels, i.e. up to 11.5% of TSP (82.5 mg protein/kg culm mass). Multiple promoters (constitutive or culm-regulated) on separate expression vectors were stacked by combinatorial plant transformation approach to boost production levels of recombinant bovine lysozyme (BvLz), which was codon-optimized for expression in monocots. A double terminator or 3′ untranslated region (UTR) was incorporated for improved transcript stability. Enzymatic activity and enzyme-linked immunosorbent assays (ELISA) of BvLz transgenic sugarcane culm protein extracts and clarified juice confirmed the presence of an intact and fully active BvLz enzyme, which accumulated in multiple vegetative generations at levels as high as 10.0 mg/kg (1.4% of TSP) in lines co-expressing BvLz from stacks of three or four different promoters on separate vectors, compared to 0.56 mg/kg (0.07% of TSP) in lines expressing BvLz from a single promoter vector. We further observed BvLz accumulation up to 82.5 mg/kg (11.5% of TSP) through event stacking by re-transforming the stacked promoter:BvLz transgenic lines with additional BvLz expression vectors.
Results and discussion
The combinatorial promoter and event stacking result in increased recombinant protein production in transgenic sugarcane culms
A salient feature of combinatorial transformation, a special case of co-transformation32, is that there is no theoretical limit to the number of expression vectors that can be co-transformed. To enable high-levels of recombinant protein production in sugarcane culms, we developed a combinatorial promoter and event stacking system and demonstrated its application in producing a high-value bovine lysozyme (BvLz) protein. This was facilitated by the availability of a set of constitutive and culm-regulated promoters previously isolated from sugarcane, in addition to the common maize ubiquitin 1 promoter (pUbi)33. These include the culm-regulated promoters for Sugarcane bacilliform virus (pSCBV21)34 and sugarcane dirigent16 (pSHDIR16) gene35, and the constitutive promoters for sugarcane proline-rich protein (pSHPRP)36 and elongation factor 1α (pSHEF1α)36 genes. Furthermore, conditions for small-scale and large-scale extraction and clarification of recombinant BvLz from sugarcane culm extracts and juice were optimized at our Pilot Plant and BioSeparation Facilities30,37.
The essential design of the resulting new combinatorial promoter stacking system is illustrated in Fig. 1. The system consisted of co-expressing the codon-optimized BvLzm, from a stack of multiple promoters on separate expression vectors in sugarcane by combinatorial transformation. A double terminator, composed of the Cauliflower mosaic virus (CaMV) 35S terminator (35ST) and the Agrobacterium tumefaciens nopaline synthase terminator (NOST), or the 3′UTR of Sorghum mosaic virus (SrMV), was fused to the coding region of BvLzm to enhance transcript stability38,39 (Fig. 1).
Design of a representative stacked multiple promoter:recombinant gene expression system developed for sugarcane. Promoter 1, 2 and 3 combinations can be any combination of the constitutive promoters maize, ubiquitin 1, sugarcane proline rich protein and sugarcane elongation factor 1α or the culm-regulated promoters from sugarcane dirigent16 and Sugarcane bacilliform virus. Vector assembly and cloning sites are indicated under “Materials and methods” section. BvLzm, maize codon-optimized bovine lysozyme gene; 35ST, terminator derived from Cauliflower mosaic virus 35S RNA; NOST, Agrobacterium tumefaciens nopaline synthase terminator; 3′UTR, 3′ untranslated region of Sorghum mosaic virus.
To test the stacking promoter gene expression system, embryogenic calli (2 month-old) and leaf roll discs (12 day-old), prepared from several commercial sugarcane varieties were co-transformed biolistically with the multiple promoter:BvLzm expression vectors, using the bar gene (phosphinothricin acetyl transferase) as a selectable marker. Several independent transgenic BvLzm lines, identified by Southern blot analysis (Fig. 2a; Supplementary Fig. S2), were generated from the combinatorial transformation of sugarcane with single, double, triple or quadruple promoter:BvLzm expression vectors (Table 1). These represent: (1) 43 lines (114 plants) expressing BvLzm from a single promoter, (2) 10 lines (52 plants) expressing BvLzm from a double promoter stack, (3) 24 lines (318 plants) expressing BvLzm from a triple promoter stack, and (4) 23 lines (76 plants) expressing BvLzm from a quadruple promoter stack (Table 1).
Stable integration, expression and yield of the bovine lysozyme (BvLzm) recombinant gene in sugarcane BvLzm transgenic lines as determined by Southern (a) and northern (b) blot analyses and enzyme-linked immunosorbent assay (ELISA) (c), respectively. Representative lines with single or multiple promoter:BvLzm-terminator cassettes are shown. BvLzm, maize codon-optimized BvLz; pU:BvLzm, BvLzm driven by the maize ubiquitin 1 promoter (pU); pUD:BvLzm, BvLzm expressed from two promoters, pU and sugarcane dirigent16 (pD); pUDE:BvLzm, BvLzm expressed from three promoters, pU, pD and sugarcane elongation factor 1α (pE); and pUPBE:BvLzm, BvLzm expressed from four promoters, pU, sugarcane proline-rich protein (pP), Sugarcane bacilliform virus (pB) and pE. DNA and RNA gel blots were hybridized to a probe corresponding to the coding region of BvLzm. The full-length uncropped DNA and RNA gel blot autoradiograms are displayed in Supplementary Figures S2 and S3, respectively. The BvLzm yield is indicated as determined by ELISA in juice extract of culms (1.0 kg of culm).
The integration and size of each respective BvLzm expression vector (promoter, BvLzm, terminator and/or 3′UTR) in the single and stacked multiple promoter:BvLzm lines were confirmed by Southern blot hybridization with a full-length BvLzm probe (Fig. 2a; Supplementary Fig. S2) and by PCR using primers encompassing each of the different promoter:BvLzm-terminator cassettes (Fig. 3; Supplementary Fig. S4, S5 and S6). All lines were analyzed for their BvLzm transcript levels by northern blot hybridization (Fig. 2b; Supplementary Fig. S3) as well as for their BvLzm accumulation by ELISA (Table 1; Fig. 2c for ELISA). For representative lines, yield was also determined by an enzyme activity assay and the results highly correlated with the ELISA data (R = 0.81–0.98; Supplementary Table S1). Furthermore, in general, a clear positive trend was observed between the BvLzm copy number, the combinatorial promoter-BvLzm cassettes transformed and the BvLzm levels (Table 2; Fig. 2; Supplementary Fig. S2). For instance, quadruple and triple promoter:BvLzm lines displayed a higher BvLzm copy number and yield than double and single promoter:BvLzm lines, as expected from co-transformation (Table 2; Fig. 2; Supplementary Fig. S2). Similarly, the double promoter pUD:BvLzm lines had a higher BvLzm copy number and accumulation than single promoter pU:BvLzm lines (Table 2; Fig. 2; Supplementary Fig. S2).
Presence and size of multiple promoter:bovine lysozyme (BvLzm)-terminator cassettes in the same BvLzm transgenic line as determined by PCR analysis. Representative lines with single or multiple promoter:BvLzm-terminator cassettes are shown. (1) pU:BvLzm-35ST line; (2) pUD:BvLzm-35ST line; (3) pUDE:BvLzm-3′UTR-35ST line; (4) pUPE:BvLzm-3′UTR-35ST line; (5) pUPBE:BvLzm-3′UTR-35ST line; (6) pUPBE:BvLzm-35STNOST line; (7) vector-transformed line; (8) non-transformed (NT; tissue culture-derived) plant; and (9) no DNA template (negative control for PCR). (a) Detection of pUbi, BvLzm, 3′UTR, 35ST and NOST using the primer sets pUbi-F/35ST-R (2.62 kilobase pairs [kb] or 2.85 kb fragment) and pUbi-F/NOST-R (2.87 kb fragment). (b) Detection of pSHDIR16, BvLzm, 3′UTR, 35ST and NOST using the primer sets pSHDIR16-F/35ST-R (3.32 kb fragment) and pSHDIR16-F/NOST-R (3.56 kb fragment). (c) Detection of pSHPRP, BvLzm, 3′UTR, 35ST and NOST using the primer sets pSHPRP-F/35ST-R (3.65 kb fragment) and pSHPRP-F/NOST-R (3.90 kb fragment). (d) Detection of pSHEF1α, BvLzm, 3′UTR, 35ST and NOST using the primer sets pSHEF1α-F/35ST-R (2.57 kb fragment) and pSHEF1α-F/NOST-R (2.82 kb fragment). (e) Detection of pSCBV21, BvLzm, 3′UTR, 35ST and NOST using the primer sets pSCBV21-F/35ST-R (2.21 kb fragment) and pSCBV21-F/NOST-R (2.46 kb fragment). BvLzm, maize codon-optimized bovine lysozyme gene; U, Ubi promoter; D, SHDIR16 promoter; P, SHPRP promoter; E, SHEF1α promoter; B, SCBV21 promoter; 3′UTR, 3′ untranslated region of Sorghum mosaic virus. 35ST, Cauliflower mosaic virus 35S terminator; NOST, Agrobacterium tumefaciens nopaline synthase terminator. Full-length uncropped gels of the PCR products are displayed in Supplementary Figures S4, S5 and S6.
The BvLzm yield from single promoter pUbi:BvLzm (pU:BvLzm) lines varied from low (0.08–0.1 mg/kg; 6.7% of plants) to moderate (0.12–0.18 mg/kg; 40.0% of plants) and high (0.2–0.4 mg/kg; 53.3% of plants) (Tables 1, 2). The BvLzm yield range was 0.08–0.4 mg/kg (0.01–0.06% of TSP), averaging 0.3 mg/kg (0.04% of TSP) ± 0.02 for the high expressers (Table 1). Other single promoter:BvLzm lines harboring pSHDIR16, pSCBV21, pSHPRP or pSHEF1α showed similar trends, with a highest BvLzm yield of 0.56 mg/kg (0.08% of TSP) (Supplementary Table S2).
Stacked double promoter pUbi-SHDIR16:BvLzm (pUD:BvLzm) lines displayed 1.8–6.3 fold higher BvLzm yield than single promoter pU:BvLzm lines, with a range of 0.5–0.7 mg/kg (0.07–0.1% of TSP) (Table 1). The BvLzm yield was further enhanced to 2.0–8.6 fold in the stacked triple promoter:BvLzm lines, with levels ranging from 1.0 to 6.0 mg/kg (0.1–0.8% of TSP) (Table 1). The majority (66.7%) of the stacked triple promoter pUbi-SHPRP-SHEF1α:BvLzm (pUPE:BvLzm) lines had a BvLzm yield of 1.0–2.0 mg/kg (0.1–0.3% of TSP) with 20.0% at 2.2–3.2 mg/kg (0.33–0.45% of TSP) and 13.3% at 3.5–4.7 mg/kg (0.5–0.7% of TSP) (Table 1). Replacing the constitutive SHPRP promoter with the culm-regulated SHDIR16 promoter in the stacked triple promoter pUbi-SHDIR16-SHEF1α:BvLzm (pUDE:BvLzm) lines boosted the BvLzm yield to 6.0 mg/kg (0.8% of TSP). Most of pUDE:BvLzm lines (62.0%) had a BvLzm yield of 2.2–3.2 mg/kg (0.33–0.45%of TSP), with 27.0% at 1.5–2.0 mg/kg (0.2–0.3% of TSP), 4.5% at 3.5–4.7 mg/kg (0.5–0.7% of TSP) and 6.5% at 5.0–6.0 mg/kg (0.7–0.8% of TSP) (Table 1).
Next, we checked if stacking another promoter to produce quadruple promoter:BvLzm lines would be helpful. The BvLzm yield increased modestly in the stacked quadruple promoter pUbi-SHPRP-SCBV21-SHEF1α:BvLzm (pUPBE:BvLzm) lines by 1.7–2.4 fold, compared to the stacked triple promoter:BvLzm lines. The highest enhancement was achieved when using a double terminator cassette, i.e. 10.0 mg/kg (1.4% of TSP) in pUPBE:BvLzm:35STNOST lines (Table 1), and the 3′UTR of SrMV with the single 35S terminator, i.e. 6.3 mg/kg (0.9% of TSP) in pUPBE:BvLzm:3′UTR35ST lines (Table 1). In fact, 24.1% of pUPBE:BvLzm:35STNOST plants had a BvLzm yield of 6.0–10 mg/kg (0.8–1.4% of TSP), and 44.5% of pUPBE:BvLzm:3′UTR35ST plants showed a BvLzm yield of 6.0–6.3 mg/kg (0.8–0.9% of TSP) (Table 1). Lastly, we evaluated if event stacking can enhance the yields of the stacked quadruple promoter lines. Event stacking, also referred to as super transformation, is a good alternative to hybridization/crossing, which is time-consuming and not a viable option in vegetatively-propagated crops like sugarcane. Stacked five promoter pUbi-SHDIR16-SHEF1α-SHPRP-SCBV21:BvLzm (pUDEPB:BvLzm) lines were generated through event stacking, by re-transforming bialaphos-resistant triple promoter pUDE:BvLzm lines with two promoter:BvLzm expression vectors, pP:BvLzm and pB:BvLzm (Table 1) using the neomycin phosphotransferase II as a selectable marker. The resulting pUDEPB:BvLzm lines showed increased BvLzm accumulation, i.e. up to 82.5 mg/kg culm mass (11.5% of TSP) (Table 1). The majority (33.3%) of these lines exhibited BvLzm levels of 26.2–32.3 mg/kg (3.6–4.5% of TSP), while 18.3% accumulated the highest BvLzm levels, i.e. 59.9–82.5 mg/kg (8.3–11.5% of TSP). The remaining 24.2% and 12.1% of the lines showed BvLzm levels of 15.9–21.1 mg/kg (2.2–2.9% of TSP) and 11.0–12.4 mg/kg (1.5–1.7% of TSP), respectively (Table 1). Notably, BvLzm accumulation was highly enhanced in the new stacked five promoter pUDEPB:BvLzm lines by 7.3–13.8-fold, compared to the receiving stacked triple promoter pUDE:BvLzm lines. Together, these experiments demonstrate that high levels of recombinant BvLzm (up to 11.5% of TSP or 82.5 mg/kg) can be successfully produced in sugarcane culms using the combinatorial promoter and event stacking strategies. Previous studies utilized multiple plant species, tissue types, and expression systems for recombinant protein production40,41. Majority of them used transient Agrobacterium- and viral vector-based approaches in Nicotiana benthamiana or N. tabacum42,43,44,45,46. While the transient systems are viable approaches, they are technically feasible only in few plant species that are amenable for infiltration and/or are hosts for the viruses used as viral vectors. In this context, transgenic plant systems are more suited for wider adoption since broad range of plant species can be transformed using latest biotechnology tools. When comparing our results of protein expression in sugarcane culms with other transgenic plant expression systems, caution was exercised particularly when comparing recovered protein yields per starting tissue weight (e.g., mg/kg). This is because not all plant tissues have similar compositions, nor the protein extractions are equally efficient among tissue types, owing to biological and biochemical differences41. For instance, sugarcane culms primarily constitute juice (sugars) and lignocellulosic fiber (bagasse). An equal amount of N. benthamiana leaves on a fresh weight basis will have less fiber, and proteins may be easier to extract from leaf tissues. We also note that biochemical properties of target proteins such as size, solubility, amino-acid composition, structural features, and protein stability may also ultimately influence the final yield. With these caveats in mind, we compared our results with other reported studies of transgenic plant systems using the % TSP unit of recovered proteins. Several studies have reported recombinant protein yields of ~ 0.002 to 0.05% of TSP in transgenic carrots47,48, ~ 0.23–2.5% of TSP in transgenic tobacco and potato49, ~ 8% TSP in transgenic tomato50, and ~ 11.9% in transgenic rice51. These comparisons suggest that higher protein yields can be achieved using the sugarcane transgenic system (up to 11.5% of TSP), which are comparable to other transgenic systems, if not greater.
In addition to the use of constitutive or tissue-specific promoters, inducible promoters can be used for expressing recombinant proteins in plants52,53,54. Several inducible promoters can be used for generating transgenic plants such as dexamethasone-, ethylene-, heat shock- and estradiol-inducible promoters52. Indeed, we have previously shown that the sugarcane DIRIGENT (SHDIR16) promoter is responsive to plant hormones such as salicylic acid or jasmonic acid35. This is promising and suggests that inducible promoters such as SHDIR16, and other well-characterized plant inducible-promoters52 can be further used in lieu or in combination with the constitutive/tissue-specific promoters that we have described, in order to robustly control and/or fine-tune the recombinant protein expression.
Increased protein levels were associated with the number of combinatorial stacked promoters and not with the copy number alone
Our results show that using multiple different promoters to drive expression of recombinant BvLzm on distinct vectors enhanced recombinant protein accumulation. It is possible that the enhanced levels may have occurred due to higher number of inserted BvLzm copies alone or it could be due to a combination of promoter-driven synergistic transcriptional activity. To test these scenarios, we performed a comparison of the BvLzm transcript and yield among the various promoter stacked lines that had similar number of insertions. This analysis showed that there is a positive correlation in BvLzm transcript and yield with combinatorial promoter:BvLzm stacks, irrespective of the number BvLzm inserts (Fig. 2; Supplementary Fig. S2). For instance, for single promoter:BvLzm line 13, double promoter:BvLzm line 42, triple promoter:BvLzm line 20 and quadruple promoter:BvLzm line 10, with all of them having about 4–5 BvLzm inserts, there was a clear enhancement in the BvLzm yield (Fig. 2a,c; Supplementary Fig. S2). Conversely, a comparison of single promoter:BvLzm transgenic lines with one or multiple inserts showed that there was no corresponding increase in BvLz yield with the copy number. For instance, line 19 with one insert (Fig. 2a,c; Supplementary Fig. S2) had a BvLzm yield of 0.2 mg/kg, while line 13 with 4 BvLzm inserts had a BvLzm yield of 0.15 mg/kg (Fig. 2a, c; Supplementary Fig. S2). Together, these results suggest that the increase in BvLzm yield is primarily attributed to the number of combinatorial stacked multiple promoters and not just with the BvLzm copy number alone.
Combinatorial promoter stacking may alleviate transcriptional occlusion and/or recombinant gene silencing
Multiple identical copies of recombinant genes or promoter transcription units (PTUs) delivered through a single construct could trigger transgene silencing55,56,57 or result in promoter occlusion or transcriptional interference, a phenomenon observed in eukaryotic systems, including plants58,59,60,61,62. For instance, a strong PTU can sequester most of the transcription factors in its immediate vicinity, limiting transcription from other promoters present in cis on the same vector63. Alternatively, homology-dependent DNA methylation within the promoter or in the coding region sequences could result in transgene silencing. For instance, in maize, transgenic lines with four copies of a cellulase gene, under control of tandemly arranged PTUs on the same vector, resulted in lowered expression than those lines with fewer copies64.
Our results here showed a positive correlation between the number of combinatorial promoter stacks of recombinant BvLzm and increase in BvLzm levels, with no apparent transgene silencing. It is likely that using different promoter sequences in separate vectors may overcome the transgene silencing or transcriptional interference. We suggest that each expression vector in the described stacked multiple promoter:BvLzm system (Fig. 1) does not negatively affect the others, as shown by a positive correlation between the combinatorial promoter:BvLzm copy number (Table 2) and enhanced steady-state BvLzm transcript accumulation (Fig. 2b; Supplementary Fig. S3) and BvLzm activity (Table 1; Fig. 2c).
Elevated recombinant BvLzm accumulation positively enhances transgenic plant growth
Analysis of the deleterious effects of recombinant protein accumulation on plant physiology and growth is crucial in order to assess the economic feasibility of using transgenic plants as biofactories, and this is largely dependent on the target protein function65. In our scenario with BvLzm, we found no deleterious effects of enhanced BvLzm expression on sugarcane growth. On the contrary, several growth characteristics of BvLzm expressing lines were better than those of non-transformed plants, such as enhanced leaf length, culm height, tiller number, culm biomass and Brix (total soluble solids) (Table 3). These differences were statistically significant (p < 0.001 and p < 0.0001) in the triple and quadruple promoter:BvLzm expressing lines (Table 3). For instance, mean culm fresh biomass per plant of the quadruple promoter:BvLzm expressing lines was nearly 2.5 times greater than that of non-transformed plants. The mean soluble solids content in juice from triple and quadruple promoter:BvLzm expressing lines was approximately 20% higher than that of non-transformed plants. Similar trends were also observed for leaf length, culm height and tiller density. The enhanced agronomic performance of the transgenic lines suggested that BvLzm, which is a well-known antimicrobial protein27, could have a growth-promoting or perhaps protective role against pathogens present in the natural growth environment.
Recombinant protein accumulation in culms increases with plant age
To monitor the temporal stability of BvLzm accumulation in sugarcane culms in a growing season, we analyzed BvLzm levels for 11-months with a selection of several representative single promoter pU:BvLzm lines. The BvLzm yields (mg of BvLzm/kg of harvested culm) in these lines after 7-, 9- and 11-month-harvest are shown in Fig. 4 (data for four representative lines) and Table 3 (data for six representative lines at the 11 month-harvest). There was a significant (p < 0.05) increase in BvLzm yield over time for all the lines tested. BvLzm accumulation was highest at the 11-month harvest, with lines 67, 108 and 114 showing the most significant (p < 0.05) increase (Fig. 4). This accumulation pattern coincides with timing of culm ripening, which is characterized by increased sucrose translocation and accumulation in culms. The age-related sucrose accumulation also was associated with the reduction in vegetative development (leaf initiation and expansion) and commences at the mature basal internodes, progressing towards the culm apex, until the entire culm reaches a stable sugar level as it approaches physiological maturity66. The age-related pattern of BvLzm accumulation may also be regulated by similar factors whereby photoassimilates and other substrates for BvLzm are diverted from vegetative growth towards metabolite synthesis and accumulation during sugarcane maturation. Regardless of the mechanisms regulating the temporal accumulation of BvLzm, our results demonstrate that the recombinant protein levels can be maintained, if not enhanced, during the development phases of sugarcane in a growing season. Similar results were observed for BvLzm accumulation in representative triple promoter:BvLzm lines, which showed sustained and stable BvLzm levels over a full growing year, as well as in successive vegetative propagations (Supplementary Table S3). Similar accumulation of recombinant proteins (human therapeutic interleukin-10) with plant maturity was observed in tobacco67.
Temporal pattern of recombinant bovine lysozyme (BvLzm) accumulation in culms of single promoter:BvLzm expressing sugarcane lines. BvLzm activity of four representative maize ubiquitin 1 promoter:BvLzm lines is shown as determined by enzyme-linked immunosorbent assay in 200.0 ml of juice extract from the 7- and 9-month-harvests, and 650.0–700.0 ml of juice extract from the 11-month-harvest (one kg of culm for all harvests). Values represent four biological samples for each BvLzm expressing line and are reported with standard errors from three technical replications. BvLzm: maize codon-optimized BvLz. Values with different letters are significantly different (p < 0.05).
High level recombinant protein accumulation requires adequate mineral nutrition to sustain the protein and biomass accumulation
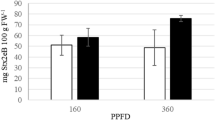
Adequate water and nutrients supply are important for crop productivity as well as quality considerations, such as protein content and other sensory traits68. Because we observed enhanced growth traits such as biomass in the BvLzm expressing lines, specifically in the triple promoter:BvLzm lines (Table 3), we next investigated the optimal fertilization regime needed to sustain the additional growth and high levels of BvLzm production. Four representative triple promoter:BvLzm lines (2-month old) were subjected to two mineral nutrient supply regimes namely, low fertility (LF or 2.4 mg N per plant, twice a week) and a high fertility (HF or 8 mg N per plant), using a balanced commercial fertilizer (Peters Professional 20–20–20; see “Materials and methods” section). BvLzm yield and growth traits were measured at 2-, 6-, and 8-months following fertilization. Supplemental fertilization increased culm biomass and BvLzm yield in the triple promoter:BvLzm lines over time. The most significant increases (p < 0.05) between LF and HF were noted at 2 months for all lines (Fig. 5). For instance, pUPE:BvLzm line 32C (CP72-1210 variety) and pUDE:BvLzm lines 19, 44 and 54 (TCP98-4454 variety) showed 4.6-, 2.5-, 3.0- and 2.0-fold increases in culm biomass and 1.7-, 1.3-, 1.1 and 1.0-fold enhancements in BvLzm yield, respectively.
Enhancement of culm biomass and yield of recombinant bovine lysozyme (BvLzm) by fertilization in triple promoter:BvLzm sugarcane lines. BvLzm activity of four representative lines is shown as determined by enzyme-linked immunosorbent assay in juice extract of culms (1.0 kg of culm). Values represented four biological samples and three technical replications at 2, 6 and 8 months following low (LF) or high (HF) fertilization. Values with different letters are significantly different (p < 0.05). BvLzm, maize codon-optimized BvLz; 32C, pUPE:BvLzm line; 18, 44 and 54, pUDE:BvLzm lines; U, maize ubiquitin 1 promoter; P, sugarcane proline-rich protein promoter; E, sugarcane elongation factor 1α promoter; and D, sugarcane dirigent16 promoter.
Leaf macronutrient contents of the triple promoter:BvLzm plants were also monitored following growth under the two fertilization regimes. Plants grown under high nutrient supply rates had significantly (p < 0.0001) higher leaf mineral nutrient contents compared to those grown under low nutrient supply rates (Table 4). Leaves of HF plants had higher levels of N, phosphorus (P), potassium (K) and magnesium (Mg), compared to leaves of LF plants (Table 4). In general, leaf nutrient content of the BvLzm expressing lines was improved by supplemental fertilization, resulting in a 1.5- to 2.2-fold increase in culm biomass and a subsequent 1.2- to 2.2-fold enhancement in BvLzm yield at 8 month-growth stage (Fig. 5). Taken together, the accumulation of BvLzm in response to fertilization and the ontogenic BvLzm accumulation pattern underscore the need for adequate input availability to sustain not only biomass production but also the yield of high-value proteins in crops such as sugarcane.
Conclusions
The genetic/biotechnology tools and resources developed in this study not only expands the utility of sugarcane for large-scale production of recombinant proteins but can be utilized with other monocots and bioenergy feedstocks. Our approach comprises stacking multiple promoters to co-express codon-optimized recombinant genes from different expression vectors using combinatorial transformation methods. This resulted in high recombinant protein yield (up to 11.5% of TSP or 82.5 mg/kg) in transgenic culms, rendering it an attractive biopharming tool for potential commercial uses69. We also showed that recombinant BvLzm levels can be maintained stably throughout the growing season and had no negative consequences on sugarcane agronomic performance. Overall, our study provides new knowledge, tools and resources to expand the utility of sugarcane beyond a food crop and bioenergy feedstock to using it as a biofactory for expressing high-value proteins25.
Materials and methods
Expression vectors
Basic vectors
A series of expression vectors were constructed, using a custom synthesized bovine lysozyme (BvLz) gene codon-optimized for expression in maize (BvLzm) (444.0 base pairs [bp])39 (GenScript, Piscataway, NJ).
The BvLzm gene was subcloned into pUC57 at BamHI and cloned at the same site into pZero2 (Invitrogen, ThermoFisher Scientific, Waltham, MA), to which the 35ST34,38,39 (197.0 bp) was added at the PstI site, resulting in the BvLzm-35ST/pZero2 plasmid.
Three basic BvLz expression vectors were generated with the constitutive promoters pUbi33, pSHPRP36 or pSHEF1α36. The first vector, pUbi-BvLzm-35ST/pZero2 was produced by cloning the pUbi fragment (1,977 bp), released from pAHC20 (pUbi:BAR/pUC8)70 (pUbi minus heat shock element; a 28.0 bp deletion at the 5′ end of pUbi) with BamHI/HindIII and filled in, into the filled-in BvLzm-35ST/pZero2. For the other two vectors, the SmaI-treated pSHPRP (3,016 bp) and pSHEF1α (1,959 bp) fragments from pSK+36 were fused to the SnaBI/BbsI-treated/filled-in BvLzm-35ST fragment from pUbi-BvLzm-35ST/pZero2 to yield pSHPRP-BvLzm-35ST/pSK+ and pSHEF1α-BvLzm-35ST/pSK+, respectively.
Two basic BvLz expression vectors were generated with the culm-regulated promoters pSHDIR1635 or pSCBV2134. The pSHDIR16-BvLzm-35ST/pSK+ vector was assembled by fusing BvLzm-35ST, excised from BamHI/EcoRI-treated BvLzm-35ST/pZero2, to the pSHDIR16 fragment35 (2,680 bp) at the same sites in pSK+. The pSCBV21-BvLzm-35ST/pGEMT-T Easy vector was produced by cloning BvLzm-35ST, excised from BamHI/EcoRI-treated BvLzm-35ST/pZero2, into the NcoI-treated/filled-in pSCVB21 (1,816 bp)/pGEM-T Easy34.
Double terminator vectors
BvLz constructs with a double terminator were generated by fusing the NOST (253 bp)39 to the 35ST of basic BvLz constructs. The pUbi-BvLzm-35STNOST/pZero2 vector was constructed by releasing the NOST from pBI221 (Accession Number AF502128) (Clontech Laboratories, Inc., Mountain View, CA) with EcoRI/SstI, filled in and cloned into the XhoI-treated/filled-in pUbi-BvLzm-35ST/pZero2. To make pSHPRP-BvLzm-35STNOST/pSK+ and pSHEF1α-BvLzm-35STNOST/pSK, the SnaBI/BbsI-treated/filled-in BvLzm-35STNOST fragment from pUbi-BvLzm-35STNOST/pZero2 was fused to the SmaI-treated pSHPRP/pSK+ and pSHEF1α/pSK+ vectors, respectively. To generate the pSCBV21-BvLzm-35STNOST/pGEM-T Easy vector, the SnaBI/BbsI-treated/filled-in BvLzm-35STNOST fragment from pUbi-BvLzm-35STNOST/pZero2 was cloned into NcoI-treated/filled-in pSCVB21/pGEM-T Easy.
Vectors with viral untranslated regions
The 3′UTR of SrMV strain H (GenBank Accession Number U57358) (235.0 bp) was custom synthesized as a fusion to BvLzm in pJI (BvLzm-SrMV 3′UTR/pJI) (ATUM, DNA2.0, Newark, CA). The pUbi-BvLzm-SrMV 3′UTR-35ST/pZero2 vector was assembled by cloning the filled-in SrMV 3′UTR, released from EcoRI/BglII-treated BvLzm-3′SrMV/pJI, into pUbi-BvLzm-35ST/pZero2 at the SmaI site. The pSHPRP-BvLzm-SrMV 3′UTR-35ST/pSK+ and pSHEF1α-BvLzm-SrMV 3′UTR-35ST/pSK+ vectors were generated by fusing the SnaBI/BbsI-treated/filled-in BvLzm-SrMV 3′UTR-35ST fragment from the pUbi-BvLzm-SrMV 3′UTR-35ST/pZero2 to pSHPRP/pSK+ and pSHEF1α/pSK+ at the SmaI site, respectively. For construction of pSHDIR16-BvLzm-SrMV 3′UTR-35ST/pSK+ vector, SrMV 3′UTR was released from BvLzm-SrMV 3′UTR/pJI by EcoRV treatment and cloned into pSHDIR16-BvLzm-35ST/pSK+ at the EcoRV site.
All DNA cloning steps were carried out as described by Sambrook71. Filling in of endonuclease-treated DNA fragments and dephosphorylation of vectors were done using T4 DNA polymerase (NEB BioLabs, Ipswich, MA) and antarctic phosphatase (NEB BioLabs), respectively.
Sugarcane transformation
Tops of field-grown sugarcane (Saccharum spp. hybrids) commercial varieties CP72-1210, CP84-1198, TCP87-3388 and TCP98-4454 were collected during the growing season, and leaf roll discs were prepared for stable transformations as previously described72. Briefly, leaf blades and sheaths were removed down to the top visible dewlap leaf, and the upper 20–30 cm portion of shoot (leaf roll culm) was surface sterilized in 70.0% (v/v) ethanol for 20 min. Immature leaf rolls close to the apical meristem were sliced transversely into 1.0 mm thick sections and cultured on MS3 medium (MS medium with 3.0 mg/l of 2,4-dichlorophenoxyacetic acid [2,4-D]) for 30–35 days (for embryogenic calli) or MS0.6 medium (MS with 0.6 mg/l of 2,4-D) for 7–10 days (for embryogenic leaf roll discs). Embryogenic calli and leaf roll discs were preconditioned on MS3- and MS0.6-osmoticum (MS3 or MS0.6 with 0.2 M d-mannitol and 0.2 M d-sorbitol), respectively, for 4 h before and after DNA particle bombardment. DNA bombardment was performed according to Beyene and colleagues38. Briefly, tungsten particles (1.1 µm; Bio-Rad Laboratories, Inc.) (1.0 mg) were coated separately with plasmid DNA (1.0 µg) of different constructs at equimolar ratios together with pUbi:BAR/pUC8 selectable marker plasmid using calcium chloride (NaCl) (1.0 M) and spermidine (14.0 mM). The DNA particle suspension (containing the selectable marker plasmid with one or more BvLzm plasmids) (4.0 μl; 0.5 µg DNA per bombardment) was placed at the center of a syringe filter and delivered into tissue with a particle inflow gun using a 26.0-inch Hg vacuum and a 7.0-cm target distance. Bombarded embryogenic calli and leaf roll discs were maintained on MS3 and MS0.6, respectively, for 10 days in the dark at 28 °C for recovery. They were later incubated in the dark at 28 °C on selection medium (MS3 or MS0.6 with bialaphos at 3.0 mg/l) for a total of 2 weeks. Shoot regeneration and root initiation were performed under bialaphos selection as previously described72. Rooted plantlets were transferred to potting soil (Sunshine Mix #1; SunGro Horticulture Distribution, Inc., Agawan, MA) in pots and maintained in the greenhouse.
Transgenic plant screening
Integration and size determination of BvLzm expression cassettes
Integration and size of each BvLzm expression cassette in the single and multiple stacked promoter:BvLzm sugarcane lines were determined by Southern blot and PCR analyses, respectively, using genomic DNA isolated according to Tai and Tanksley73 from liquid N-ground tissues (3.0 g) collected from young leaves of 3–4 month-old plants. Controls included vector-transformed lines and non-transformed plants (tissue culture-derived).
For Southern blot analysis, genomic DNA (10.0 μg per lane) was treated with HindIII endonuclease, electrophoresed on 0.8% (w/v) agarose gels and transferred to nylon membranes (Amersham Hybond-XL, GE Healthcare Bio-Sciences Corp., Piscataway, NJ) in 0.4 M sodium hydroxide74. Pre-hybridization, hybridization, washing and detection of DNA gel blots were performed using Church’s buffer75. The probe, corresponding to the BvLzm coding sequence was amplified by PCR from pUbi-BvLzm-35ST/pZero2 using the primer set BvLz-1F (5′-ATGGCGGCCCTGGTGATCCTGGGCT-3′) and BvLz-481R (5′-TCACAGGGTGCAGCCTTCCACG-3′) and labeled with [α-32P] dCTP using the Random DNA Labeling kit (Invitrogen, ThermoFisher Scientific).
PCR was performed on a C1000 Touch thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA) in a total reaction volume of 25.0 µl using 200.0 ng of DNA and Platinum Taq DNA polymerase (Invitrogen, ThermoFisher Scientific) according to the manufacturer’s instructions with the following conditions: 94 °C for 4 min, 35 cycles each at 94 °C for 30 s, 49.7–54.4 °C for 30 s, and 72 °C for 6 min. Primers encompassing the entire promoter:BvLzm-terminator cassette (Supplementary Table S4) were designed with Primer 3.0. All PCR amplicons were separated by electrophoresis on 0.7% agarose (w/v) gels stained with ethidium bromide. A “no DNA template” was included as a negative control for PCR.
Determination of BvLzm copy number
BvLzm copy number in single and multiple stacked promoter:BvLzm sugarcane lines was estimated by qPCR. qPCR was performed on a CFX384 Real-time PCR Detection System (Bio-Rad Laboratories, Inc.) using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc.), 0.4 µM of each target specific primer and 1.0 ng of genomic DNA from representative transgenic BvLzm lines, according to the manufacturer’s instructions. Primers specific to the promoter-BvLzm gene junction area (Supplementary Table S4) were designed with Primer 3.0 (https://bioinfo.ut.ee/primer3-0.4.0/primer3/). qPCR conditions were as follows: 95.0 °C for 3 min, 39 two-step cycles each at 96.0 °C for 5 s and 57 °C for 30 s, and a final melting curve of 60.0 °C to 95.0 °C for 6 min. The sugarcane anthranilate phosphoribosyltransferase and prolyl 4-hydroxylase genes were used as a reference for single copy genes76. qPCR was performed twice in triplicate with two biological replications. PCR efficiency was calculated with LinReg77. Results were analyzed and recorded as CT (threshold cycle) values. Copy number of the BvLzm gene was estimated by qPCR according to Casu et al.76 using the formula GCI = EffRefCT/EffCT, where: GCI = gene copy number index, EffRefCT = PCR efficiency using the reference gene primers to the power of the reference gene CT value for each sample, and EffCT = PCR efficiency using the test gene primers to the power of the test gene CT value generated for each sample.
Expression analysis of BvLzm
Total RNA was isolated by grinding 1.0 g of young leaves collected from 3–4 month-old plants in liquid N39,78. For northern blot analysis, RNA (15.0 μg per lane) was fractionated on 1.6% formaldehyde agarose denaturing gels in HEPES buffer and blotted onto nylon membranes (Amersham Hybond-XL) in 10x SSC75. Pre-hybridization, BvLzm probe labeling, hybridization, washing and detection of RNA gel blots were performed as described for Southern blot analysis.
Plant growth and treatment conditions
For growth cycle investigations, single-node culm cuttings of 15 single promoter pU:BvLzm transgenic lines and non-transformed plants were pre-germinated in seedling flats (Supplementary Fig. S1) for 2.5 weeks and transplanted into 37.0-l pots (four pots per line) in commercial growth medium (Sunshine Mix #1). Plants were maintained in a temperature-regulated greenhouse with average day/night temperatures of 32/22 °C and relative humidity of 60–100%. Plants were initially fertilized once per week with a commercial high-phosphorus soluble fertilizer (Peters 8%N-19.8%P-12.5%K; The Scotts Company, Marysville, OH) for 5 weeks and then with a balanced/complete soluble fertilizer (Peters Professional 20–20–20; The Scotts Company) containing N 200.0 g/kg, P 80.0 g/kg, K 166.0 g/kg, Mg 1.0 g/kg, iron 0.5 g/kg, manganese 0.3 g/kg, boron 0.1 g/kg, copper 0.13 g/kg, molybdenum 0.05 g/kg, and zinc 0.25 g/kg.
To assess the impacts of mineral nutrient supply on growth and BvLzm accumulation, plants from four representative triple promoter pUDE:BvLzm lines and one representative triple promoter pUPE:BvLzm line were pre-germinated and transplanted into 15.0-l plastic pots containing the same growth medium as described above. All pots were initially fertilized with a high-phosphorus fertilizer (Peters 8%N–19.8%P–12.5%K; Scotts, Marysville, OH; equivalent to 10.0 kg N/ha). After 2 months, pots were randomly assigned into two fertilization treatment groups, namely, high fertility (HF) and low fertility (LF), with four pots per line selected for each group. Non-transformed plants (tissue culture-derived) were included as negative controls. Fertilization treatments were achieved with a complete fertilizer (Peters Professional 20–20–20) containing macro- and micro-nutrients as described above. Plants in the LF group received an additional equivalent of 20.0 kg N/ha whereas HF plants received 50.0 kg N/ha from supplemental fertilization using Peters Professional 20–20–20 (described above). Fertilizer treatments were applied in split doses (twice per week). Transgenic culms were harvested at 2, 6 and 8 months following fertilization, processed, and their BvLz yield was determined by ELISA at the BioSeparation Facility of Texas A&M University’s Biological and Agricultural Engineering Department (College Station, Texas).
Plant physiological analysis
For inorganic mineral analysis, leaf tissue samples were collected, dried (70 °C for 48 h), ground to pass a 40-μm screen and analyzed for inorganic minerals. Total Kjeldahl N (ammonia and organic N) was determined in digested samples using the EasyChem Plus Analyzer and protocols (Systea Scientific, Chicago, IL), whereas other macronutrients such as P, K and Mg were analyzed using the Optima 7300 DV Inductively Coupled Plasma-Optical Emission Spectrometer (PerkinElmer, Shelton, CT) after partial digestion (hydrolysis) on a HotBlock Digestion System (Environmental Express, Inc., Charleston, SC).
Total protein extraction
Large-scale extraction and size fractionation of total soluble proteins (TSPs) from culms (300.0 lbs) of BvLzm transgenic sugarcane were performed at our Pilot Plant Facility mainly as described previously79. Bench-scale extraction and purification of BvLzm from extracts of transgenic sugarcane culms (100.0 g), using a single-step hydrophobic interaction chromatography, were performed at our BioSeparation Facility (College Station, Texas) as previously described30.
For small-scale extraction of TSP from BvLzm transgenic sugarcane leaf tissue (200.0 mg) was homogenized in 600.0 µl of sodium acetate buffer (50 mM NaOAc, pH 4.4, 0.1 M NaCl) in 2.0 ml tubes for 30 s at 5,000 rpm with the Precellys 24 homogenizer (MO BIO Laboratories, Carlsbad, CA) using ceramic spherical beads (0.64 cm-diameter). TSP supernatants were collected by centrifugation at 13,000g for 25 min at 4 °C.
Determination of BvLzm accumulation by enzyme activity and enzyme-linked immunosorbent assays
To determine the levels of recombinant BvLzm, enzyme activity and enzyme-linked immunosorbent assays (ELISA) were performed on TSP from culm extract juice. Juice was extracted from 1.0 kg of culms of greenhouse grown BvLzm transgenic plants at 7, 9 and 11 months for the growth cycle experiment and at 2, 6 and 8 months for the fertilization experiment. For enzyme activity determination, culm extract juice was tested for its ability to lyse Micrococcus lysodeikticus cells using the standard protocol from Sigma-Aldrich (St. Louis, MO). Rabbit anti-BvLz antibody used in the ELISA was synthesized by Bethyl Laboratories, Inc. (Montgomery, TX) using tobacco-derived BvLz31 and further purified through an SP-Sepharose column (GE Healthcare, Piscataway, NJ). ELISA of culm extract juice was performed as previously described30. Briefly, a sandwich ELISA consisting of anti-BvLz antibody was used to capture BvLz in juice. Detection was performed using a biotinylated anti-BvLz antibody and horseradish peroxidase-labeled NeutrAvidin (Pierce, ThermoFisher Scientific). The standard curve was generated using BvLz produced in Pichia pastoris as in Digan et al.80.
Statistical analysis
Agronomic data were collected from 3 to 4 independent experiments, with 3–4 replicates per experiment and subjected to an analysis of variance (ANOVA) using the General Linear Model procedure of the Statistical Analysis System 9.4 (SAS Institute Inc., Cary, NC). Mean separation was performed using the Student–Newman–Keuls (SNK) test.
References
Demain, A. & Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv.27, 297–306. https://doi.org/10.1016/j.biotechadv.2009.01.008 (2009).
Basaran, P. & Rodríguez-Cerezo, E. Plant molecular farming: opportunities and challenges. Crit. Rev. Biotechnol.28, 153–172. https://doi.org/10.1080/07388550802046624 (2008).
Rybicki, E. P. Plant-made vaccines for humans and animals. Plant Biotechnol. J.8, 620–637 (2010).
Wiktorek-Smagur, A. et al. Green way of biomedicine—how to force plants to produce new important proteins. In Transgenic Plants-Advances and Limitations (ed. Çiftçi, Y. O.) 63–90 (InTech, Rijeka, 2012).
Twyman, R. M., Stoger, E., Schillberg, S., Christou, P. & Fischer, R. Molecular farming in plants: host systems and expression technology. Trends Biotechnol.21, 570–578 (2003).
Brumbley, S. M., Purnell, M. P., Petrasovits, L. A., Nielsen, L. K. & Twine, P. H. Developing the sugarcane biofactory for high-value biomaterials. Int. Sugar J.1297, 5 (2007).
Ando, S. et al. Overwintering ability and dry matter production of sugarcane hybrids and relatives in the Kanto Region of Japan. Jpn. Agric. Res. Q.45, 259–267. https://doi.org/10.6090/jarq.45.259 (2011).
Matsuoka, S., Kennedy, A. J., Santos, E. G. D., Tomazela, A. L. & Rubio, L. C. S. Energy cane: its concept, development, characteristics, and prospects. Adv. Bot.2014, 597275 (2014).
Gallo-Meagher, M. & Irvine, J. E. Effects of tissue type and promoter strength on transient GUS expression in sugarcane following particle bombardment. Plant Cell Rep.12, 666–670. https://doi.org/10.1007/BF00233416 (1993).
Lakshmanan, P. et al. Sugarcane biotechnology: the challenges and opportunities. Vitro Cell. Dev. Biol. Plant41, 345–363 (2005).
Anderson, D. J. et al. Synthesis of short-chain-length/medium-chain length polyhydroxyalkanoate (PHA) copolymers in peroxisomes of transgenic sugarcane plants. Trop. Plant Biol.4, 170–184. https://doi.org/10.1007/s12042-011-9080-7 (2011).
McQualter, R. B. et al. Initial evaluation of sugarcane as a production platform for p-hydroxybenzoic acid. Plant Biotechnol. J.3, 29–41 (2005).
Petrasovits, L. A. et al. Enhanced polyhydroxybutyrate production in transgenic sugarcane. Plant Biotechnol. J.10, 569–578 (2012).
Purnell, M. P., Petrasovits, L. A., Nielsen, L. K. & Brumbley, S. M. Spatio-temporal characterization of polyhydroxybutyrate accumulation in sugarcane. Plant Biotechnol. J.5, 173–184 (2007).
Tilbrook, K., Gebbie, L., Schenk, P. M., Poirier, Y. & Brumbley, S. M. Peroxisomal polyhydroxyalkanoate biosynthesis is a promising strategy for bioplastic production in high biomass crops. Plant Biotechnol. J.9, 958–969 (2011).
Chong, B. F. et al. Co-ordinated synthesis of gentiobiitol and sorbitol, evidence of sorbitol glycosylation in transgenic sugarcane. Phytochemistry71, 736–741. https://doi.org/10.1016/j.phytochem.2010.01.014 (2010).
Mudge, S. R. et al. Mature-stem expression of a silencing-resistant sucrose isomerase gene drives isomaltulose accumulation to high levels in sugarcane. Plant Biotechnol. J.11, 502–509 (2013).
Wu, L. & Birch, R. G. Doubled sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotechnol. J.5, 109–117. https://doi.org/10.1111/j.1467-7652.2006.00224.x (2007).
Wang, M.-L., Goldstein, C., Su, W., Moore, P. H. & Albert, H. H. Production of biologically active GM-CSF in sugarcane: a secure biofactory. Transgenic Res.14, 167–178 (2005).
Henrique-Silva, F. & Soares-Costa, A. Transgenic Plants 437–450 (Springer, Berlin, 2012).
Gianotti, A. et al. Recombinant expression, purification, and functional analysis of two novel cystatins from sugarcane (Saccharum officinarum). Protein Expr. Purif.47, 483–489. https://doi.org/10.1016/j.pep.2005.10.026 (2006).
Ribeiro, C. W. et al. Production of a His-tagged canecystatin in transgenic sugarcane and subsequent purification. Biotechnol. Prog.24, 1060–1066 (2008).
Harrison, M. D. et al. Accumulation of recombinant cellobiohydrolase and endoglucanase in the leaves of mature transgenic sugar cane. Plant Biotechnol. J.9, 884–896. https://doi.org/10.1111/j.1467-7652.2011.00597.x (2011).
Harrison, M. D. et al. Recombinant cellulase accumulation in the leaves of mature, vegetatively propagated transgenic sugarcane. Mol. Biotechnol.56, 795–802 (2014).
Palaniswamy, H. et al. Vacuolar targeting of r-proteins in sugarcane leads to higher levels of purifiable commercially equivalent recombinant proteins in cane juice. Plant Biotechnol. J.14, 791–807 (2016).
Jolles, P. et al. Stomach lysozymes of ruminants. II. Amino acid sequence of cow lysozyme 2 and immunological comparisons with other lysozymes. J. Biol. Chem.259, 11617–11625 (1984).
Sahoo, N. R. et al. Lysozyme in livestock: a guide to selection for disease resistance: a review. J. Anim. Sci. Adv.2, 347–360 (2012).
Lemos, F. J. A., Ribeiro, A. F. & Terra, W. R. A bacteria-digesting midgut-lysozyme from Musca domestica (Diptera) larvae. Purification, properties and secretory mechanism. Insect Biochem. Mol. Biol.23, 533–541 (1993).
Mirkov, T. E. & Fitzmaurice, L. C. Google patents (1995).
Barros, G. O. F. et al. Recovery of bovine lysozyme from transgenic sugarcane stalks: extraction, membrane filtration, and purification. Bioprocess Biosyst. Eng.36, 1407–1416. https://doi.org/10.1007/s00449-012-0878-y (2013).
Wilcox, C. P. et al. Production and purification of an active bovine lysozyme in tobacco (Nicotiana tabacum): utilization of value-added crop plants traditionally grown under intensive agriculture. J. Agric. Food Chem.45, 2793–2797 (1997).
Bock, R. Strategies for metabolic pathway engineering with multiple transgenes. Plant Mol. Biol.83, 21–31. https://doi.org/10.1007/s11103-013-0045-0 (2013).
Christensen, A. H., Sharrock, R. A. & Quail, P. H. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol.18, 675–689. https://doi.org/10.1007/BF00020010 (1992).
Gao, S.-J. et al. A novel Sugarcane bacilliform virus promoter confers gene expression preferentially in the vascular bundle and storage parenchyma of the sugarcane culm. Biotechnol. Biofuels10, 172–172. https://doi.org/10.1186/s13068-017-0850-9 (2017).
Damaj, M. B. et al. Sugarcane DIRIGENT and O-METHYLTRANSFERASE promoters confer stem-regulated gene expression in diverse monocots. Planta231, 1439–1458. https://doi.org/10.1007/s00425-010-1138-5 (2010).
Yang, M., Bower, R., Burow, M. D., Paterson, A. H. & Mirkov, T. E. A rapid and direct approach to identify promoters that confer high levels of gene expression in monocots. Crop Sci.43, 1805–1813 (2003).
Woodard, S. L. et al. American Society of Agricultural and Biological Engineers.
Beyene, G. et al. Unprecedented enhancement of transient gene expression from minimal cassettes using a double terminator. Plant Cell Rep.30, 13–25. https://doi.org/10.1007/s00299-010-0936-3 (2011).
Damaj, M. B. & Mirkov, T. E. Compositions, organisms, systems, and methods for expressing a gene product in plants. U.S. patent (2019).
Moon, K.-B. et al. Development of systems for the production of plant-derived biopharmaceuticals. Plants9, 30 (2020).
Merlin, M., Gecchele, E., Capaldi, S., Pezzotti, M. & Avesani, L. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. BioMed Res. Int.2014, 1–14 (2014).
Jiang, M.-C., Hu, C.-C., Lin, N.-S. & Hsu, Y.-H. Production of human IFNγ protein in Nicotiana benthamiana plant through an enhanced expression system based on bamboo mosaic virus. Viruses11, 509 (2019).
Zischewski, J., Sack, M. & Fischer, R. Overcoming low yields of plant-made antibodies by a protein engineering approach. Biotechnol. J.11, 107–116 (2016).
Buyel, J. F., Twyman, R. M. & Fischer, R. Very-large-scale production of antibodies in plants: the biologization of manufacturing. Biotechnol. Adv.35, 458–465 (2017).
Sainsbury, F. et al. Rapid transient production in plants by replicating and non-replicating vectors yields high quality functional anti-HIV antibody. PLoS ONE5, e13976 (2010).
Lai, H., He, J., Engle, M., Diamond, M. S. & Chen, Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol. J.10, 95–104 (2012).
Porceddu, A. et al. Transgenic plants expressing human glutamic acid decarboxylase (GAD65), a major autoantigen in insulin-dependent diabetes mellitus. Mol. Breed.5, 553–560 (1999).
Uvarova, E. A. et al. Oral immunogenicity of plant-made Mycobacterium tuberculosis ESAT6 and CFP10. BioMed Res. Int.2013, 1–13 (2013).
Mason, H. S. et al. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl. Acad. Sci.93, 5335–5340 (1996).
Zhang, X., Buehner, N. A., Hutson, A. M., Estes, M. K. & Mason, H. S. Tomato is a highly effective vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol. J.4, 419–432 (2006).
Tokuhara, D. et al. Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J. Clin. Investig.123, 3829–3838 (2013).
Borghi, L. Plant Developmental Biology 65–75 (Springer, Berlin, 2010).
Maruyama, K. et al. Design of an optimal promoter involved in the heat-induced transcriptional pathway in Arabidopsis, soybean, rice and maize. Plant J.89, 671–680 (2017).
Gatz, C. Chemically inducible promoters in transgenic plants. Curr. Opin. Biotechnol.7, 168–172 (1996).
Butaye, K. M. J., Cammue, B. P. A., Delauré, S. L. & De Bolle, M. F. C. Approaches to minimize variation of transgene expression in plants. Mol. Breed.16, 79–91. https://doi.org/10.1007/s11032-005-4929-9 (2005).
Kohli, A. et al. The quest to understand the basis and mechanisms that control expression of introduced transgenes in crop plants. Plant Signal. Behav.1, 185–195. https://doi.org/10.4161/psb.1.4.3195 (2006).
Rajeev Kumar, S., Anunanthini, P. & Ramalingam, S. Epigenetic silencing in transgenic plants. Front. Plant Sci.6, 693. https://doi.org/10.3389/fpls.2015.00693 (2015).
Greger, I. H., Proudfoot, N. J., Demarchi, F. & Giacca, M. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res.26, 1294–1300. https://doi.org/10.1093/nar/26.5.1294 (1998).
Ingelbrecht, I. et al. Transcriptional interference in transgenic plants. Gene109, 239–242 (1991).
Kadesch, T. & Berg, P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol. Cell. Biol.6, 2593–2601 (1986).
Maqbool, S. B. & Christou, P. Multiple traits of agronomic importance in transgenic indica rice plants: analysis of transgene integration patterns, expression levels and stability. Mol. Breed.5, 471–480 (1999).
Shearwin, K. E., Callen, B. P. & Egan, J. B. Transcriptional interference—a crash course. Trends Genet.21, 339–345 (2005).
Horlick, R. A. et al. Combinatorial gene expression using multiple episomal vectors. Gene243, 187–194 (2000).
Egelkrout, E. et al. Enhanced expression levels of cellulase enzymes using multiple transcription units. BioEnergy Res.6, 699–710 (2013).
Urreta, I. & Castañón, S. Transgenic plants as biofactories for the production of biopharmaceuticals: A case study of human placental lactogen. In Transgenic Plants-Advances and Limitations (ed. Çiftçi, Y. O.) 305–328 (InTech, Rijeka, 2012).
Legendre, B. L. Ripening of sugarcane: effects of sunlight, temperature, and rainfall 1. Crop Sci.15, 349–352 (1975).
Duwadi, K., Chen, L., Menassa, R. & Dhaubhadel, S. Identification, characterization and down-regulation of cysteine protease genes in tobacco for use in recombinant protein production. PLoS ONE10, e0130556 (2015).
Marschner, H. Mineral Nutrition of Higher Plants (Academic Press, NY, 1995).
Davies, H. M. Review article: commercialization of whole-plant systems for biomanufacturing of protein products: evolution and prospects. Plant Biotechnol. J.8, 845–861. https://doi.org/10.1111/j.1467-7652.2010.00550.x (2010).
Christensen, A. H. & Quail, P. H. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res.5, 213–218. https://doi.org/10.1007/BF01969712 (1996).
Sambrook, H. C. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1989).
Ramasamy, M. et al. A biolistic-based genetic transformation system applicable to a broad-range of sugarcane and energycane varieties. GM Crops Food9, 211–227 (2018).
Tai, T. H. & Tanksley, S. D. A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. Plant Mol. Biol. Report.8, 297–303 (1990).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2001).
Mangwende, T. et al. The P0 gene of Sugarcane yellow leaf virus encodes an RNA silencing suppressor with unique activities. Virology384, 38–50 (2009).
Casu, R. E., Selivanova, A. & Perroux, J. M. High-throughput assessment of transgene copy number in sugarcane using real-time quantitative PCR. Plant Cell Rep.31, 167–177. https://doi.org/10.1007/s00299-011-1150-7 (2012).
Ruijter, J. M. et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res.37, e45–e45 (2009).
Damaj, M. B. et al. Reproducible RNA preparation from sugarcane and citrus for functional genomic applications. Int. J. Plant Genom.2009, 13. https://doi.org/10.1155/2009/765367 (2009).
Mirkov, T. E., Monclin, J. P., Barrilleaux, A., Irvine, J. E. & Moonan, F. Google patents (2002).
Digan, M. E. et al. Continuous production of a novel lysozyme via secretion from the yeast, Pichia pastoris. Biotechnology7, 160 (1989).
Acknowledgements
We would like to dedicate this manuscript to late Professor T. Erik Mirkov (1959–2018), a pioneer in sugarcane biotechnology. We gratefully acknowledge Xavier Gonzales for the assembly of three genetic constructs, Renesh Bedre for the statistical analysis of data and Sonia Irigoyen (Texas A&M AgriLife Research) for critical review of the manuscript. We are grateful to Denise Rossi, Hyun Park Kang, Ninfa Ramos, Soledad Al-Varez, Gerleene Acuna, Adan Solis and Abigail Cruz for excellent technical assistance. This research was supported by funds from BioCane, Inc., a subsidiary of Grower Research Group LLC (Soledad, CA) and Texas A&M AgriLife Research Grants (124738-96210; 124190-96210) to K.K.M.
Author information
Authors and Affiliations
Contributions
M.B.D., J.L.J., S.L.W., C.V.-B., G.O.F.B., J.M., Z.L.N., and K.K.M. designed the experiments. B.B.D. designed the combinatorial gene expression system and developed total protein enrichment protocols. M.B.D. made the genetic constructs and prepared the manuscript. M.B.D. and J.M. conducted transformation experiments. M.B.D., J.M. and C.V.-B. conducted transgenic plant screening analyses. J.L.J. conducted growth cycle experiments. M.B.D. and J.M. conducted fertilization experiments. S.L.W., G.O.F.B. and S.G.W. conducted protein extraction, ELISA and purification experiments. S.L.W., J.L.J., Z.L.N., and K.K.M. supervised the study and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Damaj, M.B., Jifon, J.L., Woodard, S.L. et al. Unprecedented enhancement of recombinant protein production in sugarcane culms using a combinatorial promoter stacking system. Sci Rep 10, 13713 (2020). https://doi.org/10.1038/s41598-020-70530-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70530-z
This article is cited by
-
Media Optimization for Direct Somatic Embryogenesis, Regeneration in Sugarcane and Genetic Transformation using cry1Ac Gene
Sugar Tech (2024)
-
Escherichia coli methionine-tRNAi/methionyl tRNA synthetase pairs induced protein initiation of interest (PII) expression
Applied Biological Chemistry (2022)
-
Enhancement of healthful novel sugar contents in genetically engineered sugarcane juice integrated with molecularly characterized ThSyGII (CEMB-SIG2)
Scientific Reports (2022)
-
Effects of plant growth regulators on transient expression of foreign gene in Nicotiana benthamiana L. leaves
Bioresources and Bioprocessing (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.