Abstract
In this report, the utilization of mixed methanol–ethanol system for the production of biodiesel from waste cooking oil (WCO) using enhanced eggshell-derived calcium oxide (CaO) nano-catalyst was investigated. CaO nano-catalyst was produced by calcination of eggshell powder at 900 °C and followed by hydration-dehydration treatment to improve its catalytic activity. The particle size, morphology, and elemental composition of a catalyst were characterized by using XRD, SEM, and EDX techniques, respectively. After hydration-dehydration the shape of a catalyst was changed from a rod-like to honeycomb-like porous microstructure. Likewise, average particle size was reduced from 21.30 to 13.53 nm, as a result, its surface area increases. The main factors affecting the biodiesel yield were investigated, accordingly, an optimal biodiesel yield of 94% was obtained at 1:12 oil to methanol molar ratio, 2.5 wt% catalyst loading, 60 °C, and 120-min reaction time. A biodiesel yield of 88% was obtained using 6:6 equimolar ratio of methanol to ethanol, the yield even increased to 91% by increasing the catalyst loading to 3.5 wt%. Moreover, by slightly increasing the share of methanol in the mixture, at 8:4 ratio, the maximum biodiesel yield could reach 92%. Therefore, we suggest the utilization of methanol–ethanol mixture as a reactant and eggshell-derived CaO as a catalyst for enhanced conversion of WCO into biodiesel. It is a very promising approach for the development of low-cost and environmentally friendly technology. Properties of the biodiesel were also found in good agreement with the American (ASTM D6571) fuel standards.
Similar content being viewed by others
Introduction
Energy is among the key pillars for one’s country’s economic and social development. Currently, lion shares of the world energy demand still supplied through non-renewable sources, which accounts for about 78% of the total share. Nowadays they are being consumed very quickly and have limited reserves1. Immense carbon dioxide (CO2) and greenhouse gas emissions (GHGs) from different anthropogenic activities trigger environmental pollution, environmental deterioration, and global warming2,3. Moreover, these trace gases are also responsible for climate change. Consequently, reducing these emissions is critical, this can be achieved by focusing on the mitigation strategies including the utilization of renewable energy systems. Searching for an alternative non-fossil fuel-based energy system that is renewable, sustainable, economical, and environmentally benign is inevitable. Biodiesel is a liquid biofuel explored as a conventional mitigation technology with low-carbon emissions. Therefore, substituting petroleum fuels with biodiesel could be a plausible means to mitigate CO2 and other GHGs emissions particularly in the transportation sector, and thus, it plays a vital role in reducing environmental related problems, global warming, and climate change4.
Biodiesel is a renewable, clean-burning liquid fuel, which can be produced by transesterification reaction from biomass sources such as vegetable oil, animal oil/fat, and waste cooking oil2,5,6. The main problem facing biodiesel commercialization and its market competitiveness is its production cost and the expensiveness of the raw materials used in the production of biodiesel2,6,7,8. Compared to petrol diesel, biodiesel from common foodstuff is more expensive, and hence to tackle this problem it is vital to focus on searching for economical and readily available feedstocks, alcohols, and catalysts. Biodiesel production from waste such as waste cooking oil (WCO) and eggshell-derived calcium oxide (CaO) is a promising alternative for the production of low-cost and environmentally sustainable products5,6,7,8,9,10,11,12. Using waste cooking oil and eggshell for biodiesel production has a dual advantage which is an effective way to reduce the production cost of biodiesel and reduce environmental pollution problems due to the disposal of waste cooking oil and eggshell.
CaO is the most widely used heterogeneous base catalyst naturally abundant as limestone. In addition to limestone, there are other effective sustainable natural sources of CaO such as crab shells, eggshells, capiz shells, abalone shells, snail shells, oyster shells, mussel shells, and goat bone2,10,11,13,14,15,16,17,18,19. Besides, apart from CaO-based catalyst other catalysts such as MgO20, Mg/Al hydrotalcite21,22,23,24, nano-magnetic catalyst KF/CaO–Fe3O425, hydrated lime-derived CaO26, and CaO–Fe2O327 have been explored for biodiesel production. Transesterification is the reaction of a fat or oil triglyceride with an alcohol to form esters and glycerol which is a reversible process and thus an excess of alcohol is required to force the equilibrium to the product side. Theoretically, the stoichiometry for the transesterification reaction is 3:1 alcohol to oil, however, in practice, the amount can vary, as it is experimentally determined. Several alcohols have been studied for biodiesel production of which methanol and ethanol are the two most frequently used in the production of biodiesel8,12,28,29,30. Using methanol or ethanol has its advantage and disadvantage related to the physical and chemical properties of the transesterification reaction and its product.
Methanol is the most preferred alcohol chosen due to reactivity and high equilibrium conversion, but it is a toxic fossil fuel-based product and exhibits mass transfer limitations in transesterification reaction due to its limited solubility in oil31. On the other hand, transesterification using ethanol has limitations due to the formation of stable emulsion and difficulty in separation of biodiesel and glycerol associated with high solubility and less reactivity of ethanol32. The main advantage of using ethanol is; it can be obtained from a renewable source and is not toxic.
Several previous works have reported on the utilization of CaO material as a catalyst in the transesterification reaction from different sources. CaO catalyst is prepared from natural sources, mostly from different waste shells. As stated by Rezaei et al. (2013), a biodiesel yield of 94.1% was obtained from soybean oil using CaO catalyst prepared from mussel shell with pronounced purity at a specified experimental setting18. Likewise, Chen et al. (2014) also reported a 93% biodiesel yield from palm oil using CaO catalyst made from ostrich eggshell prepared through an ultrasonic technique33. Moreover, Chouhan et al. (2011) achieved a biodiesel yield of more than 97% from soybean oil using an actual CaO catalyst prepared from chicken waste eggshell at optimal experimental conditions. They demonstrated the practicality of waste chicken eggshell-based CaO catalyst for high yield and good quality biodiesel production34. Similarly, Gupta et al. (2016) also reported a biodiesel yield of 96% from soybean oil using snail eggshell-based CaO catalyst at a particular experimental condition35.
As witnessed from several previous studies, the CaO catalyst obtained from the natural source has effectively catalyzed the transesterification of high-grade or virgin vegetable oil. Nevertheless, consuming high-grade or unused vegetable oil for biodiesel production is not economical. This is one of the critical challenges that impede entering into the biodiesel market. Towards overwhelmed this constraint many research activities are carried-out for searching an alternative feedstock that is renewable, environmentally friendly, sustainable, and economical. In this regard, the utilization of waste cooking oil and a variety of non-edible plant oils such as jatropha, castor, linseed, and tobacco for the transesterification reaction are important feedstock. Even if the use of waste cooking oil for biodiesel production has been well explored in several previous works, there are still some shortcomings such as the existence of high free fatty acid content and impurities. These limitations must be overcome or reduce for it to be considered as a promising candidate for conventional homogenously-catalyzed biodiesel production36. In conjunction, different studies were conducted on biodiesel production from waste cooking oil by using eggshell catalysts and they confirmed an optimal yield of 91%11 and 100%37 at different reaction conditions. Unlike homogenous catalyzed transesterification, the heterogeneously-catalyzed transesterification reactions were relatively taken longer reaction time. This happened due to the mixing problem of the three-phases systems (oil-alcohol-catalyst)38. The low basicity and surface area of a CaO potentially limit its catalytic activity. As a consequence, higher catalyst loading and large reaction time might be required for the transesterification process to complete, which encounters an additional cost of production. The catalytic activity of CaO can be improved for an enhanced biodiesel yield by increasing the basicity and surface area of a catalyst. The latter can be achieved by reducing a crystal size, for example synthesizing a nanoparticle can remarkably increase the catalytic performance of a heterogeneous catalyst. Hence, to alleviate this problem reducing the heterogeneous catalyst size is one key step.
Currently, several studies have been conducted to improve the catalytic activity of CaO with different techniques relevant for transesterification reaction. Yoosuk et al. (2010) reported that the decomposed hydrated CaO exhibited higher catalytic activity even at shorter reaction time compared to CaO produced from direct calcination of CaCO3 under mild reaction conditions. About 93.9% biodiesel yield was accomplished at oil to methanol molar ratio 1:15, catalyst loading 7 wt%, reaction temperature 60 °C, and a reaction time of an hour. Interestingly, high biodiesel yield was achieved within an hour of reaction time mainly due to an improvement of catalytic activity of hydrated CaO catalysts39. Moreover, Asikin-Mijan et al. (2015) showed that hydration treated clam shell derived CaO effectively convert palm oil into biodiesel with 98% yield after 2 h of reaction time at methanol to oil molar ratio of 9:1, catalyst loading of 1wt%, and temperature of 65 °C40. Similarly, Niju et al. (2016) showed high activity with a biodiesel yield of around 94% using 7 wt% of hydrated white bivalve clam shell derived catalyst with the methanol to oil molar ratio of 12:1, reaction temperature of 65 °C, and reaction time of an hour41.
More investigation is still needed to search for sustainable, low-cost, and efficient catalysts, alcohols, and feedstocks for improved and viable biodiesel production. In this study, mixed methanol–ethanol and eggshell-derived calcium oxide nano-catalyst were utilized for enhanced biodiesel production from waste cooking oil at different reaction parameters. Chicken eggshell-based CaO is prepared by calcination at 900 °C which is also followed by hydration- dehydration method to enhance its catalytic activity2,6,39,42.
Experimental
Waste cooking oil sample preparation
WCO was collected from nearby restaurants originally from the mixture of palm and sunflower oil. The oil mixture was filtered to remove food particles and followed by heating at 110 °C inside an oven for about half an hour to remove water present in the WCO. Moreover, the physicochemical properties of WCO such as like density, ash content, acid value, free fatty acid (FFA) value, saponification value, kinematic viscosity, and molecular weight43 were estimated using standard procedures.
Preparation of CaO nano-catalyst
Chicken eggshell waste was collected from nearby restaurants and soaked in boiled water for 10-min to solidify the gelatinous materials adhering to the inner wall of the eggshell for easy removal. It was then washed repeatedly with tap water and rinsed with distilled water to remove impurities and followed by oven-dried at 105 °C for 24 h. After drying it was grounded and sieved by 63 µm sieve. As it can be seen in Fig. 1, the grounded eggshell powder is taken into a crucible and calcinated in a muffle furnace at 900 °C for 3 h. The calcinated eggshell powder was refluxed in the water at 60 °C for 6 h which was followed by oven dried at 105 °C over-night41,44.The powder was further dehydrated by calcination at 800 °C for 3 h to convert the hydroxide form into a highly porous calcium oxide nano-particle.
Characterization of catalyst
The two different samples of CaO (the one prepared by the method of calcination of eggshell at a temperature of 900 °C and the other which undergoes further hydration-dehydration process) were characterized using X-Ray Diffraction (XRD) to determine the crystal structure and size. The XRD analysis of the sample was conducted on a diffractometer with Ni filtered CuKα radiation at λ = 0.154 nm in the range of 2 theta 5–50°. Scanning electron microscopy (SEM) (FEI INSPECT 50) and electron dispersive X-ray spectroscopy (EDX) were used to determine the morphological structure and Elemental composition of the prepared CaO nano-catalyst. The XRD results were analyzed by using origin software and the average crystal size of the catalyst was determined using the Debye Scherrer equation45.
Transesterification of waste cooking oil
The transesterification process was carried out in a three‐necked 500 ml round‐bottom flask immersed in a dish as a water bath to control the temperature in the hot plate. The middle neck is used to insert a water-cooled condenser and the other neck is fixed with a thermometer to control the reaction temperature. During the reaction waste cooking oil was measured and poured into a three-necked flask and it was preheated at 50 °C and the catalyst weighed and dissolved in the required amount of preheated alcohol (calcium ethoxide/methoxide) was then, added. The transesterification process was performed to obtain the maximum yield of biodiesel by varying the operating variables such as the catalyst loading, alcohol/oil molar ratio, reaction temperature, and time. The biodiesel yield was estimated using the following equation.
In order to investigate and optimize the effect of catalyst loading, alcohol to oil molar ratio, mixing ratio of ethanol and methanol, reaction temperature, and reaction time different experimental runs were conducted. All experiments were conducted with a constant volume of waste cooking oil of 100 ml and agitation speed of 600 rpm. To investigate the effect of catalyst loading, in each run catalyst loading varied in the range 1–4 wt% (the mass of catalyst was determined based on the weight of waste cooking oil) and the other parameters were kept constant. The initial parameter values for oil to alcohol molar ratio of 1:9, the temperature of 60 °C, and reaction time of 3 h were taken from the literature46.
In the next stage of determination, catalyst loading was kept constant throughout this experiment. To determine the effect of oil to alcohol molar ratio the experiment was carried out by keeping constant reaction temperature, reaction time, and previously determined catalyst loading. Oil to alcohol molar ratio was varied as 1:6, 1:8, 1:10, 1:12, 1:14, and 1:16, based on the highest biodiesel yield the value of oil to alcohol ratio was selected. A similar procedure was carried to determine the mixed ratio of methanol to ethanol by varying the ratios of alcohols as 2:10, 4:8, 6:6, and 8:4 with constant oil to alcohol ratio of 1:12 which is optimized using methanol. The reaction temperature was optimized by varying the temperature to 50, 55, 60, and 65 °C. The reaction temperature with highest biodiesel yield was taken as the optimum value.
Finally, to investigate the optimum reaction time with the highest biodiesel yield the three values such as catalyst loading, oil to alcohol molar ratio, reaction temperature which have been previously determined in this study were taken as constant value, and reaction time was varied as 60, 90, 120, and 180-min. After the reaction has been completed, the solution was poured into separating funnel. The separate layers of glycerol, catalyst, and methyl ester/ethyl ester were distinguished. For efficient separation, the product was kept standing for over-night. After a one-night stand, As shown in Fig. 2, after the over-night stand separation of the three phases was very clear.
Results and discussion
Characteristics of waste cooking oil
After filtration and dehydration, the density, kinematic viscosity, acid value, free fatty acid value, saponification value, ash content, and molecular weight of waste cooking oil were determined, obtained results are presented in Table 1.
Catalyst characterization
XRD analysis
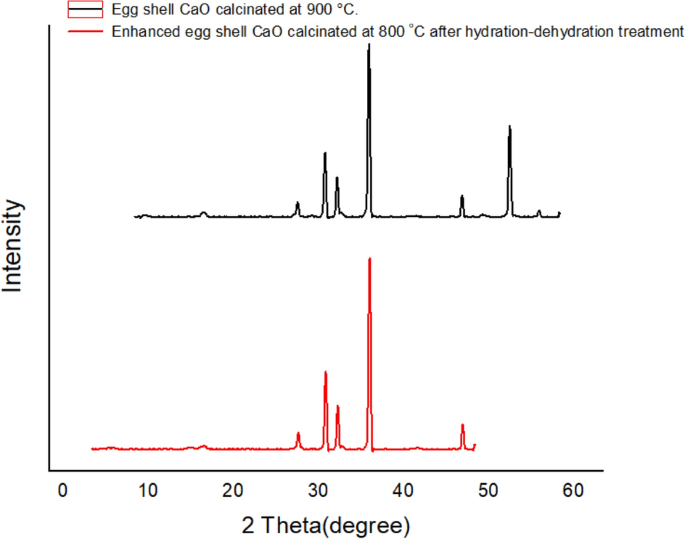
As shown in Fig. 3, the XRD diffraction pattern exhibit sharp peaks at 2-theta (2θ) values at 29.19°, 32.4°, 33.85°, 37.55°, and 48.48° for eggshell powder calcinated at 900 °C. Similarly, high-intensity peaks of 2θ values at 32.31°, 33.76°, 37.48°, 48.48°, and 54.01° were exhibited for eggshell powder prepared by calcination at 900 °C and followed by hydration-dehydration process, see Fig. 3. The presence of these sharp peaks in both materials revealed the presence of dominant CaO nanoparticles in the crystallinity of the particles. The XRD results are in a very good agreement with a similar previous report on eggshell-derived calcium oxide powder prepared from eggshell 46.
The calculated average crystallite size was obtained from XRD peak width analysis using the Debye Scherrer equation. Accordingly, the average crystallite sizes of 21.30 nm and 13.53 nm were obtained for eggshell-derived CaO particles prepared by calcination at 900 °C and for CaO calcinated at 800 °C followed by the hydration-dehydration method, respectively. Thus, the later catalyst preparation method provides lesser particle size, as a result, increases the surface area which in turn enhances the catalytic activity of the catalyst in biodiesel production.
The result suggests that the water treatment (hydration followed by dehydration step) has a strong effect on the crystallinity and crystalline size of the catalyst. This considerably decreases crystallinity (increase porosity) and crystalline size as a result surface area increases. This is likely associated with the evolution of water molecules, whose expulsion from the lattice during the calcination of the hydrated samples would be expected to fracture the crystallites. This phenomenon has also been demonstrated in the study by improving the transesterification activity of CaO using the hydration method. Yoosuk et al. (2010) proved that the water treatment has a strong effect on porosity, crystallinity, and crystalline size39. Furthermore, during hydration CaO converted to Ca (OH)2 then, after dehydration strong basic CaO was obtained. This is related to the fact that the basic strength and reactivity of CaO depend on its precursor. Thus, CaO obtained from Ca(OH)2 has strong basic characteristics than obtained from CaCO347.
EDX analysis
The EDX analysis revealed that the major constituent of the eggshell-derived CaO nano-catalyst is calcium and oxygen with 54.74 and 39.76 mass percentages, respectively. The remaining trace elements in the catalyst might have not any negative impact on transesterification reaction in their oxide form rather these oxides have been reported to be active materials for transesterification48. The basic oxides (MgO, K2O, and Na2O) will improve the catalyst’s basic strength while the acidic components (SiO2, SO3, and P2O5) have the capacity to mediate esterification of the feedstock’s free fatty acid (FFA) content49.
SEM analysis
The morphology of the obtained eggshell-derived CaO catalyst was investigated using SEM image. As shown in Fig. 4, the calcinated eggshell powder at 900 °C showed an agglomerate of rod-like regular structure. When calcinated eggshell powder was treated with water and followed by dehydration, the morphology was changed to honeycomb-like porous microstructure as shown in Fig. 5. The change in morphology after water treatment is probably due to the fact that numerous water vapor molecules is liberated from the decomposition of calcium hydroxide (Ca(OH)2) and the gaseous water molecules creates high porosity and more activity42. Besides, calcination of the catalyst after dehydration contributes to homogenize its textural properties.
Effect of reaction parameters on biodiesel yield using methanol
Effect of catalyst loading
Catalyst loading is one of the key factors that determine the usefulness of the transesterification reaction. The effects of catalyst loading on the biodiesel yield are presented in Fig. 6. Based on the results, the biodiesel yield was increased with the increasing amount of catalyst loading from 1–2.5 wt%. The result indicates that increasing of catalyst loading can improve the active surface area of the catalyst that involves in transesterification reaction and enhance the biodiesel yield26. However, the yield was slightly decreased after 2.5 wt% up to 4 wt%. The decrement of the biodiesel after reaching optimum value can be due to larger catalyst amounts exceeding the average value makes the transesterification reaction product stickier which usually impede the mass transfer process in the liquid(oil)–liquid(alcohol)–solid(catalyst) structure. At low catalyst loading, it also deceases biodiesel yield this can be related to the amount of catalyst that is insufficient for full conversion and formation of methyl ester. In this study, the maximum yield of 80% biodiesel was obtained at an optimum value of 2.5 wt% catalyst loading.
Effect of oil to methanol molar ratio
Oil to methanol molar ratio has a significant impact on the biodiesel yield12. As can be seen in Fig. 7, as the molar ratio of oil to methanol increase from 1:6 to 1:12 the percentage biodiesel yields also increased. Above the 1:12 ratio the biodiesel yield decreases which can be related to the high molar ratio of oil to methanol interferes with the separation of glycerin because there is an increase in solubility of glycerol in excess methanol. When glycerin remains in solution it helps drive the equilibrium back to the left, lowering the yield of esters and promoting the displacement of the balance in the opposite direction toward the formation of mono, di, and triglycerides thereby decreasing the production of esters. The overloading of methanol above optimum value would inactivate the catalyst and therefore favor the backward reaction of the transesterification process, similar effect also observed for alcohol48. As shown in Fig. 7, biodiesel yield decreases with an increase in the oil to methanol ratio beyond optimum value. In this study, the optimum molar ratio of oil to methanol was found to be 1:12 for a maximum biodiesel yield of 89%.
Effect of reaction temperature
The temperature has a significant effect on biodiesel production. As shown in Fig. 8, a maximum yield of biodiesel was obtained at 60 °C. Increasing the temperature above 60 °C resulted in a decrease in the biodiesel yield due to the fact that continuous vaporization of methanol hence methanol remained in the gas phase in the reflux, causing a decrease of methanol in the reaction media3. The highest yield of biodiesel was obtained at the optimum temperature of 60 °C which gave a maximum yield of 89.5%.
Effect of reaction time
The effect of reaction time on the transesterification process is presented in Fig. 9. The maximum biodiesel yield was attained at a 2 h reaction time. After the optimum reaction time, the biodiesel yield slightly decreases which can be due to the fact that longer reaction time leads to the reduction of biodiesel yield because of the reversible reaction of transesterification causing in loss of product. Longer reaction time may also result in the hydrolysis of esters and formed additional fatty acids to make soap consequently it reduces the biodiesel yield50.
Eggshell-based CaO nano-catalyst enhanced by hydration and dehydration method was used and the transesterification of waste cooking oil was carried out. The optimum value of catalyst loading 2.5 wt%, oil to methanol ratio 1:12, reaction temperature 60 °C, and reaction time 2 h was determined, and the biodiesel yield of 94% achieved. Many studies have reported the production of biodiesel by using waste shell-derived CaO. However, the catalytic performances are still lower51. Although many authors have reported successful transesterification reaction catalyzed by CaO, the reaction rate was slow and thus required a longer reaction time of at least 3–8 h (reaction temperature of 60–65 °C) to complete. Higher amount of catalyst and methanol is also required which resulted in high biodiesel production cost26. The reason behind on waste shell derived CaO required longer reaction time in order to reach high triglycerides conversion to biodiesel is due to lower basicity and surface area of waste shell derived catalyst which is known to be the major problem in heterogenous catalyst. When compared with present study the effect of enhanced eggshell based CaO on the rate of transesterification reaction is very high this is due to the improvement of the activity of CaO with hydration and dehydration technique. The effectiveness of water treatment on improving the catalytic activity of CaO has also been proved in literature51 by showing the hydration treated clam shell derived CaO could convert palm oil to biodiesel with approximately 98% yield after 2 h of reaction time with molar ratio of 9:1 methanol to oil, catalyst amount of 1 wt% and temperature of 65 °C.
Effect of reaction parameters on biodiesel yield using methanol–ethanol mixture
Effect of oil to mixed methanol–ethanol molar ratio
Mixing ethanol with methanol is one of the key ways to improve the limitation of ethanol in biodiesel production due to difficulty in product separation after transesterification. As can be seen in Fig. 10, at 2:10 and 4:8 methanol to ethanol molar ratios the separation of glycerol from the biodiesel layer was very difficult and requires a long waiting time to separate, as a result, the obtained yield was low 54% and 63%, respectively. The result indicates that domination in the amount of ethanol in the reaction causes emulsification and complicate biodiesel separation after transesterification and causing decreasing in the biodiesel yield21. Nevertheless, when equimolar methanol–ethanol mixture 6:6 and 8:4 ratios were used in the transesterification the separation of glycerol and biodiesel layer became easier and as a consequence, the yield significantly increased and was found to be 88% and 92%, respectively. The increase in the biodiesel yield is mainly related to the decrease in the amount of ethanol in the reaction mixture. The high biodiesel yield obtained of biodiesel at the ratio of 6:6 and 8:4 can be attributed to a combined effect of the high reactivity of methanol and better solubility of ethanol. The solubility of ethanol in oil is better than methanol and thus minimizes the mass transfer limitation between oil, alcohol, and heterogeneous catalyst. Likewise, methanol lessening the emulsification effect of ethanol and able to breakdown emulsion faster to enhance the formation of biodiesel and glycerol. Therefore, the mixed effect of methanol and ethanol was observed in this study and the study is in agreement with the previous reports21. The result suggests the potential replacement of methanol alcohol with renewable product bioethanol as a transesterification agent.
Effect of catalyst loading
As shown in Fig. 11, the amount of catalyst loading is varied at a different mixed ratio of methanol and ethanol. 91% biodiesel yield was obtained with mixed methanol–ethanol ratio of 6:6 using 3.5 wt% catalyst loading. However, in the case of 8:4, the highest biodiesel yield of 92% was obtained with relatively less catalyst loading 2.5 wt%. It is can be concluded from the results that when the amount of ethanol in the mixture increases, the catalyst loading slightly increases. This can be related to the high solubility of ethanol causing emulsion and as a result, relatively increase the catalyst consumption and decreasing biodiesel yield. When the alcohol mixture dominated by methanol (8:4) the maximum biodiesel obtained was relatively high (92%) with lower catalyst loading (2.5 wt%) compared to 6:6 with biodiesel yield of 88% obtained using 2.5 wt% catalyst loading. Moreover, with further increase in the amounts of catalyst, the reaction with dominant ethanol is more sensitive and the yield decreases rapidly after reaching the optimum value of catalyst (Fig. 11). This can be due to the formation of an emulsion and the viscosity which at the same time makes the biodiesel and glycerin separation very difficult49.
Characteristics of produced biodiesel
Fuel properties of obtained biodiesel produced by CaO nano-catalyst synthesized from calcination and hydration-dehydration method at optimal reaction conditions were evaluated along with the American Society for Testing and Material method (ASTM). As can be seen in Table 2, biodiesel produced from methanol alone and also methanol–ethanol mixture both processes showed high-quality fuel properties within the range of biodiesel standard also in good agreement with the previous reports52.
Conclusion
Biodiesel was produced from low-cost waste cooking oil using an enhanced CaO nano-catalyst prepared from chicken eggshell using hydration-dehydration treatment followed by calcination. SEM results revealed the change in morphology from a rod-like to a honeycomb-like porous surface with a larger surface area. Moreover, the XRD analysis disclosed a reduction in the average crystalline particle size of a catalyst from 21.3 to 13.53 nm. Hence, the additional water treatment introduced to the catalyst preparation method substantially improved the biodiesel production from waste cooking oil with mixed methanol–ethanol by enhancing its catalytic activity. As a result, a biodiesel yield of 94% was achieved under the reaction conditions of 1:12 oil to methanol molar ratio, 2.5 wt% catalyst loading, 60 °C, and 120 min reaction time. Moreover, 91% of biodiesel yield was achieved when a mixture of alcohols (i.e., ethanol-methanol), was used in the transesterification process. The ratio of methanol to ethanol affects the yield and product separation, the 6:6 and 8:4 ratios have been found to be ideal to produce high-yield with reasonable quality. The use of waste cooking oil, waste eggshell catalyst, and a mixture of methanol–ethanol in biodiesel production is relevant from the economic and environmental point of view. Hence, we can conclude that ethanol, produced from a renewable source, can feasibly substitute the common alcohol, methanol, at a sensible portion in the transesterification of waste cooking oil using enhanced CaO nano-catalyst.
References
International Energy Agency. Key world energy statistics (2017).
Bankovi, I. B., Miladinovi, M. R., Stamenkovi, O. S. & Veljkovi, V. B. Application of nano CaO–based catalysts in biodiesel synthesis. Renew. Sustain. Energy Rev. 72, 746–760. https://doi.org/10.1016/j.rser.2017.01.076 (2017).
Yang, X. et al. Catalytic transesterification to biodiesel at room temperature over several solid bases. Energy Convers. Manag. 164, 112–121. https://doi.org/10.1016/j.enconman.2018.02.085 (2018).
Osman, A. I., Hefny, M., Maksoud, M. I. A. A., Elgarahy, A. M. & Rooney, D. W. Recent advances in carbon capture storage and utilisation technologies: a review. Environ. Chem. Lett. https://doi.org/10.1007/s10311-020-01133-3 (2020).
Mansir, N., Hwa, S., Rashid, U., Izham, M. & Ping, Y. Modified waste eggshell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 82, 3645–3655. https://doi.org/10.1016/j.rser.2017.10.098 (2018).
Laca, A., Laca, A. & Díaz, M. Eggshell waste as catalyst: a review. J. Environ. Manage. 197, 351–359. https://doi.org/10.1016/j.jenvman.2017.03.088 (2017).
Kawashima, A., Matsubara, K. & Honda, K. Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresour. Technol. 100, 696–700. https://doi.org/10.1016/j.biortech.2008.06.049 (2009).
Leung, D. Y. C., Wu, X. & Leung, M. K. H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 87, 1083–1095. https://doi.org/10.1016/j.apenergy.2009.10.006 (2010).
Degfie, T. A., Mamo, T. T. & Mekonnen, Y. S. Optimized biodiesel production from waste cooking oil (WCO) using calcium oxide (CaO) nano-catalyst. Sci. Rep. 9, 18982. https://doi.org/10.1038/s41598-019-55403-4 (2019).
Mamo, T. T. Microwave-assisted biodiesel production from Microalgae, Scenedesmus species, using goat bone: made nano-catalyst. Appl. Biochem. Biotechnol. https://doi.org/10.1007/s12010-019-03149-0 (2019).
Tshizanga, N. & Funmilayo, E. Optimisation of biodiesel production from waste vegetable oil and eggshell ash. S. Afr. J. Chem. Eng. 23, 145–156. https://doi.org/10.1016/j.sajce.2017.05.003 (2017).
Verma, P. & Sharma, M. P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 62, 1063–1071. https://doi.org/10.1016/j.rser.2016.04.054 (2016).
Nakatani, N., Takamori, H., Takeda, K. & Sakugawa, H. Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresour. Technol. 100, 1510–1513. https://doi.org/10.1016/j.biortech.2008.09.007 (2009).
Poves, M. D. Z., Martı, D. & Lo, M. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Cata. B Envir. 73, 317–326. https://doi.org/10.1016/j.apcatb.2006.12.017 (2007).
Mirghiasi, Z., Bakhtiari, F. & Darezereshki, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 20, 113–117. https://doi.org/10.1016/j.jiec.2013.04.018 (2014).
Tang, Z., Claveau, D., Corcuff, R., Belkacemi, K. & Arul, J. Preparation of nano-CaO using thermal-decomposition method. Mater. Lett. 62, 2096–2098. https://doi.org/10.1016/j.matlet.2007.11.053 (2008).
Marques, L. et al. Characterization of calcium oxide catalysts from natural sources and their application in the transesterification of sunflower oil. Bioresour. Technol. 151, 207–213. https://doi.org/10.1016/j.biortech.2013.10.046 (2014).
Rezaei, R., Mohadesi, M. & Moradi, G. R. Optimization of biodiesel production using waste mussel shell catalyst. Fuel 109, 534–541. https://doi.org/10.1016/j.fuel.2013.03.004 (2013).
Chen, G. et al. Remarkably enhancing the biodiesel yield from palm oil upon abalone shell-derived CaO catalysts treated by ethanol. Fuel Process Technol. 143, 110–117. https://doi.org/10.1016/j.fuproc.2015.11.017 (2016).
Du, L. et al. Synthesis and characterization of carbon-based MgO catalysts for biodiesel production from castor oil. Fuel 258, 116122. https://doi.org/10.1016/j.fuel.2019.116122 (2019).
Ma, Y. et al. Mixed methanol / ethanol on transesterification of waste cooking oil using Mg–Al hydrotalcite catalyst. Energy 107, 523–531. https://doi.org/10.1016/j.energy.2016.04.066 (2016).
Manivannan, R. & Karthikeyan, C. Synthesis of biodiesel from neem oil using Mg–Al nano hydrotalcite. Adv. Mater. Res. 678, 268–272 (2013).
Navajas, A. et al. Applied catalysis B: environmental synthesis of biodiesel from the methanolysis of sunflower oil using Mg–Al hydrotalcites as catalyst precursors. Appl. Catal. B Environ. 100, 299–309. https://doi.org/10.1016/j.apcatb.2010.08.006 (2010).
Zeng, H., Feng, Z., Deng, X. & Li, Y. Activation of Mg–Al hydrotalcite catalysts for transesterification of rape oil. Fuel 87, 3071–3076. https://doi.org/10.1016/j.fuel.2008.04.001 (2008).
Hu, S., Guan, Y., Wang, Y. & Han, H. Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 88, 2685–2690. https://doi.org/10.1016/j.apenergy.2011.02.012 (2011).
Roschat, W., Siritanon, T., Yoosuk, B. & Promarak, V. Biodiesel production from palm oil using hydrated lime-derived CaO as a low-cost basic heterogeneous catalyst. Energy Convers. Manag. 108, 459–467. https://doi.org/10.1016/j.enconman.2015.11.036 (2016).
Shi, M., Zhang, P., Fan, M., Jiang, P. & Dong, Y. Influence of crystal of Fe2O3 in magnetism and activity of nanoparticle CaO@Fe2O3 for biodiesel production. Fuel 197, 343–347. https://doi.org/10.1016/j.fuel.2017.02.060 (2017).
Karki, S., Sanjel, N., Poudel, J., Choi, J. H. & Oh, S. C. Supercritical transesterification of waste vegetable oil: characteristic comparison of ethanol and methanol as solvents. Appl. Sci. 7, 632. https://doi.org/10.3390/app7060632 (2017).
Fadhil, A. B. & Ahmed, A. I. Production of mixed methyl/ethyl esters from waste fish oil through transesterification with mixed methanol/ethanol system. Chem. Eng. Commun. https://doi.org/10.1080/00986445.2018.1434514 (2018).
Husain, S. A. Transesterification of castor oil by using methanol and ethanol (50/50) mixture. Eng. Technol. J. 36, 59–63. https://doi.org/10.30684/etj.36.1B.10 (2018).
Musa, I. A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt J. Pet. 25, 21–31. https://doi.org/10.1016/j.ejpe.2015.06.007 (2016).
Kim, M., Yan, S., Salley, S. O. & Ng, K. Y. S. Competitive transesterification of soybean oil with mixed methanol/ethanol over heterogeneous catalysts. Bioresour. Technol. 101, 4409–4414. https://doi.org/10.1016/j.biortech.2010.01.099 (2010).
Chen, G., Shan, R., Shi, J. & Yan, B. Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Bioresour. Technol. 171, 428–432. https://doi.org/10.1016/j.biortech.2014.08.102 (2014).
Chouhan, A. P. S. & Sarma, A. K. Modern heterogeneous catalysts for biodiesel production: a comprehensive review. Renew. Sustain. Energy Rev. 15, 4378–4399. https://doi.org/10.1016/j.rser.2011.07.112 (2011).
Gupta, J. & Agarwal, M. Preparation and characterization of CaO nanoparticle for biodiesel production. AIP Conf. Proc. 1724, 020066. https://doi.org/10.1063/1.4945186 (2016).
Zhang, Y., Dubé, M. A., McLean, D. D. & Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 89, 1–16. https://doi.org/10.1016/S0960-8524(03)00040-3 (2003).
Navajas, A., Issariyakul, T., Arzamendi, G., Gandía, L. M. & Dalai, A. K. Development of eggshell derived catalyst for transesteri fi cation of used cooking oil for biodiesel production. Asia-Pacific J. Chem. Eng. 8, 742–748. https://doi.org/10.1002/apj (2013).
Xie, W., Peng, H. & Chen, L. Transesterification of soybean oil catalyzed by potassium loaded on alumina as a solid-base catalyst. Appl. Catal. A Gen. 300, 67–74. https://doi.org/10.1016/j.apcata.2005.10.048 (2006).
Yoosuk, B., Udomsap, P., Puttasawat, B. & Krasae, P. Improving transesterification activity of CaO with hydration technique. Bioresour. Technol. 101, 3784–3786. https://doi.org/10.1016/j.biortech.2009.12.114 (2010).
Asikin-mijan, N., Lee, H. V. & Taufiq-yap, Y. H. Synthesis and catalytic activity of hydration: dehydration treated clamshell derived CaO for biodiesel production. Chem. Eng. Res. Des. 102, 368–377. https://doi.org/10.1016/j.cherd.2015.07.002 (2015).
Niju, S., Meera Sheriffa Begum, K. M. & Anantharaman, N. Enhancement of biodiesel synthesis over highly active CaO derived from natural white bivalve clam shell Enhancement of biodiesel synthesis over highly active CaO. Arab J. Chem. 9, 633–639. https://doi.org/10.1016/j.arabjc.2014.06.006 (2016).
Roschat, W., Phewphong, S., Thangthong, A. & Moonsin, P. Catalytic performance enhancement of CaO by hydration-dehydration process for biodiesel production at room temperature. Energy Convers. Manag. 165, 1–7. https://doi.org/10.1016/j.enconman.2018.03.047 (2018).
Ejim, IF., & Kamen, FL. Physiochemical characterization of algae oil from microalgae of Nike Lake Enugu. J. Eng. Appl. Sci. 5 (2013).
Niju, S., Begum, M. M. M. S. & Anantharaman, N. Modification of eggshell and its application in biodiesel production. J. Saudi. Chem. Soc. 18, 702–706. https://doi.org/10.1016/j.jscs.2014.02.010 (2014).
Gopal, V. R. V. & Kamila, S. Effect of temperature on the morphology of ZnO nanoparticles: a comparative study. Appl. Nanosci. 7, 75–82. https://doi.org/10.1007/s13204-017-0553-3 (2017).
Ayodeji, A. A., Modupe, O. E., Rasheed, B. & Ayodele, J. M. Data on CaO and eggshell catalysts used for biodiesel production. Data Br. 19, 1466–1473. https://doi.org/10.1016/j.dib.2018.06.028 (2018).
Bok, Y., Seo, G. & Rae, D. Transesterification of tributyrin with methanol over calcium oxide catalysts prepared from various precursors. Fuel Process Technol. 90, 1252–1258. https://doi.org/10.1016/j.fuproc.2009.06.007 (2009).
Birla, A., Singh, B., Upadhyay, S. N. & Sharma, Y. C. Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour. Technol. 106, 95–100. https://doi.org/10.1016/j.biortech.2011.11.065 (2012).
Boey, P., Pragas, G. & Abd, S. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: a review. Chem. Eng. J. 168, 15–22. https://doi.org/10.1016/j.cej.2011.01.009 (2011).
Eevera, T., Rajendran, K. & Saradha, S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew. Energy 34, 762–765. https://doi.org/10.1016/j.renene.2008.04.006 (2009).
Asikin-mijan, N., Lee, H. V. & Taufiq-yap, Y. H. Chemical engineering research and design synthesis and catalytic activity of hydration: dehydration treated clamshell derived CaO for biodiesel production. Chem. Eng. Res. Des. 102, 368–377. https://doi.org/10.1016/j.cherd.2015.07.002 (2015).
Yaşar, F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: its effects on some critical fuel properties and comparison with CaO. Fuel 255, 115828. https://doi.org/10.1016/j.fuel.2019.115828 (2019).
Acknowledgements
The authors would like to acknowledge financial support from the thematic research project (Project No. TR/012/2016) funded by Addis Ababa University. The authors also would like to thank Dr. Behabtu E. Tebkachew for his valuable support.
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.S.E., T.T.M., G.A.W., and Y.S.M.; writing—original draft preparation. Y.S.E., T.T.M., G.A.W., and Y.S.M.; writing—review and editing; All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erchamo, Y.S., Mamo, T.T., Workneh, G.A. et al. Improved biodiesel production from waste cooking oil with mixed methanol–ethanol using enhanced eggshell-derived CaO nano-catalyst. Sci Rep 11, 6708 (2021). https://doi.org/10.1038/s41598-021-86062-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86062-z
This article is cited by
-
Biodiesel production potential of Eichhornia crassipes (Mart.) Solms: comparison of collection sites and different alcohol transesterifications
Scientific Reports (2024)
-
Production of biodiesel from waste fish fat through ultrasound-assisted transesterification using petro-diesel as cosolvent and optimization of process parameters using response surface methodology
Environmental Science and Pollution Research (2024)
-
Synthesis of Pure and High-Quality Biodiesel from Waste Frying Oil Using CaO Prepared from Waste Coralline Sand
Waste and Biomass Valorization (2024)
-
Faidherbia Albida Role in Green Catalysis for Sustainable Energy
Catalysis Letters (2024)
-
Waste Chicken Eggshell-Derived CaO Based Magnetic Solid Base Catalysts for the One-Pot Synthesis of Tetrahydro-4H-chromenes and Benzopyranopyrimidines
Catalysis Letters (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.