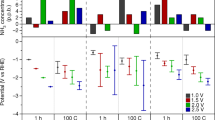
Abstract
Stimulated by the growing demand for sustainable and/or economical distributed ammonia synthesis, the electrochemical nitrogen reduction reaction has attracted considerable interest. The nitrogen-containing impurities in commercial metal-based nitrogen reduction reaction catalysts such as metal oxides and metallic irons have, however, been overlooked. Herein we report the presence of nitrogen-containing species in NOx− or nitrides at substantial levels revealed from many commercial catalysts. We call attention to the necessity to screen the NOx−/nitrides impurities in commercial catalysts, as the nitrogen impurities are not commonly listed in vendors’ assay documents. A simple two-step procedure (alkaline/acidic treatment followed by HPLC/UV–vis analysis) is recommended as a reliable protocol for screening NOx−/nitrides impurities in catalyst materials. A case analysis is also carried out on the previously reported H2O–NaOH–KOH system with both 15N-isotopic labelling and nitrogen elemental tracking, reassigning the true nitrogen source of the electrochemically produced NH3 from gaseous N2 to nitrogen-containing impurities in catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. All data supporting the findings of this study are available from the corresponding author on reasonable request.
Change history
23 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41929-021-00588-z
References
Apodaca, L. E. Nitrogen (Fixed)—Ammonia. U.S. Geological Survey, Mineral Commodity Summaries (2020).
Nørskov, J., Chen, J., Miranda, R., Fitzsimmons, T. & Stack, R. Sustainable Ammonia Synthesis—Exploring the Scientific Challenges Associated with Discovering Alternative, Sustainable Processes for Ammonia Production (US DOE Office of Science, 2016).
Wang, L. et al. Greening ammonia toward the solar ammonia refinery. Joule 2, 1055–1074 (2018).
Foster, S. L. et al. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 1, 490–500 (2018).
Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal. 2, 377–380 (2019).
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, eaar6611 (2018).
Martín, A. J., Shinagawa, T. & Pérez-Ramírez, J. Electrocatalytic reduction of nitrogen: from Haber–Bosch to ammonia artificial leaf. Chem 5, 263–283 (2019).
Tang, C. & Qiao, S.-Z. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev. 48, 3166–3180 (2019).
Shipman, M. A. & Symes, M. D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 286, 57–68 (2017).
Greenlee, L. F., Renner, J. N. & Foster, S. L. The use of controls for consistent and accurate measurements of electrocatalytic ammonia synthesis from dinitrogen. ACS Catal. 8, 7820–7827 (2018).
Suryanto, B. H. R. et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2, 290–296 (2019).
Andersen, S. Z. et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 570, 504–508 (2019).
Du, H.-L., Gengenbach, T. R., Hodgetts, R., MacFarlane, D. R. & Simonov, A. N. Critical assessment of the electrocatalytic activity of vanadium and niobium nitrides toward dinitrogen reduction to ammonia. ACS Sustain. Chem. Eng. 7, 6839–6850 (2019).
Hu, B., Hu, M., Seefeldt, L. & Liu, T. L. Electrochemical dinitrogen reduction to ammonia by Mo2N: catalysis or decomposition? ACS Energy Lett. 4, 1053–1054 (2019).
Zhao, Y. et al. Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates? Adv. Sci. 6, 1802109 (2019).
Manjunatha, R., Karajić, A., Teller, H., Nicoara, K. & Schechter, A. Electrochemical and chemical instability of vanadium nitride in the synthesis of ammonia directly from nitrogen. ChemCatChem 12, 438–443 (2020).
Wang, Y. et al. Generating defect-rich bismuth for enhancing the rate of nitrogen electroreduction to ammonia. Angew. Chem. Int. Ed. 58, 9464–9469 (2019).
Lv, C. et al. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions. Angew. Chem. Int. Ed. 57, 6073–6076 (2018).
Licht, S. et al. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 345, 637–640 (2014).
Zhou, F. et al. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids. Energy Environ. Sci. 10, 2516–2520 (2017).
Hu, L. et al. Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe oxide catalyst. ACS Catal. 8, 9312–9319 (2018).
Furuya, N. & Yoshiba, H. Electroreduction of nitrogen to ammonia on gas-diffusion electrodes loaded with inorganic catalyst. J. Electroanal. Chem. Interfacial Electrochem. 291, 269–272 (1990).
Gadalla, A. M. & Yu, H. F. Thermal decomposition of Fe(iii) nitrate and its aerosol. J. Mater. Res. 5, 1233–1236 (1990).
Kodama, H. Synthesis of a new compound, Bi5O7NO3, by thermal decomposition. J. Solid State Chem. 112, 27–30 (1994).
Sarkas, H., Murray, P., Fay, A. & Brotzman, R. Nanocrystalline mixed metal oxides—novel oxygen storage materials. MRS Proc. 788, L4.8 (2011).
Birkeland, K. On the oxidation of atmospheric nitrogen in electric arcs. Trans. Faraday Soc. 2, 98–116 (1906).
Smil, V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production 62–64 (MIT Press, 2004).
Verdouw, H., Van Echteld, C. J. A. & Dekkers, E. M. J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 12, 399–402 (1978).
Ertl, G., Huber, M. & Thiele, N. Formation and decomposition of nitrides on iron surfaces. Z. fur Naturforsch. A 34, 30–39 (1979).
Torres, J., Perry, C. C., Bransfield, S. J. & Fairbrother, D. H. Low-temperature oxidation of nitrided iron surfaces. J. Phys. Chem. B 107, 5558–5567 (2003).
Baltrusaitis, J., Jayaweera, P. M. & Grassian, V. H. XPS study of nitrogen dioxide adsorption on metal oxide particle surfaces under different environmental conditions. Phys. Chem. Chem. Phys. 11, 8295–8305 (2009).
Davies, J. A., Boucher, D. L. & Edwards, J. G. The question of artificial photosynthesis of ammonia on heterogeneous catalysts. Adv. Photochem. 19, 235–310 (1995).
Li, L., Tang, C., Yao, D., Zheng, Y. & Qiao, S.-Z. Electrochemical nitrogen reduction: identification and elimination of contamination in electrolyte. ACS Energy Lett. 4, 2111–2116 (2019).
Nash, J. et al. Electrochemical nitrogen reduction reaction on noble metal catalysts in proton and hydroxide exchange membrane electrolyzers. J. Electrochem. Soc. 164, F1712–F1716 (2017).
Dabundo, R. et al. The contamination of commercial 15N2 gas stocks with 15N–labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS One 9, e110335 (2014).
Kim, K., Chen, Y., Han, J.-I., Yoon, H. C. & Li, W. Lithium-mediated ammonia synthesis from water and nitrogen: a membrane-free approach enabled by an immiscible aqueous/organic hybrid electrolyte system. Green. Chem. 21, 3839–3845 (2019).
Chou, S.-S., Chung, J.-C., And, D.-F. & Hwang, D.-F. A high performance liquid chromatography method for determining nitrate and nitrite levels in vegetables. J. Food Drug Anal. 11, 11 (2003).
Haynes, W. M. CRC Handbook of Chemistry and Physics (CRC, 2016).
Watt, G. W. & Chrisp, J. D. Spectrophotometric method for determination of hydrazine. Anal. Chem. 24, 2006–2008 (1952).
Acknowledgements
This research was partly supported by ARPA-E agency through REFUEL program (grant no. DE-AR0000812) and by Iowa Economic Development Authority (IEDA, grant no. AWD-019199). We are grateful to S. D. Cady, D. Jing, and B. W. Boote from Iowa State University for their generous assistance in NMR and material characterization. We also acknowledge fruitful discussions with J. Li, E. A. Smith, H. Lin, B. H. Shanks, R. C. Brown, J. L. Trettin (Iowa State University), K. Kim (University of Illinois at Urbana-Champaign) and G. Soloveichik (ARPA-E) on the electrosynthesis of ammonia. W. Li thanks his Bailey Research Career Development Award and Richard Seagrave Professorship. Y. Chen acknowledges his Catron Graduate Fellowship from Catron Center for Solar Energy Research at Iowa State University.
Author information
Authors and Affiliations
Contributions
W.L., S.G. and S.L. proposed the research and supervised the project. Y.C. performed material characterization. H.L. carried out HPLC measurements. Y.C. and N.H. set up the electrolytic cell system with the assistance from S.G. and S.L. and performed the electrochemical studies. Y.C., S.G., S.L. and W.L. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Figs. 1–8 and references.
Supplementary Data 1
Experimental Source Data for Supplementary Table 1 and Supplementary Figs. 2, 3 and 5–7.
Source data
Source Data Fig. 2
Experimental Source Data.
Source Data Fig. 3
Experimental Source Data.
Rights and permissions
About this article
Cite this article
Chen, Y., Liu, H., Ha, N. et al. Revealing nitrogen-containing species in commercial catalysts used for ammonia electrosynthesis. Nat Catal 3, 1055–1061 (2020). https://doi.org/10.1038/s41929-020-00527-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00527-4