ABSTRACT
The basal activity of JNK is low in normal growing cells and inactivated JNK targets p53 for ubiquitination. To elucidate if the C-terminal part of JNK is responsible for its binding to p53, the low background tet-off inducible NIH3T3 cell line was selected by luciferase reporter gene and a double stable C-JNK Aa (203-424) cell line was established. After withdrawing tetracycline, the C-JNK fragment expression was induced and cell growth was dramatically inhibited 24 h later. However, the expresion of p53 was found to be increased after the induction of C-JNK fragment, evaluated by transfecting p21waf-luciferase reporter genes. Our further studies showed that C-JNK fragment could form complex with p53 both in vivo and in vitro. Induction of C-JNK fragment in vivo can increase p53 stability by inhibiting p53 ubiquitination.
Similar content being viewed by others
INTRODUCTION
The superfamily of mitogen-activated protein kinase (MAPK) consists of evolutionarily conserved proteins which play an important role in the regulation of intracellular metabolism and gene expression, therefore they are correlated with a variety of cellular functions including cell growth, differentiation, apoptosis as well as cellular response to environmental stresses and pro-inflammatory cytokines. MAPK signal transduction pathways have been proved to be ubiquitous in eukaryotes 1, 2, 3, 4, 5. As major components, there are three Ser/Thr kinases within the MAPK family: extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNKs)/stress-activated protein kinases (SAPKs) and p38 MAPKs 6, 7. ERKs are activated preferentially by mitogens and EGF 7, 8, while JNK and p38 pathways are stimulated mainly by proinflammantory cytokines and diverse array of cellular stresses 9, 10, 11, 12.
JNK plays a pivotal role in cellular stresses such as UV light, heat, anisomycin, hydrogen peroxide or hyperosmolarity. In normal growing cells, the efficiency of JNK is limited due to the influence of glutahione S-transferase pi (GSTp), a natural inhibitor of JNK 13. Under this condition, JNK mediates the ubiquitination and degradation of its associated proteins such as c-Jun, ATF2 and p53 14, 15, 16, 17. When responding to environmental stress, JNK is activated by dual phosphorylation at the Thr-Pro-Tyr motif by up-stream kinases MKK4/7, leading to the phosphorylation of its substrates such as c-Jun 18, 19, ATF2 20, c-Myc 21, Bcl-2 22 and p53 23, and then breaks cellular balance.
So far, ERKs have been profoundly studied 24, but the respective functions of JNK and its domains remain unclear. Several reports demonstrated that the effect of JNK on p53 in both normal cells and those exposed to external stresses. The association of JNK with p53 could efficiently inhibit p53 ubiquitination and this process is Mdm2 independent 15. In order to elucidate the regulation of p53 by effective domains of JNK, a NIH 3T3/Tet-off inducible expression system was established, and the C-JNK (aa 203-424) positive clones were selected by G418 and hygromycin. By removing tetracycline, we studied its effect on stability and transcription activity of p53 as well as cell growth.
MATERIALS AND METHODS
Cells lines
The mouse fibroblast cell line NIH 3T3 that stably expresses the pSV40-Hyg plasmid (Clontech) was maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen) at 37 °C in a humidified atmosphere with 5% CO2.
The selection of high expression and low basal level NIH3T3 cells
NIH 3T3 cells plated in a 6-well plate at 3×105 cells/well was changed to DMEM containing 1 μg/ml tetracycline (tet) (Calbiochem) 24 h prior to the experiment. Luciferase-pUHD-10-3 reporter vector (0.5 μg) as a gift from Dr. Bujard H (University of Heidelberg, Germany) was incubated with LipofectAMINE (Invitrogen) for 15 min and then was added into each well. After 24 h incubation, tet was removed and 8 h later cells were harvested for quantitation of activity of luciferase.
Establishment of double stable C-JNK cell lines and fragment expression
The pTet-C-JNK was constructed by subcloning the cDNA of the C-terminal 222 amino acids of wild type JNK2 into the pUHD-10-3 vector. The C-JNK stable clones were selected in 600 μg/ml geneticin (Calbiochem) in the presence of hygromycin (100 μg/ml) after transfection. To suppress the expression of C-JNK, tet (final concentration 1 μg/ml) was added to the medium every 3 d. While in order to turn on the expression of C-JNK, cells were washed 3 times with PBS to remove tet and allowed to incubate in DMEM supplemented with 10% fetal bovine serum.
Immunoprecipitation (IP) and immunoblots
Ployclonal antibodies for JNK and p53 were generated by using bacterially expressed JNK and p53 as antigens. Anti-p21 mAb was from Labvision company. For immunoprecipitation, whole cell extract (WCE) was incubated with the above antibodies for 16 h at 4 °C respectively, and than treated by 20 ml protein G-beads (Invitrogen) for 30 min at room temperature. Following 4 times intensive wash with PBS/0.5M LiCl supplemented with Tween 100 (0.5%), the extract was subjected to Western blot analysis.
Immunoblot analysis was performed as follows: 50-100 μg of WCE separated by SDS-PAGE and than electrotransfered to a nitrocellulose membrane. After ponceau staining to confirm equal loading, the membrane was blocked by 5% non-fat milk, washed with TBST for 4 times, and incubated with anti-HA, anti-p53, or anti-p21waf monoclonal antibodies as indicated and then the HRP-conjugated goat anti mouse IgG antibody (Calbiochem) for 1 h each. Results were visualized using ECL (Amersham Pharmacia Biosciences).
Cell proliferation detection
NIH3T3 double stable cells cultured in 6-well plates were induced for the expression of JNK by removing tet. After 24 h, tet was added back to inhibit C-JNK expression. To determine cell proliferation, cells in each well were incubated with 1 μCi 3H-TdR for 24 h and then harvested. The radioactivity of 3H-TdR incorporation into DNA was measured using liquid scintillography (Perkin Elmer).
In vitro ubiquitination assay
For in vitro ubiquitination assay, 50 μg of whole cell lysates prepared from C-JNK expressing cells that were incubated with bacterially expressed human tagged his-p53 (5 μg on ice) and then with NTA beads for 1 h. After intensive washes, the substrate bound beads were equilibrated with ubiquitin buffer (50 mM Tris-HCl, pH 8.0, 5 mM MgCl2, 0.5 mM dithiothreitol, 2 mM NaF and 3 μM okadaic) and incubated in the same buffer supplemented with 2 mM ATP, 10 mM creatine phosphate, 0.02 unit of creatine phosphokinase, 2 μg of hemagglutinin (HA)-tagged ubiquitin, 1.5 mM [γ-32P]ATP (Sigma) and 33% reticulocyte lysate(v/v) (Promega) in a total volume of 30 μl at 37 °C for 5 min.
RESULTS
Establishment of double stable C-JNK cell lines and fragment expression
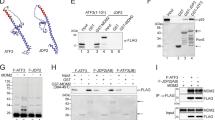
The HA-C-JNK cDNA inserted pUHD-10-3 plasmid was transfected into the cells with high expression and low basal level and tet was added immediately to a final concentration of 1 μg/ml. Resistant clones were selected by using 600 μg/ml geneticin (G418) and 100 μg/ml hygromycin. There is an exogenous HA tag at the N-terminus of C-JNK for avoiding the endogenous JNK, so when tet (1 μg/ml) was present, C-JNK fragment expression was suppressed. On the other hand, if tet was withdrawn by three times washing with PBS and adding normal medium, the expression of C-JNK increased with time, as detected by Western blot (Fig. 1). As a control, GST protein level during such period kept stable (data not shown).
Expression of C-JNK in NIH 3T3 cells after removal of tetracycline. HA-tagged C-JNK was used to established 3T3 cells that are tet-regulated. Mixed populations of cells were selected based on drug resistance and analyzed for C-JNK expression at the indicated time points after tet removal. Western blotting analysis revealed the expression of a 25 kD protein detected by anti-HA.
The effect of C-JNK fragment induction on cell proliferation
The radioactivity of 3H-TdR was measured to determine cell proliferation when tet was added back 24 h after being removed to stop further expression. We showed that cell proliferation decreased obviously compared to the control cells with the induction of C-JNK fragment (Fig. 2). This result is consistent with the finding that forced expression of C-JNK fragment increased p21waf-luciferase activity (Fig. 4 A) and p53 stability (Fig. 3A).
3H-TdR incopration into DNA before and after C-JNK induction. C-JNK expressing NIH 3T3 cells (105 in 6-well plates) were cultured in the presence or absence of tet. Cells were prepared for analysis 24 h after 3H-TdR being added. Each analysis was performed in triplicate. The data represents three independent experiments.
(A) Transcriptional activates of p53 in C-JNK-expressing cells. Cells (105 in 6-well plates) were cotransfected with p21waf-luciferase and α-gal constructs (0.5 μg of each) and maintained in presence or absence of tet. After 24h, cells were harvested at the indicated time points. Luciferase assays were carried out using proteins normalized as per α-gal activates to reflect equal transfection efficiency. Data shown represents three experiments performed in duplicates. (B) The p21 protein level at different time points before and after removing tet. Cells were harvested at different time points before and after removing tet. The level of p21 levels were detected by western blot using p21 antibody.
(A) The effect of C-JNK on p53 protein level. Cells expressing C-JNK were maintained in tet-free medium and harvested at indicated time points. Western blot analysis was carried out using monoclonal pAb421 antibodies against p53. (B) C-JNK effect on in vitro ubiqutination of p53. Ubiquitination of bacterially expressed purified p53 incubated with cell lysates that either contained or lacked C-JNK fragment was carried out as described under “Material and Methods”. A high molecular weight smear was detected by antibodies to HA recognized HA-ubiquitin, which formed a poly-ubiquitin chain covalently bound to p53.
Decrease of p53 ubiquitination and its upregulation after forced expression of C-JNK fragment
As shown in Fig. 3A, after induction of C-JNK fragment, the endogenous p53 protein was obviously up-regulated. In order to determine whether such increase of p53 protein level was resulted from the inhibition of ubiquitination of p53, the degree of smear above p53-reacting band representing polyubiquitin chains of HA-ubiquitin was detected to describe changes in the level of p53 ubiquitination according to C-JNK expression. Results showed the ubiquitin bound to p53 was evidently reduced with the expression of C-JNK (Fig. 3B), indicating that C-JNK may be involved in the ubiquitination of p53.
The effect of forced expression of C-JNK fragment on p53
p21waf, which can be activated by p53 and lead to cell apoptosis or growth arrest, was used to measure p53 activity. In order to study the effect of forced expression of C-JNK fragment on p53, p21waf-luciferase reporter vector was transfected into the selected cells. Then the expression of C-JNK fragment was induced by removing tet, the luciferase activity were measured before and after induction. Our result showed clearly that p53 activity increased after the induction of C-JNK fragment, which is supported not only by elevated p21waf-luciferase activity, but also the p21 protein level (Fig. 4).
Association of C-JNK and p53 in vitro and in vivo
The inducible C-JNK system and the bacterially expressed p53 and C-JNK were used to further detect if C- JNK could efficiently interact with p53. For in vitro studies, bacterially expressed his-tagged p53 protein was incubated with beads-bound HA-C-JNK. As shown in fig. 5A, co-precipitating p53 was detected by immuno-blot analysis with anti-p53 antibody (Fig. 5A lanes 1-3). Reciprocal experiments showed C-JNK was also detected in the nickel-beads-bound his-p53 pull-down complexes (Fig. 5A lanes 4-9). In vivo experiment of measuring the association between C-JNK and p53 was demonstrated in cells expressing inducing C-JNK, using anti-p53 antibody to immunoprecipitate p53. Fig. 5B shows that C-JNK was detected in tet-induced cells, wheareas absent in non-induced cells. These results suggested that JNK had some docking sites for p53 association.
(A) In vitro association of bacterially expressed C-JNK with p53. Bacterially expressed his-p53 and HA-C-JNK were purified on nickel or HA-antibody-coupled beads, respectively. Soluble C-JNK was incubated with the bead-bound p53 for 15 min on ice before beads were subjected to three washes. Antibodies to either p53 or to HA were used to immunoprecipitate respective complexes. Immunoprecipitated material was washed and analyzed by western blots using HA or p53 antibodies as indicated. (B) In vivo association of immunoprecipitated p53 with C-JNK. C-JNK expressing cells were grown in the presence or absence of tet. Immunoprecipitation was carried out using monoclonal pAb421 antibodies against p53. Bound C-JNK was detected using HA antibodies which identified HA-tagged C-JNK. Data shown here represents three independent analyses.
DISSCUSSION
The most comman ones among mechanisms underlying the regulation of stress kinases are their dimerization and abrogation of intramolecular inhibition, both of which are dependent upon their phosphorylation by each respective upstream kinase 24. However, for JNK, the characters and functions of its domains were still kept rarely informed. This study aims to elucidate which part of JNK is responsible for the binding of p53, by using the low background tet-off inducible NIH3T3 cell line selected by luciferase reporter gene and a double stable C-JNK Aa (203-424) cell line. The finding that the induction of C-JNK fragment could lead to JNK phosphorylation (data not show) hinted that C-JNK opened the phosphorylation sites of JNK, as similar as other MAPKs. In normal growing cells, one of the mechanisms to keep low base level of p53 in normal cells lies in the bingding of inactivated JNK to p53, which in turn leads to the degradation of p53 15. Previous studies revealed that wide type JNK contributes to the stability as well as the activity of p53 14, 15. In the present study, our findings demonstrated that the forced expression of C-JNK fragment obviously inhibited cell proliferation. So next we focus on whether C-JNK could regulate p53. We found that C-JNK fragment could form complex with p53 both in vivo and in vitro and the induction of C-JNK fragment in vivo can increase p53 stability by altering p53 ubiquitination. It is obvious that the forced expression of C-JNK fragment could stabilize p53, and furthermore, the induction of C-JNK fragment could affect cell cycle.
What we found in the function of C-JNK fragment in inhibiting p53 stability is quite similar with that of N-JNK fragment 25, which may hint that both terminals of JNK hold sites for p53 association. The difference between N-JNK and C-JNK fragment is that induction of N-JNK fragment increases the sensitivity of cells to H2O2 while induction of C-JNK regulates cell proliferation 25. Although the docking sites of c-Jun and p53 for JNK are clear 16, 26, which domain of JNK is crutial for the association of p53 and c-Jun still remains unknown. Our results suggest that both N-JNK and C-JNK are responsible for full length JNK to bind with p53.
MDM2 is another cellular protein which mediates p53 ubiquitination and degradation. The relationship between C-JNK and MDM2 should be carried out in our further studies.
References
Errede B, Levin BE . A conserved kinase cascade for MAP kinase activation in yeast. Curr Opin Cell Biol 1993; 5: 254–60.
Davis RJ . Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–52.
Marshall CJ . MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev 1994; 4: 82–9.
Kyriakis JM, Avruch J . Sounding the alarm: protein kinases cascades activated by stress and inflammation. J Biol Chem 1996; 271: 24313–6.
Schaeffer HJ, Weber MJ . Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 1999; 19: 2435–44.
Widmann C, Gibson S, Jarpe MB, Johnson GL . Mitogen-activated protein: conservation from yeast to human. Physiol Rev 1999; 79: 143–80.
Jiang Y, Chen C, Li Z, et al. Characterization of the structure and function of a new mitogen-activated protein kinase(p38β). J Biol Chem 1996; 271: 17920–6.
Lopez-Ilasaca M . Signalling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol 1998; 56: 269–77.
Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 1994; 76: 1025–37.
Karin M . The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 1995; 270: 16483–16486.
Minden A, Karin M . Regulation and function of the JNK subgroup of MAP kinases. Biochem Biophys Acta 1997; 1333: F85–104.
Adler V, Yin Z, Fuchs SY, et al. Regulation of JNK signaling by GSTp. EMBO J 1999; 18: 1321–34.
Fuchs SY, Fried VA, Ronai Z . Stress-activated kinases regulate protein stability. Oncogene 1998; 17: 1483–90.
Fuchs SY, Xie B, Adler V, et al. c-Jun N-terminal kinases target the ubiquitination of their associated transcription factors. J Biol Chem 1997; 272: 32163–8.
Fuchs SY, Adler V, Thomas B, et al. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev 1998; 12: 2658–63.
Hibi M, Lin A, Smeal T, Minden A, Karin M . Identification of an oncoprotein-and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev 1993; 7: 2135–48.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME . Opposing effects of ERK and JNK-p38 MAPK kinases on apoptosis. Science 1995; 270: 1326–31.
Verheij M, Bose R, Lin XH, et al. Requirement for ceramide-initiated SAPK/JNK signaling in stress induced apoptosis. Nature 1996; 380: 75–9.
Gupta S, Barrett T, Whitmarsh AJ, et al. Selective interation of JNK protein kinase isoforms with transcription factors. EMBO J 1996; 15: 2760–70.
Westwick JK, Bielawska AE, Dbaibo G, et al. Ceramide activates the stress-activated protein kinase. J Biol Chem 1995; 270: 22689–92.
Noguchi K, Kitanaka C, Yamana H, Hannun YA, Brenner DA . Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase. J Biol Chem 1999; 274: 32580–7.
Yamamoto K, Ichijo H, Korsmeyer SJ . BCL-2 is phosphorylated and inactivated by ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol 1999; 19: 8469–78.
Milne DM, Campbell DG, Meek DW, Meek DW . p53 is phosphorylated in vitro and in vivo by an ultraviolet radiation induced protein kinase characteristic of c-Jun kinase JNK1. J Biol Chem 1995; 270: 5511–8.
Tanoue T, adachi M, Moriguchi T, Nishida E . A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol 2000; 2:270: 110–6.
Thomas B, Yin Z, Anindita B, Ze'ev Ronai . Amino-terminal-derived JNK Fragment Alters Expression and Activity of c-Jun, ATF2, and p53 and Increases H2O2-induced Cell Death. J Biol Chem. 2000; 275: 16590–6.
Fuchs SY, Adler V, Pincus MR, Ronai Z . MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci USA 1998; 95: 10541–6.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No.30270556) and The National Basic Research Program (No.2002CB513004). We would also like to thank Dr. Ze'ev Ronai (Department of Oncological Sciences, Mount Sinai School of Medicin. NY, USA) for the constructions of some plasmids and cell lines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
YIN, Z., SIMA, J., WU, Y. et al. The effect of C-terminal fragment of JNK2 on the stability of p53 and cell proliferation. Cell Res 14, 434–438 (2004). https://doi.org/10.1038/sj.cr.7290244
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290244