Abstract
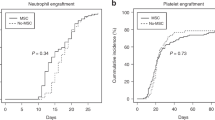
In this open-label randomized clinical trial, HLA-identical sibling-matched hematopoietic stem cells (HSC) were transplanted (non-MSCs group, n=15) or cotransplanted with mesenchymal stem cells (MSCs) (MSCs group, n=10) in hematologic malignancy patients. The median number of MSCs infused was 3.4 × 105 kg−1 (range, 0.3–15.3 × 105 kg−1). MSCs infusions were well tolerated. The median time to neutrophil engraftment (absolute neutrophil count >0.5 × 109 l−1) was 16 days for MSCs group and 15 days for non-MSCs group. The median time to platelet engraftment (platelet count >50 × 109 l−1) was 30 and 27 days, respectively. Grades II–IV acute graft-versus-host disease (GVHD) was observed respectively, in one (11.1%) and eight (53.3%) evaluable patients. Chronic GVHD was found in one (14.3%) and four (28.6%) evaluable patients. The number of patients who relapsed were six (60.0%) and three (20.0%), and the 3-year disease-free survivals were 30.0 and 66.7%, respectively. Thus cotransplantation of MSCs and HSCs may prevent GVHD, but the relapse rate is obviously higher than the control group. We conclude that use of MSCs must be handled with extreme caution before a large-scale clinical trial is performed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Armitage JO . Bone marrow transplantation. N Engl J Med 1994; 330: 827–838.
Yeager AM . Allogeneic hematopoietic cell transplantation in inborn metabolic diseases. Ann Hematol 2002; 81: S16–S19.
Burt RK, Traynor AE, Craig R, Marmont AM . The promise of hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant 2003; 31: 521–524.
Wingard JR, Vogelsang GB, Deeg HJ . Stem cell transplantation: supportive care and long-term complications. Hematology 2002, 422–444.
Tabbara IA, Zimmerman K, Morgan C, Nahleh Z . Allogeneic hematopoietic stem cell transplantation: complications and results. Arch Intern Med 2002; 162: 1558–1566.
Antin JH, Chen AR, Couriel DR, Ho VT, Nash RA, Weisdorf D . Novel approaches to the therapy of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant 2004; 10: 655–668.
Van Lint MT, Milone G, Leotta S, Uderzo C, Scime R, Dallorso S et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood 2006; 107: 4177–4181.
Ferrara JL, Deeg HJ . Graft-versus-host disease. N Engl J Med 1991; 324: 667–674.
Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW et al. Depletion of HLA-identical transplants in leukemia. Blood 1991; 78: 2120–2130.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147.
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy 2005; 7: 393–395.
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI et al. Rapid hematopoietic recovery after co-infusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307–316.
Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol 2003; 31: 413–420.
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30: 42–48.
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722–3729.
Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC . Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389–397.
Frassoni F, Labopin M, Bacigalupo A, Gluckman E, Rocha V, Bruno B et al. Expanded mesenchymal stem cells (MSC), co-infused with HLA identical hematopoietic stem cells transplants, reduce acute and chronic graft-versus-host disease: a matched pair analysis in hematologic malignancy patients. Bone Marrow Transplant 2002; 29 (suppl 2): S2 [Abstract 75].
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441.
Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003; 102: 3837–3844.
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 9: 1269–1274.
Olerup O, Zetterquist H . HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 h: an alternative to serological DR typing in clinical practice including donorrecipient matching in cadaveric transplantation. Tissue Antigens 1992; 39: 225–235.
Copelan EA, Biggs JC, Szer J, Thompson JM, Crilley P, Brodsky I et al. Allogeneic bone marrow transplantation for acute myelogenous leukemia, acute lymphocytic leukemia, and multiple myeloma following preparation with busulfan and cyclophosphamide (BuCy2). Semin Oncol 1993; 20 (4 suppl 4): 33–38.
Socié G, Clift RA, Blaise D, Devergie A, Ringden O, Martin PJ et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood 2001; 98: 3569–3574.
Clift RA, Buckner CD, Thomas ED, Bensinger WI, Bowden R, Bryant E et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood 1994; 84: 2036–2043.
Storb R, Deeg HJ, Fisher L, Appelbaum F, Buckner CD, Bensinger W et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood 1988; 71: 293–298.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM . Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Bone Marrow Transplant 1989; 4: 247–254.
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30: 42–48.
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838–3843.
Troeger A, Meisel R, Moritz T, Dilloo D . Immunotherapy in allogeneic hematopoietic stem cell transplantation—not just a case for effector cells. Bone Marrow Transplant 2005; 35: S59–S64.
Ramasamy R, Lam EW-F, Soeiro I, Tisato V, Bonnet D, Dazzi F . Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia 2007; 21: 304–310.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11: 389–398.
Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H et al. Mesenchymal stem cells for treatment of therapy resistant graft-versus-host disease. Transplantation 2006; 81: 1390–1397.
Schmitz N, Bacigalupo A, Hasenclever D, Nagler A, Gluckman E, Clark P et al. Allogeneic bone marrow transplantation vs filgrastim-mobilised peripheral blood progenitor cell transplantation in patients with early leukaemia: first results of a randomised multicentre trial of the European group for blood and marrow transplantation. Bone Marrow Transplant 1998; 21: 995–1003.
Powles R, Mehta J, Kulkarni S, Treleaven J, Millar B, Marsden J et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet 2000; 355: 1231–1237.
Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 2001; 344: 175–181.
Champlin RE, Schmitz N, Horowitz MM, Chapuis B, Chopra R, Cornelissen JJ et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. Blood 2000; 95: 3702–3709.
Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100: 1525–1531.
Tisato V, Naresh K, Girldstone J, Navarrete C, Dazzi F . Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia 2007; 21: 1992–1999.
Lazarus HM, Koc ON . Culture-expanded human marrow derived MSCs in clinical hematopoietic stem cell transplantation. Graft 2001; 3: 329–333.
Koc ON, Lazarus HM . Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant 2001; 27: 235–239.
Park S-K, Won J-H, Kim H-J, Bae S-B, Kim C-K, Lee K-T et al. Co-transplantation of human mesenchymal stem cells promotes human CD34+ cells engraftment in a dose-dependent fashion in NOD/SCID mice. J Korean Med Sci 2007; 22: 412–419.
Acknowledgements
We thank Dr Lianming Liao, for reading, commenting and correcting the manuscript. We also thank Professor Liangping Hu for help on statistical analysis. This work was supported by the National 863 Program, Ministry of Science and Technology, People's Republic of China (No: 2001AA217131).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ning, H., Yang, F., Jiang, M. et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 22, 593–599 (2008). https://doi.org/10.1038/sj.leu.2405090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2405090
Keywords
This article is cited by
-
Mesenchymal stromal cells plus basiliximab improve the response of steroid-refractory acute graft-versus-host disease as a second-line therapy: a multicentre, randomized, controlled trial
BMC Medicine (2024)
-
A review of the application of mesenchymal stem cells in the field of hematopoietic stem cell transplantation
European Journal of Medical Research (2023)
-
Stem cell therapy: a novel approach against emerging and re-emerging viral infections with special reference to SARS-CoV-2
Molecular Biology Reports (2023)
-
Mesenchymal stromal cells plus basiliximab, calcineurin inhibitor as treatment of steroid-resistant acute graft-versus-host disease: a multicenter, randomized, phase 3, open-label trial
Journal of Hematology & Oncology (2022)
-
Infusion of haploidentical HSCs combined with allogenic MSCs for the treatment of ALL patients
Bone Marrow Transplantation (2022)