Abstract
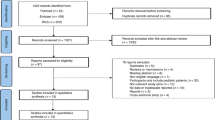
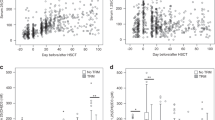
Bone loss occurs frequently following allogeneic haematopoietic stem cell transplantation (alloSCT). The Australasian Leukaemia and Lymphoma Group conducted a prospective phase II study of pretransplant zoledronic acid (ZA) and individualised post-transplant ZA to prevent bone loss in alloSCT recipients. Patients received ZA 4 mg before conditioning. Administration of post-transplant ZA from days 100 to 365 post alloSCT was determined by a risk-adapted algorithm based on serial bone density assessments and glucocorticoid exposure. Of 82 patients enrolled, 70 were alive and without relapse at day 100. A single pretransplant dose of ZA prevented femoral neck bone loss at day 100 compared with baseline (mean change −2.6±4.6%). Using the risk-adapted protocol, 42 patients received ZA between days 100 and 365 post alloSCT, and this minimised bone loss at day 365 compared with pretransplant levels (mean change −2.9±5.3%). Femoral neck bone loss was significantly reduced in ZA-treated patients compared with historical untreated controls at days 100 and 365. This study demonstrates that a single dose of ZA pre-alloSCT prevents femoral neck bone loss at day 100 post alloSCT, and that a risk-adapted algorithm is able to guide ZA administration from days 100 to 365 post transplant and minimise further bone loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ebeling PR, Thomas DM, Erbas B, Hopper JL, Szer J, Grigg AP . Mechanisms of bone loss following allogeneic and autologous hemopoietic stem cell transplantation. J Bone Miner Res 1999; 14: 342–350.
Stern JM, Sullivan KM, Ott SM, Seidel K, Fink JC, Longton G et al. Bone density loss after allogeneic hematopoietic stem cell transplantation: a prospective study. Biol Blood Marrow Transplant 2001; 7: 257–264.
Yao S, McCarthy PL, Dunford LM, Roy DM, Brown K, Paplham P et al. High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transplant 2008; 41: 393–398.
Petropoulou AD, Porcher R, Herr AL, Devergie A, Brentano TF, Ribaud P et al. Prospective assessment of bone turnover and clinical bone diseases after allogeneic hematopoietic stem-cell transplantation. Transplantation 2010; 89: 1354–1361.
Pundole XN, Barbo AG, Lin H, Champlin RE, Lu H . Increased incidence of fractures in recipients of hematopoietic stem-cell transplantation. J Clin Oncol 2015; 33: 1364–1370.
Ebeling PR . Approach to the patient with transplantation-related bone loss. J Clin Endocrinol Metab 2009; 94: 1483–1490.
Quach JM, Askmyr M, Jovic T, Baker EK, Walsh NC, Harrison SJ et al. Myelosuppressive therapies significantly increase pro-inflammatory cytokines and directly cause bone loss. J Bone Miner Res 2015; 30: 886–897.
Välimäki MJ, Kinnunen K, Tähtelä R, Löyttyniemi E, Laitinen K, Mäkelä P et al. A prospective study of bone loss and turnover after cardiac transplantation: effect of calcium supplementation with or without calcitonin. Osteoporos Int 1999; 10: 128–136.
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–1822.
Grigg AP, Shuttleworth P, Reynolds J, Schwarer AP, Szer J, Bradstock K et al. Pamidronate reduces bone loss after allogeneic stem cell transplantation. J Clin Endocrinol Metab 2006; 91: 3835–3843.
Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001; 19: 558–567.
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 2003; 98: 1735–1744.
Chae YS, Kim JG, Moon JH, Kim SN, Lee SJ, Kim YJ et al. Pilot study on the use of zoledronic acid to prevent bone loss in allo-SCT recipients. Bone Marrow Transplant 2009; 44: 35–41.
Ganguly S, Divine CL, Aljitawi OS, Abhyankar S, McGuirk JP, Graves L . Prophylactic use of zoledronic acid to prevent early bone loss is safe and feasible in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation. Clin Transplant 2012; 26: 447–453.
Hari P, DeFor TE, Vesole DH, Bredeson CN, Burns LJ . Intermittent zoledronic acid prevents bone loss in adults after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2013; 19: 1361–1367.
Hausmann A, Hill W, Stemmler HJ, Ledderose G, Baur-Melnyk A, Fritsch S et al. Bone loss after allogeneic haematopoietic stem cell transplantation: a pilot study on the use of zoledronic acid. Chemother Res Pract 2012; 2012: 858590.
Tauchmanova L, Ricci P, Serio B, Lombardi G, Colao A, Rotoli B et al. Short-term zoledronic acid treatment increases bone mineral density and marrow clonogenic fibroblast progenitors after allogeneic stem cell transplantation. J Clin Endocrinol Metab 2005; 90: 627–634.
D'Souza AB, Grigg AP, Szer J, Ebeling PR . Zoledronic acid prevents bone loss after allogeneic haemopoietic stem cell transplantation. Intern Med J 2006; 36: 600–603.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Anandi P, Jain NA, Tian X, Wu CO, Pophali PA, Koklanaris E et al. Factors influencing the late phase of recovery after bone mineral density loss in allogeneic stem cell transplantation survivors. Bone Marrow Transplant 2016; 51: 1101–1106.
Falch JA, Odegaard OR, Finnanger AM, Matheson I . Postmenopausal osteoporosis: no effect of three years treatment with 1,25-dihydroxycholecalciferol. Acta Med Scand 1987; 221: 199–204.
Arthur RS, Piraino B, Candib D, Cooperstein L, Chen T, West C et al. Effect of low-dose calcitriol and calcium therapy on bone histomorphometry and urinary calcium excretion in osteopenic women. Miner Electrolyte Metab 1990; 16: 385–390.
Gómez Alonso C, Díaz Curiel M, Hawkins Carranza F, Pérez Cano R, Díez Pérez A . Femoral Bone Mineral Density, Neck-Shaft Angle and Mean Femoral Neck Width as Predictors of Hip Fracture in Men and Women. Osteoporos Int 2000; 11: 714–720.
Cummings SR, Melton LJ . Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359: 1761–1767.
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125–1132.
Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012; 48: 3082–3092.
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E . FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008; 19: 385–397.
Acknowledgements
This study was supported with funding and the provision of zoledronic acid by Novartis Pharmaceuticals Australia Pty Ltd.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
BB received payment for statistical services from the Australasian Leukaemia and Lymphoma Group (ALLG), and received consulting fees from Novartis for other unrelated work. AB has received honoraria from Novartis. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Grigg, A., Butcher, B., Khodr, B. et al. An individualised risk-adapted protocol of pre- and post transplant zoledronic acid reduces bone loss after allogeneic stem cell transplantation: results of a phase II prospective trial. Bone Marrow Transplant 52, 1288–1293 (2017). https://doi.org/10.1038/bmt.2017.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.108
This article is cited by
-
Predictors of impaired bone health in long-term survivors after allogeneic stem cell transplantation
Bone Marrow Transplantation (2019)
-
Bone management in hematologic stem cell transplant recipients
Osteoporosis International (2018)