Abstract
As many oncogenic changes, such as Myc overexpression, promote apoptosis, the survival of emerging neoplastic clones may often initially depend upon endogenous levels of particular pro-survival members of the Bcl-2 protein family. Pertinently, we recently showed that in lymphoma-prone Eμ-myc transgenic mice, which overexpress Myc in all B-lymphoid cells, endogenous Bcl-xL is critical for the survival, as well as the expansion of preneoplastic B-lymphoid cells and the development of malignant disease. This discovery raised the possibility that pharmacological blockade of Bcl-xL might impede Myc-driven lymphoma development. Indeed, we report here that treatment of preleukaemic Eμ-myc transgenic mice with the Bcl-2 homology (BH)3 mimetic drug ABT-737, which inhibits Bcl-xL, as well as Bcl-2 and Bcl-w, augmented apoptosis of preneoplastic B-lymphoid cells, reduced their numbers and greatly prolonged lymphoma-free survival. These findings reveal that BH3 mimetic drugs may provide a prophylactic strategy to prevent the development of certain tumours, particularly those driven by deregulated Myc expression. Moreover, such treatment may help in the management of patients with hereditary cancer syndromes and perhaps also in the prevention of tumour relapses.
Similar content being viewed by others
Main
Evasion of apoptosis is a prerequisite for the development of most, possibly all, malignancies.1, 2, 3 Apoptosis is controlled largely by opposing factions of the Bcl-2 family.4, 5 The members promoting cell survival include Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1, which all have four Bcl-2 homology (BH) domains. Largely owing to their diverse expression patterns, particular pro-survival Bcl-2 family members are critical to sustain survival of specific cell types.6 One pro-apoptotic faction includes Bax and Bak, which also bear four BH domains and share extensive structural similarity with their pro-survival relatives,6 and their activation is essential for the pivotal step of mitochondrial outer membrane permeabilisation, which unleashes the caspase cascade that demolishes the cell.4, 7 The members of the more distantly related second pro-apoptotic group, the so-called BH3-only proteins (e.g. Bim, Puma, Bad), share with each other and the wider Bcl-2 family only the BH3 domain. The BH3-only proteins, which are activated by diverse stress stimuli, including cytokine deprivation and DNA damage, are essential to initiate apoptosis signalling. They are thought to activate Bax and Bak either by binding them directly (e.g. in the case of Bim, tBid and Puma) or by liberating them from guardian pro-survival Bcl-2 relatives or both ways.4, 5, 8
As recently reviewed,5 both genetic alterations in human tumours and analysis of transgenic mice overexpressing Bcl-2 or a pro-survival homologue leave no doubt that these proteins can contribute to neoplastic transformation. However, on its own, the tumorigenic impact of Bcl-2 is relatively low,9, 10 unless another oncogene, such as Myc, is coexpressed.11 As many of the mutations that initiate oncogenesis, like those imposing Myc overexpression, disturb cell cycle checkpoints and thereby render cells more sensitive to apoptosis,12 we reasoned that emerging neoplastic clones could be particularly dependent upon the endogenous levels of certain Bcl-2 pro-survival proteins. We examined this hypothesis using the well-studied lymphoma-prone Eμ-myc transgenic mice, in which enforced Myc expression creates an expanded pool of proliferating (preleukaemic) pro-B and pre-B lymphocytes from which malignant clones emerge.13, 14, 15 To test the consequences of loss of Bcl-2 or Bcl-xL in these mice, we circumvented the early postnatal or embryonic lethality provoked, respectively, by loss of Bcl-216, 17 or Bcl-xL18 by generating cohorts of chimaeric mice in which the haemopoietic system had an Eμ-myc/bcl-2−/−, Eμ-myc/bcl-x−/− or control genotype.19, 20
Bcl-2 and Bcl-xL both proved critical for the survival of preleukaemic Eμ-myc mature (sIg+) B cells, but only Bcl-xL was required for the survival and accumulation of preleukaemic Eμ-myc pro-B and pre-B cells. Remarkably, whereas loss of Bcl-2 affected neither the incidence nor rate of lymphoma development,19 loss of Bcl-xL abrogated the lymphomagenesis.20 These observations are consistent with the notion that the pro-B and pre-B cell stages are critical for neoplastic progression in this mouse lymphoma model, most likely due to their high rate of proliferation and the genomic instability caused by immunoglobulin gene rearrangement, which both facilitate acquisition of mutations.21
Our demonstration that Bcl-xL was essential for the survival of Myc-driven lymphoma-initiating cells while they acquire the additional oncogenic lesions that propel neoplastic transformation raised the possibility that pharmacological blockade of Bcl-xL might, similar to loss of the bcl-x gene,20 inhibit Myc-induced lymphomagenesis. BH3 mimetics, synthetic compounds that mimic the BH3-only proteins by engaging and inhibiting one or more of the pro-survival proteins, are showing great promise for cancer therapy,5, 22, 23 particularly in chronic lymphocytic leukaemia,24 but their potential for preventing cancer has not yet been explored. Here we demonstrate that prophylactic treatment of preleukaemic Eμ-myc mice with the BH3 mimetic ABT-737, which neutralises Bcl-xL, Bcl-2 and Bcl-w but not Mcl-1 or A1,22, 25 markedly delayed the onset and greatly reduced the incidence of lymphoma development.
Results
To generate standardised cohorts of lymphoma-prone and control animals, the haemopoietic system of lethally irradiated C57BL/6-Ly5.1 mice was reconstituted with haemopoietic stem/progenitor cells derived from the fetal liver of E14.5 Eμ-myc mice. Six weeks post reconstitution, the recipients (hereafter called Eμ-myc mice) were treated with either a single dose of ABT-737 (75 mg/kg body weight)22 or for 2 weeks with three doses per week to monitor short-term effects on the preleukaemic B-lymphoid compartment (Figure 1). Alternatively, mice were treated for an 8-week period (three doses per week) to assess long-term effects on lymphomagenesis (Figure 1).
Experimental strategy to assess the impact of ABT-737 on Eμ-myc-induced lymphoma development. C57BL/6-Ly5.1 mice reconstituted with Eμ-myc fetal liver-derived stem/progenitor cells were treated from 6 weeks post reconstitution with ABT-737 (75 mg/kg – three times per week) for the periods indicated. Mice treated with a single dose of ABT-737 were killed after 24 h and analysed for the induction of apoptosis in the bone marrow. Mice treated for 2 weeks were investigated for the impact of this compound on the numbers of preleukaemic B-lymphoid cells in the bone marrow and spleen. Mice treated for 8 weeks with ABT-737 were examined for its impact on the rate of onset and incidence of lymphoma
ABT-737 reduces the numbers of preleukaemic B-lymphoid cells in E μ -myc mice
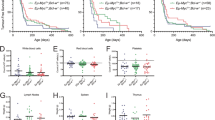
The enforced Myc expression in Eμ-myc mice generates a several-fold elevation in preleukaemic pro-B and pre-B cell numbers in haemopoietic tissues,15 but the elevated Myc also renders these lymphocytes more susceptible to apoptotic stimuli, such as growth factor deprivation.26 To assess whether ABT-737 affected the preleukaemic abnormalities caused by Myc overexpression, the reconstituted mice were injected six times with either ABT-737 (75 mg/kg) or vehicle over a 2-week period (three doses per week) and their content of preleukaemic B-lymphoid cells was determined by flow cytometric analysis. Remarkably, Figure 2 shows that all B-lymphoid subsets in the ABT-737-treatment cohort were significantly decreased, compared with the vehicle-treated animals, in both the bone marrow (*Ppro-B <0.05, ***Ppre-B <0.001, **PsIg+-B <0.01) and the spleen (*Ppro-B <0.05, *Ppre-B <0.05, *PsIg+-B <0.05).
ABT-737 reduced the numbers of preleukaemic Eμ-myc B-lymphoid cells in vivo. The numbers of preleukaemic B-lymphoid cells in the bone marrow and spleen of Eμ-myc reconstituted mice after six injections of ABT-737 or vehicle over a period of 2 weeks (three doses per week) were determined by flow cytometric analysis. Data represent mean±S.E.M., 4–5 mice per treatment group (*P<0.05, **P<0.01, ***P<0.001)
The drop in preleukaemic B-lymphoid cells elicited by ABT-737 almost certainly reflects increased apoptosis, because TUNEL staining for DNA breaks, a hallmark of apoptosis, revealed that Eμ-myc animals treated with a single dose of ABT-737 contained significantly (**P<0.01) more apoptotic cells in their bone marrow than vehicle-treated mice (Figure 3).
ABT-737-induced apoptosis of preleukaemic Eμ-myc B lymphoid cells in vivo. TUNEL staining for DNA strand breaks to enumerate apoptotic preleukaemic B-lymphoid cells in the bone marrow of Eμ-myc reconstituted mice 24 h after treatment with a single dose of ABT-737. (a) Data represent mean±S.E.M., for at least five mice per treatment group. **P<0.01. (b) Representative example of TUNEL analyses of the extent of apoptosis in bone marrow sections of Eμ-myc reconstituted mice treated prophylactically with ABT-737 or vehicle. Slides were counterstained with haematoxylin. As a control for the TUNEL reaction, staining was performed without using the enzyme TdT
Collectively, these results demonstrate that ABT-737 treatment causes a significant reduction in the preleukaemic Myc overexpressing B-lymphoid cells within the whole animal by increasing their propensity to undergo apoptosis.
ABT-737 substantially delays the onset and reduces the incidence of Myc-driven lymphoma
Next, we investigated whether the reduction in preleukaemic B-lymphoid cells and their increased rate of apoptosis elicited by ABT-737 treatment translated into a delay in Myc-induced lymphomagenesis. Six weeks post reconstitution, cohorts of transgenic mice were treated three times a week for 8 consecutive weeks with ABT-737 (75 mg/kg) or vehicle (Figure 1) and were then monitored for up to 18 months to determine the impact of the drug on the incidence and rate of lymphoma development. The vehicle-treated Eμ-myc mice developed lymphoma as early as 9 weeks post reconstitution and reached a tumour incidence of ∼80% by 60 weeks (Figure 4). In striking contrast, all 15 ABT-737-treated Eμ-myc mice remained tumour-free until 24 weeks post reconstitution and only 2 of them (13.4%) succumbed to lymphoma by 60 weeks (***P<0.001; Figure 4).
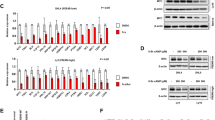
Notably, the only two lymphomas that arose in the drug-treated arm developed 8 and 40 weeks after cessation of ABT-737 treatment (Figure 4), and therefore both almost certainly developed in the complete absence of the drug. Furthermore, in these two lymphomas the expression of Bcl-2 family members and sensitivity in culture to apoptotic stimuli closely resembled that of conventional Eμ-myc lymphomas (Figure 5). Hence, these lymphomas most likely represent tumours that were initiated after ABT-737 blockade had ceased.
The two Eμ-myc lymphomas that arose in mice treated prophylactically with ABT-737 show similar Bcl-2 protein family expression profiles and similar sensitivity to apoptotic stimuli in vitro compared with conventional Eμ-myc lymphomas. (a) Western blots showing that the two lymphomas that arose at a late time after cessation of ABT-737 treatment (Figure 4) and a conventional Eμ-myc tumour had comparable expression of the indicated pro-survival and pro-apoptotic Bcl-2 family members. Probing with an antibody to Hsp-70 provided a loading control. (b) Cell survival assays showing that the two lymphomas from mice treated earlier with ABT-737 behaved similarly to conventional Eμ-myc tumours in culture when they were left untreated or exposed to etoposide (0.5 μg/ml), ABT-737 (10 μM) or 0.5 μg/ml etoposide plus 1 μM ABT-737. Cell viability after the indicated times of treatment was monitored by flow cytometric analysis
Discussion
The now widely accepted concept that cells must evade apoptosis to become malignant1, 2, 3, 5 was engendered by the discoveries that bcl-2, commonly translocated in human follicular lymphoma, promotes cell survival27 and that its overexpression in transgenic mice promotes lymphomagenesis.9, 10, 11, 28 However, compared with the tumorigenesis driven by enforced expression of the Myc transgene,14 overexpression of Bcl-2 alone causes only a low incidence of tumours with long latency.9, 10, 11, 28 Hence, we surmise that the initial mutations leading to most cancers enhance proliferation or self-renewal, and those impairing apoptosis are selected later, for example, to counter the stress imposed by disrupted cell cycle checkpoints. If so, the emerging neoplastic clones must initially be sustained by endogenous levels of expression of Bcl-2 pro-survival proteins. Accordingly, Bcl-xL proved to be essential for the emergence of Eμ-myc lymphomas.20
That discovery prompted us to test whether a BH3 mimetic that can neutralise Bcl-xL could retard tumorigenesis, and indeed, we show here that treatment with ABT-73722 provided effective prophylaxis against Myc-induced lymphomagenesis. We believe that its prophylactic effect can be ascribed largely (possibly solely) to its ability to antagonise Bcl-xL and not its other targets, Bcl-2 and Bcl-w.22 Bcl-w is probably not important in this setting because it is only poorly expressed in both normal lymphocytes29 and in Myc-overexpressing preleukaemic pro-B and pre-B lymphoid cells,30 the cell types from which the Eμ-myc lymphomas are thought to arise.13, 14, 15 Bcl-2 is ruled out as a significant target, because its complete loss did not lower the numbers of the pro-B and pre-B cells in preleukaemic Eμ-myc mice and did not notably impact the onset or incidence of Eμ-myc lymphoma.19 In contrast, both loss of Bcl-xL20 and ABT-737 prophylaxis reduced the numbers of Eμ-myc pre-B cells, rendered them sensitive to apoptosis and potently inhibited Myc-induced lymphomagenesis.
Notably, the only two mice treated prophylactically with ABT-737 that developed lymphoma presented 8 and 40 weeks after ABT-737 treatment had ceased (Figure 4). As the drug has a half-life in vivo of only about 18 h, these tumours clearly arose from Myc-driven preleukaemic precursors that acquired cooperating oncogenic lesions long after ABT-737 was gone. In other words, these two lymphomas most likely arose de novo from preneoplastic Myc-driven lymphoid cells rather than from transformed lymphoid cells that became resistant to ABT-737. Consistent with this hypothesis, neither of these two lymphomas displayed any overt abnormalities in the expression of pro-survival or pro-apoptotic Bcl-2 family members or behaviour in culture that might be expected if they had undergone neoplastic progression under selective pressure exerted by ABT-737, such as marked elevation in Mcl-125 or resistance to apoptosis. These observations indicate that several shorter intervals of ABT-737 prophylaxis might inhibit Eμ-myc-induced lymphoma development even more efficiently than the single-period treatment strategy that we employed.
Overexpression of Myc increases expression of the pro-apoptotic BH3-only Bcl-2 family members Bim and Puma,30, 31 and loss of Bim or Puma markedly accelerates Eμ-myc-induced lymphomagenesis.30, 31, 32, 33, 34 Hence, in the preleukaemic pro-B and pre-B cells of Eμ-myc mice treated with ABT-737, we surmise that the elevated levels of Bim and Puma elicited by Myc overexpression overwhelm the pro-survival Bcl-2 proteins that this agent cannot inhibit, namely Mcl-1 and possibly A1, and perhaps also directly activate Bax or Bak. The resulting apoptosis is expected to reduce the target population for transformation and also eliminate nascent neoplastic clones.20 It will be interesting to test whether loss of Bim, Puma or both of these BH3-only proteins will abrogate the ability of ABT-737 to inhibit Myc-driven lymphoma development.
It is interesting to note that, although ABT-737 greatly impedes the emergence of Myc-induced lymphomas (Figure 4), the fully fledged pre-B/B-cell malignancies induced solely by the Eμ-myc transgene are instead highly refractory to treatment with ABT-737.35 The loss in sensitivity may be because the malignant Eμ-myc lymphomas commonly have acquired mutations that block function of p53 or ARF,36 impairing their ability to induce the key p53 pro-apoptotic targets Puma and Noxa,37, 38 which together with Bim are critical for the killing of Eμ-myc lymphoma cells by chemotherapeutic drugs that cause DNA damage.39 Alternatively, some Eμ-myc lymphomas may be resistant to ABT-737 because they have acquired mutations that increase the level of Mcl-1 or A1, which ABT-737 does not inhibit. Mcl-1 levels can be augmented by higher copy numbers of its gene,40 by loss of one of the E3 ubiquitin ligases that promote its proteasomal degradation, such as the tumour suppressor FBW741 or conversely by upregulation of one of its deubiquitinases, such as USP9x.42
Our findings indicate that drugs that target the expression or activity of specific pro-survival Bcl-2 family members, such as the BH3 mimetic ABT-73722 and the closely related compound ABT-263,43 which is currently undergoing clinical trials, may not only help to eliminate established cancers but may also prevent the development of certain types of tumours by unleashing the pro-apoptotic impetus of the oncogenic changes they have sustained (e.g. enforced c-Myc expression). Hence, in principle, such drugs could contribute to the management of individuals with hereditary predispositions to malignancy, such as individuals bearing germline mutations in BRCA1, BRCA2 or p53. With p53-deficient mice, a model for the inherited human Li-Fraumeni syndrome, we recently tested prophylaxis with ABT-737, although the relevant Bcl-2 pro-survival target(s) for this condition is (are) unknown. ABT-737 treatment did significantly delay lymphoma development but only in the γ-irradiated p53−/− mice.44 Perhaps in p53 heterozygous individuals, a BH3 mimetic targeting Mcl-1 or A1 would have a more marked prophylactic effect. In any case, our present results strongly suggest that BH3 mimetics could have a prophylactic role in certain familial cancer syndromes, particularly those in which deregulated Myc expression contributes to neoplastic progression.
With certain types of malignancies, BH3 mimetics might also have a prophylactic role in reducing the frequency of recurrences. In human acute B-lymphoblastic leukaemias, which derive from cells of the same stage as those yielding the Eμ-myc lymphomas, genome-wide comparisons of paired primary and relapse samples suggest that relapses often arise from distinct minor subclones present at diagnosis but lacking some of the mutations present in the predominant diagnostic clone.45, 46 Conceivably, some recurrences arise from clones that, like the preneoplastic Myc-driven pro-B/pre-B lymphocytes, still rely initially upon endogenous levels of particular pro-survival proteins. If so, prophylactic use of a BH3 mimetic could prevent the re-emergence of malignant clones.
A concern for prophylactic use of a BH3 mimetic is the possibility of unacceptable toxicities in some normal cells. However, the only recognised toxicity so far identified in clinical trials with ABT-263 is an acute thrombocytopenia,24 produced by its on-target inhibition of Bcl-xL, the level of which controls platelet lifespan.47 Fortunately, as the drop in platelets is transient, dose dependent and reduced during chronic treatment,47 the thrombocytopenia can be largely managed by dose and scheduling. Whether other adverse effects would arise on prolonged prophylactic treatment remains to be determined by clinical trial.
Another issue for the prophylactic use of BH3 mimetics would be whether they compromise defence against infections by damaging the immune system. For example, ABT-737 treatment of mice reduces certain lymphoid populations and some newly arising immune responses but not those already established.48 However, the impact on immunity will depend greatly upon the specificity of the BH3 mimetic. For example, conditional deletion of bcl-x in antigen-activated B cells of mice had no effect on the development of germinal centre or memory B cells and little on plasma cells, whereas deletion of mcl-1 essentially abolished all three populations.49 Hence, a BH3 mimetic that targets Bcl-xL would not be expected to compromise immunity unacceptably and could have prophylactic potential.
Materials and Methods
Mice
Experiments with mice were conducted according to the guidelines of the Walter and Eliza Hall Institute Animal Ethics Committee. Eμ-myc transgenic mice have been described earlier.13, 14 The strain had been backcrossed to the C57BL/6-Ly5.2 genetic background for >30 generations.
Haemopoietic stem cell reconstitution and prophylactic treatment of preleukaemic mice with ABT-737
Eμ-myc E14.5 (Ly5.2) embryos were generated by mating Eμ-myc transgenic males with (wt) C57BL/6-Ly5.2 females. The day when the vaginal plug was detected was deemed embryonic day 0.5. Embryos from timed matings were harvested at E14.5 and genotyped by PCR on tail-derived DNA. Immediately before injection, single cell suspensions were prepared from fetal livers and ∼2 × 106 cells injected into the tail vein of lethally irradiated (2 × 5.5 Gy at 3 h interval) C57BL/6-Ly5.1 mice. Mice were maintained on neomycin sulphate-supplemented drinking water for 14 days post irradiation to prevent infection. Six weeks post reconstitution, the recipients were treated either once with ABT-737 (75 mg/kg body weight) or vehicle, or treated with ABT-737 for 2 or 8 week (75 mg/kg, three times per week; see the scheme in Figure 1).
Lymphoma monitoring and statistical analysis
Reconstituted mice that had been treated with ABT-737 or vehicle were monitored daily for signs of lymphoma development. Tumour-free survival was defined as the time from lethal irradiation and haemopoietic reconstitution with fetal liver cells to the time the animal was deemed ill by an experienced animal technician. To verify that mice used for preleukaemic analysis lacked malignant cells, 1 × 106 of their bone marrow and/or spleen cells were transplanted into non-irradiated histocompatible recipient mice, which were then monitored for 90 days for the development of any tumour. Kaplan–Meier curves were constructed using GraphPad Prism (version 5.0; GraphPad Software Inc., La Jolla, CA, USA), and statistical analysis performed using a Log-rank (Mantel-Cox) test.
Analysis of the haemopoietic system of reconstituted mice treated with ABT-737 or vehicle
Bone marrow (both femora), spleen and lymph nodes (combined mesenteric, inguinal and axillary) were harvested from the reconstituted mice that had been treated with one dose of ABT-737 or vehicle. Single cell suspensions were prepared and total cell numbers determined by trypan blue staining and counting in a haemocytometer as described earlier.50 Absolute numbers in each cellular compartment were calculated by multiplying the percentage of a cell type (as determined by fluorescence-activated cell sorting (FACS) analysis) by the total organ cellularity. Donor-derived cell numbers were calculated by multiplying the percentage of donor-derived (Ly5.2+) cells by the total organ cellularity. Peripheral blood was harvested via retro-orbital bleed or at the time of killing by cardiac puncture and collected into heparinized vessels. The numbers of total white blood cells were determined by using an Advia 120 blood analyser equipped with a mouse analysis software module (Bayer/Siemens, Deerfield, IL, USA).
Immunofluorescent staining, flow cytometric analysis and cell sorting
Before FACS-based immuno-phenotyping, peripheral blood was depleted of red blood cells by incubation (2 × 5 min) at 4 °C with red cell lysis buffer (56 mM NH4Cl, 0.1 mM EDTA, 12 mM NaHCO3, pH 7.3). To prevent non-specific antibody binding, cells were incubated in the presence of 2.4G2 (anti-FcγRII) antibody plus 2% normal rat serum. Host (Ly5.1+) and donor-derived (Ly5.2+) cells were discriminated by staining with anti-Ly5.1 (A201.1) and anti-Ly5.2 (5.450.15.2) monoclonal antibodies. Preleukaemic pro-B (B220+c-Kit+sIgM−sIgD−), pre-B (B220+c-Kit−sIgM−sIgD−) and sIg+ (B220+c-Kit-sIgM+sIgD+) B-lymphoid populations were purified by FACS sorting by staining with monoclonal antibodies to: B220 (RA3-6B2), c-Kit (ACK2 or ACK4), IgM (5.1 or 333.12) and IgD (11-26C). Antibodies were produced in our laboratory and conjugated to biotin, fluorescein isothiocyanate (FITC, both from Molecular Probes, Inc., Eugene, OR, USA), cyanine 5 (Cy5, Amersham Biosciences, Piscataway, NJ, USA), R-phycoerythin (R-PE) or allophycocyanin (APC, both from Prozyme, Hayward, CA, USA) according to the manufacturers’ instructions. Biotinylated antibodies were detected by secondary staining with FITC-, PE- or Tricolor-coupled streptavidin (Caltag Laboratories, Carlsbad, CA, USA). Dead cells were excluded by staining with propidium iodide (PI, 2 μg/ml). Purification of B-lymphoid subpopulations was performed by multi-parameter FACS sorting using a MoFlo (DAKO Cytomation Ltd, Ely, Cambridgeshire, UK) or DiVa (BD Biosciences, San Jose, CA, USA) high-speed flow cytometer.
Cell culture and cell survival analysis
Eμ-myc pre-B or B-lymphoma cells were cultured in a humidified incubator (10% CO2) at 37 °C in flat bottom 96-well microtitre plates at 2–5 × 104 cells/100 μl (per time point) in the high-glucose version of Dulbecco’s modified Eagle’s medium supplemented with 250 μM L-asparagine, 50 μM 2-mercaptoethanol and 10% heat-inactivated fetal calf serum (FCS, JRH Biosciences Pty Ltd, Melbourne, VIC, Australia). Cells were cultured in simple medium (no added growth factors) to assess the effects of cytokine deprivation. Cells were harvested after 4 h and the viability determined by staining with PI and FITC-conjugated annexin-V followed by flow cytometric analysis using a FACScan analyser (BD Biosciences).
TUNEL analysis on bone marrow
Sternum specimens were collected in 80% Histochoice for histological analysis. Sections for TUNEL analysis were de-paraffinised, treated with proteinase K (20 μg/ml for 10 min at room temperature) and endogenous peroxidases blocked by incubation in 10% hydrogen peroxide in methanol for 5 min at room temperature. They were then incubated with 0.6 U/μl terminal deoxynucleotidyl transferase (Promega, Madison, WI, USA), 20 μM biotin-16-dUTP (Roche, Castle Hill, NSW, Australia), 1 mM CoCl2 (Sigma-Aldrich Pty, Sydney, NSW, Australia) in terminal transferase buffer (Promega) for 1 h at 37 °C, followed by blocking with 2% FCS (FCS, JRH Biosciences) in PBS for 10 min at room temperature. The blocking solution was then removed and Vectorstain ABC (Vector Laboratories, Burlingame, CA, USA) reagents applied according to the manufacturer’s instructions. Sections were stained using DAB reagent (Vector Laboratories) according to the manufacturer’s instructions. Finally, sections were counterstained with haematoxylin.
Abbreviations
- BH:
-
Bcl-2 homology
- FACS:
-
fluorescence-activated cell sorting
References
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–656.
Letai AG . Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer 2008; 8: 121–132.
Chipuk JE, Green DR . How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 2008; 18: 157–164.
Strasser A, Cory S, Adams JM . Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J 2011; 30: 3667–3683.
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389–1399.
Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol 2009; 186: 355–362.
McDonnell TJ, Korsmeyer SJ . Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18). Nature 1991; 349: 254–256.
Strasser A, Harris AW, Cory S . Eμ-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells.. Oncogene 1993; 8: 1–9.
Strasser A, Harris AW, Bath ML, Cory S . Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990; 348: 331–333.
Evan GI, Vousden KH . Proliferation, cell cycle and apoptosis in cancer. Nature 2001; 411: 342–348.
Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985; 318: 533–538.
Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM . The Eμ-myc transgenic mouse: a model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med 1988; 167: 353–371.
Langdon WY, Harris AW, Cory S, Adams JM . The c-myc oncogene perturbs B lymphocyte development in Eμ-myc transgenic mice. Cell 1986; 47: 11–18.
Nakayama K-i Nakayama K, Izumi N, Kulda K, Shinkai Y, Louie MC et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science 1993; 261: 1584–1588.
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ . Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993; 75: 229–240.
Motoyama N, Wang FP, Roth KA, Sawa H, Nakayama K, Nakayama K et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science 1995; 267: 1506–1510.
Kelly PN, Puthalakath H, Adams JM, Strasser A . Endogenous bcl-2 is not required for the development of Eμ-myc-induced B-cell lymphoma. Blood 2007; 109: 4907–4913.
Kelly PN, Grabow S, Delbridge ARD, Strasser A, Adams JM . Endogenous Bcl-xL is essential for Myc-driven lymphomagenesis in mice. Blood 2011; 118: 6380–6386.
Wang JH, Gostissa M, Yan CT, Goff P, Hickernell T, Hansen E et al. Mechanisms promoting translocations in editing and switching peripheral B cells. Nature 2009; 460: 231–236.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681.
Lessene G, Czabotar PE, Colman PM . BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 2008; 7: 989–1000.
Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase i study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 2012; 30: 488–496.
van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006; 10: 389–399.
Strasser A, Elefanty AG, Harris AW, Cory S . Progenitor tumours from Em-bcl-2-myc transgenic mice have lymphomyeloid differentiation potential and reveal developmental differences in cell survival. EMBO J 1996; 15: 3823–3834.
Vaux DL, Cory S, Adams JM . Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988; 335: 440–442.
Strasser A, Harris AW, Vaux DL, Webb E, Bath ML, Adams JM et al. Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Curr Top Microbiol Immunol 1990; 166: 175–181.
O'Reilly LA, Print C, Hausmann G, Moriishi K, Cory S, Huang DCS et al. Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ 2001; 8: 486–494.
Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684–696.
Egle A, Harris AW, Bouillet P, Cory S . Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA 2004; 101: 6164–6169.
Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW . Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA 2004; 101: 9333–9338.
Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, Cordon-Cardo C et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature 2005; 436: 807–811.
Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 2008; 28: 5391–5402.
Mason KD, Vandenberg CJ, Scott CL, Wei AH, Cory S, Huang DC et al. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci USA 2008; 105: 17961–17966.
Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL . Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev 1999; 13: 2658–2669.
Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 2003; 302: 1036–1038.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003; 4: 321–328.
Happo L, Cragg MS, Phipson B, Haga JM, Jansen ES, Herold MJ et al. Maximal killing of lymphoma cells by DNA-damage inducing therapy requires not only the p53 targets Puma and Noxa but also Bim. Blood 2010; 116: 5256–5267.
Beroukhim R, Mermel C, Porter D, Wei G, Raychaudhuri S, Donovan J et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463: 899–905.
Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 2011; 471: 110–114.
Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 2010; 463: 103–107.
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–3428.
Grabow S, Waring P, Happo L, Cook M, Mason KD, Kelly PN et al. Pharmacological blockade of Bcl-2, Bcl-x(L) and Bcl-w by the BH3 mimetic ABT-737 has only minor impact on tumour development in p53-deficient mice. Cell Death Differ 2012; 19: 623–632.
Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 2008; 322: 1377–1380.
van Delft FW, Horsley S, Colman S, Anderson K, Bateman C, Kempski H et al. Clonal origins of relapse in ETV6-RUNX1 acute lymphoblastic leukemia. Blood 2011; 117: 6247–6254.
Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S et al. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128: 1173–1186.
Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA 2010; 107: 10967–10971.
Vikstrom I, Carotta S, Luethje K, Peperzak V, Jost PJ, Glaser S et al. Mcl-1 is essential for germinal center formation and B cell memory. Science 2010; 330: 1095–1099.
Strasser A, Harris AW, Cory S . Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 1991; 67: 889–899.
Acknowledgements
We thank Drs S Cory, D Huang and P Bouillet for providing mice, reagents and advice; M Cook, for preparing the ABT-737 for injections; K Vella, G Siciliano, D Cooper, N Iannarella, J Coughlin and Lisa Reid for animal care and help with ABT-737 treatment; J Corbin for automated blood analysis; B Helbert and C Young for genotyping; Dr. F Battye and his team for cell sorting, Dr. S Mihajlovic and his team for histological services and D Quilici, T Nikolaou and G Thomas for irradiation. This work was supported by grants and fellowships from the Cancer Council of Victoria (to PNK), the National Health and Medical Research Council (Program Grant No. 461221, NHMRC Australia Fellowship), the Leukemia and Lymphoma Society (SCOR Grant No. 7413) and operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS. We acknowledge collaboration with Genentech and Abbott Laboratories on the development of BH3 mimetic drugs.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Kelly, P., Grabow, S., Delbridge, A. et al. Prophylactic treatment with the BH3 mimetic ABT-737 impedes Myc-driven lymphomagenesis in mice. Cell Death Differ 20, 57–63 (2013). https://doi.org/10.1038/cdd.2012.92
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2012.92
Keywords
This article is cited by
-
Human antibody-based chemically induced dimerizers for cell therapeutic applications
Nature Chemical Biology (2018)
-
Hematologic malignancies: newer strategies to counter the BCL-2 protein
Journal of Cancer Research and Clinical Oncology (2016)
-
The BCL-2 protein family, BH3-mimetics and cancer therapy
Cell Death & Differentiation (2015)