Abstract
Purpose
The purpose of this study was to compare the reliability of the ‘gold standard’ Goldmann applanation tonometer (GAT), with that of the ocular response analyser (ORA), and the dynamic contour tonometer (DCT).
Patients and methods
A total of 694 subjects were recruited to participate from the TwinsUK (UK Adult Twin Registry) at St Thomas’ Hospital, London. Intraocular pressure (IOP) was measured using GAT, ORA, and the DCT. The agreement between the three methods was assessed using the Bland–Altman method. Repeatability coefficients and coefficient of variation between first and second readings of the same eye were used to assess reliability.
Results
Mean age was 57.5 years (SD, 13.1; range, 16.1–88.5). The mean IOPs, calculated using the mean of two readings from the right eye were as follows: Goldmann (GAT), 14.1±2.8 mm Hg; IOPg (ORA), 15.9±3.2 mm Hg; IOPcc (ORA), 16.6±3.2 mm Hg; and DCT, 16.9±2.7 mm Hg. The 95% limits of agreement were for ORA (IOPcc): GAT, −2.07 to 7.18 mm Hg; for DCT: GAT, −0.49 to 6.21 mm Hg; and for DCT: ORA (IOPcc), −3.01 to 4.85 mm Hg. Coefficients of variation for the three tonometers were GAT, 8.3%; ORA, 8.2%; DCT, 6.3%. The repeatability coefficients were 3.4 mm Hg for GAT, 3.57 mm Hg for ORA and 3.09 mm Hg for DCT. GAT and ORA (IOPg) readings showed a positive correlation with central corneal thickness (P<0.005).
Conclusions
This study found similar reliability in all three tonometers. Bland–Altman plots showed the three instruments to have 95% limits of agreement outside the generally accepted limits, which means they are not interchangeable. GAT measurements were found to be significantly lower than the two newer instruments.
Similar content being viewed by others
Introduction
Glaucoma is the second leading cause of blindness, affecting an estimated 70 million people worldwide.1 Although it no longer forms part of the definition of the disease, elevated intraocular pressure (IOP) is the principal modifiable risk factor for the development and progression of open-angle glaucoma.2, 3, 4
Although the Goldmann applanation tonometer (GAT) has been the ‘gold standard’ for measuring IOP in the clinical setting for several decades, recently there has been a growing interest in developing new technology to measure a more accurate IOP.5 It is well known that GAT is affected by the central corneal thickness (CCT).6, 7 The Ocular Hypertension Treatment Study showed that CCT is an important and independent risk factor for the progression of ocular hypertension to a first diagnosis of glaucoma.8 It has been estimated that a measurement error of between 0.119 and 0.71 mmHg7 occurs per 10 μm deviation from the average CCT of 550 μm.
We set out to compare the GAT with two relatively new instruments that are designed to produce IOP readings less dependent on CCT; the ocular response analyser (ORA; Reichert, Buffalo, NY—software version 1.10) and the Dynamic Contour Tonometer (DCT, Pascal; Swiss Microtechnology AG, Port, Switzerland—software version 2.2). The ORA is a non-contact air-puff tonometer that ejects an air impulse lasting 20 ms and monitors the time course changes of the cornea by an electro-optical collimation detector system. The difference between ‘inward’ and ‘outward’ applanation pressure depends on the viscoelastic properties of the corneoscleral shell, because opposition forces absorb energy from the air impulse (corneal hysteresis).10, 11 The ORA derives two IOPs: the Goldmann-correlated IOP (IOPg), which the manufacturers have calibrated with GAT, and the corneal-compensated IOP (IOPcc), which is designed to be independent of the CCT.
The DCT is a contact tonometer with a concave surface with radius of curvature of 10.5 mm, which creates a distribution of forces between the central contour of the tip and the cornea that equals the forces generated by the internal pressure of the eye.12 Its measure of IOP is supposedly independent of CCT.
To our knowledge, this is the largest sample to date comparing the ORA to the GAT and the second largest sample comparing the DCT and GAT, in the same group of unselected individuals.
Materials and methods
Data were available on 694 individuals who were recruited from the TwinsUK (UK Adult Twin Registry), based at St Thomas’ Hospital, London. The subjects were twin volunteers from the general population, and were part of a twin study on glaucoma heritability.13, 14 Subjects were recruited for studies other than eye studies, and subsequently asked to attend for an eye examination. All subjects provided informed consent, and the study was reviewed by the Local Research Ethics Committee. The methods adopted in this study adhered to the tenets of the Declaration of Helsinki Principles. All 694 subjects had two readings performed on both eyes with all three instruments, by a single investigator (FC).
A drop of proxymetacaine 0.5% with fluorescein was instilled in both eyes before testing. IOP measurement with the ORA was performed first, as it is a non-contact device—first and second readings were taken for the right eye and if the accuracy was poor a third reading was taken, and the same for the left eye. Poor accuracy was defined by abnormal symmetry between the signal peaks of the distribution graph. IOP readings above 50 or below 5 mm Hg were discarded and the measurement repeated. In the case of the DCT, two readings per eye were again taken; with this instrument, readings were alternated between eyes. For each individual reading displayed, the DCT uses the average of 5 or 6 IOP readings. Only those measures with reliability score recommended by the manufacturer (⩽3 on a scale 1–5) were accepted (mean reliability was 1.8±0.9). For the GAT, the gauge was set at 0 mm Hg and dialled up to the subject's IOP; this was repeated on the contralateral eye. The dial was next set at 40 mm Hg and moved down to the individual's IOP to provide a second reading for each eye. A sample of individuals was assessed in random order to see if there was any applanation effect induced by using this order of IOP measurement; however, none was found. All measurements were taken in immediate succession to eliminate bias due to diurnal fluctuation.
Data handling and preliminary analyses were undertaken using STATA (Intercooled Stata for Windows 95, version 5.0; StataCorp, College Station, TX; 1997). Pearson correlation coefficients between right and left eyes were calculated and as right and left eyes showed significant correlation, right eye measures were arbitrarily used for further analyses, including coefficient of variation between first and second readings of the right eye, and repeatability coefficients between first and second readings of the right eye.15 The coefficient of variation between first and second readings of one eye is the standard deviation of the difference between the two readings, divided by the total mean of both readings. The lower the coefficient of variation, the less variation between readings and therefore the more reliable the instrument. The repeatability coefficient can be calculated as 1.96 times the standard deviations of the differences between the two measurements. The agreement between each instrument and GAT and between the two newer instruments was assessed using the Bland–Altman method.16 For the ORA, only IOPcc was used for the coefficient of variation, repeatability coefficient, and Bland–Altman plots, as this is the measure that is supposed to be independent of CCT. The 95% limits of agreement, estimated by mean difference ±1.96 standard deviations of the differences, provide an interval within which 95% of differences between measurements by the two methods are expected to lie. Proportional bias was determined from the gradient of the Bland–Altman plot of the mean difference between the two instruments against the mean of the two instruments. The correlation between each instrument's IOP readings and CCT was also compared using Pearson's correlation coefficient. For the mean IOPs of all four measurements and for the correlations between IOP and other parameters and for the Bland–Altman plots, the mean of four readings, two from each eye, was used.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
The study sample consisted of 1388 eyes, of 694 individual twin subjects. Mean age was 57.5 years (SD 13.1, range 16.1–88.5). Of these, 95% were women and all were Caucasian. A previous diagnosis of glaucoma had been made in seven subjects (1%), but exclusion of these individuals did not alter the mean values or the correlations and so they remained in further analyses. In total 13 eyes required a third measurement for the GAT, 9 for the ORA, and 24 for the DCT. Results were also unchanged if the familial relationships were taken into account, randomly selecting only one of each pair of twins. However, as this study is comparing measurement variation within individuals, all twin subjects are included in the results reported.
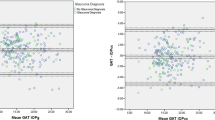
The mean IOPs were calculated using the mean of four readings, two from the right eye and two from the left. The mean IOPs for all measures were as follows: Goldmann (GAT), 14.1±2.8 mm Hg; IOPg (ORA), 15.9±3.2 mm Hg; IOPcc (ORA), 16.6±3.2 mm Hg; and for DCT, 16.9±2.7 mm Hg. The mean CCT was 542±34 μm. The range of IOPs and mean differences between the instruments are shown in Table 1; both the newer instruments gave significantly higher IOP readings than GAT. Figure 1 shows the IOP frequency distribution for GAT.
Right/left eye correlations were the following: GAT, 0.83 (P<0.001); IOPg, 0.79 (P<0.001); IOPcc, 0.67 (P<0.0001); DCT, 0.82 (P<0.0001). The coefficients of variation for the three tonometers were the following: GAT, 8.3%; ORA, 8.2%; DCT, 6.3%. The mean of absolute differences between the first and second readings of the right eye were as follows: for GAT, 1.5 mm Hg (SD: 1.2); ORA (IOPg), 1.4 mm Hg (SD: 1.2); ORA (IOPcc), 1.7 mm Hg (SD: 1.5); and DCT: 1.2 mm Hg (SD: 1.2). The repeatability coefficients were calculated between readings one and two of the right eye. These were found to be, for GAT, 3.4 mm Hg; ORA, 3.57 mm Hg; and DCT, 3.09 mm Hg. Bland–Altman plots were computed between ORA (IOPcc) and GAT; DCT and GAT; and DCT and ORA (IOPcc); the results are shown in Figures 2,3,4. The limits of agreement for ORA (IOPcc)–GAT were −2.1 to 7.2 mm Hg; for DCT–GAT were −0.5 to 6.2 mm Hg; and for DCT–ORA (IOPcc) were −3.0 to 4.9 mm Hg. We found a proportional bias between GAT readings and ORA (IOPcc). As the IOP reading increases, the difference between ORA (IOPcc) and GAT also increased, so that with higher IOPs, the GAT and ORA (IOPcc) readings were more dissimilar (r=0.19 P<0.001). In the case of the DCT, there did not appear to be any proportional bias (r=0.04, P=0.26).
Comparing correlations between IOPs generated and CCT, as expected the GAT (r=0.13, P<0.001) and the ORA IOPg (r=0.32, P<0.0001) readings were significantly correlated with CCT. Neither the IOPcc (ORA) (r=0.02, P=0.67) nor DCT (r=0.06, P=0.14) readings were significantly correlated with CCT. All four instruments gave IOP readings that showed a highly significant (all with P<0.0001) positive correlation with age (GAT, 0.2; IOPg, 0.14; IOPcc, 0.29; DCT, 0.21). All four IOP readings also showed strongly significant positive correlations with both systolic [GAT, 0.14 (P<0.0001); IOPg, 0.12 (P<0.01); IOPcc, 0.21 (P<0.0001); and DCT, 0.13 (P<0.002)] and diastolic blood pressures (GAT, 0.14 (P<0.0001); IOPg, 0.11 (P<0.01); IOPcc, 0.16 (P<0.0001); and DCT, 0.17 (P<0.001)). When CCT, systolic blood pressure, and diastolic blood pressure were included in the regression analysis, age was no longer found to be significantly correlated with GAT (P=0.19) or IOPg (P=0.06); however, it was still significantly correlated with IOPcc (P<0.001) and DCT (P=0.01). CCT was also found to have a significant negative correlation with age of −0.12 (P<0.005).
Discussion
Comparison of the reliability of these two newer tonometers with the ‘gold standard’ GAT has shown that the DCT had a marginally greater reliability, with a coefficient of variation (6.3%) less than that of the GAT and ORA (8.3 and 8.2%, respectively). We ascribe this lower coefficient of variation in the DCT to the fact that each individual IOP reading is an average taken over approximately 5 s. In the case of the GAT and ORA, each IOP point estimate is taken at a single, static point in time, although in reality the GAT IOP is taken as the average within the ocular pulse cycle.
It is usually considered that a tonometer with limits of agreement within ±3 mm Hg compared to GAT is clinically acceptable and therefore interchangeable; the two newer tonometers had limits of agreement outside this range, and there was proportional bias, suggesting they are not interchangeable with GAT.17 In a study by Pepose et al,18 comparing the same three instruments, the DCT was the only tonometer that did not show a significant change in IOP measurement pre- and post-LASIK. The wide limits of agreement found in this study are similar to the literature for both ORA17, 19 and DCT.20, 21
There are conflicting findings in the literature regarding the difference in IOP measurement between GAT and ORA; our ORA IOPg measures were on average 1.8 mm Hg higher than the GAT pressures, similar to another study that found IOPcc to overestimate GAT by 1.7 mm Hg.22 In contrast, a study by Medeiros and Weinreb23 found very similar mean IOP between GAT and IOPcc, with the difference being almost zero, similar to a Chinese cohort study.17 Yet another study looking at 48 eyes of 48 patients found mean IOPg to be 7.2 mm Hg higher than GAT and IOPcc 8.3 mm Hg higher.19 This study found a proportional bias between GAT and ORA; that is, as the IOP increased, the difference between ORA and GAT increased proportionately, with the ORA measures being even higher. There is conflicting evidence in the literature regarding proportional bias between these two instruments; two studies have reported a proportional bias,17, 19 with the difference between ORA and GAT increasing with higher IOP. Another study has found no proportional bias.23
The mean IOP of 14.1 mm Hg was comparable to large population-based studies (the Los Angeles Latino Eye Study21 of 14.4 mm Hg and similarly aged females (55–59 years) from the Beaver Dam Eye Study24 of 15.1±3 mm Hg). We feel, therefore, that significant ascertainment or other biases in this twin cohort is unlikely. The mean DCT IOP was significantly higher than GAT. The manufacturer recommends discarding the first reading with the DCT, because it is usually higher than the subsequent readings. We did not do this because we did not find a significant difference in the mean IOP between first and subsequent readings. Most studies have found DCT readings to be higher than GAT.25, 26 In contrast, a recent study on African-American subjects did not find a significant difference in mean IOPs between GAT and DCT; with mean DCT IOP only 0.4 mm Hg higher than GAT.27
This study found no proportional bias between the GAT and DCT readings. There are varied results in the literature for proportional bias; some studies have, like us, found no bias between DCT and GAT measurement,25, 28 others have found increasing differences between DCT and GAT as IOP increased,29, 30 or decreasing differences in another.31 DCT values for IOP are similar to intracameral manometric IOP in vivo32 and are more similar than GAT (which are significantly lower) in cadaveric eyes.33, 34 This may mean that the higher IOP readings obtained with DCT are in fact the ‘true’ IOP.
As expected in our study, the GAT measurements are positively correlated with CCT. The IOPg reading of the ORA was also positively correlated with CCT. As proposed by the manufacturers, we have found that IOPcc is an IOP reading that is independent of CCT.22, 23 There is conflicting evidence on the relation of ORA readings with CCT; two studies have shown that it is able to provide IOP readings that are independent of CCT. Kotecha et al22 found that IOPcc showed a positive, albeit not significant, trend with CCT. They proceeded to refine the calculation for IOPcc, calling this new value IOPccf (corneal constant factor) and this was found to have no association with CCT.22 Another study, performed on patients undergoing glaucoma treatment showed that ORA readings were not independent of CCT,19 although GAT was not correlated with CCT in this study, in contrast to most published studies. In the case of the DCT, the literature has consistently showed that IOP readings are less dependent on CCT than GAT,21, 25 and our study has re-confirmed the finding.
Our findings that IOP readings from all the instruments are positively correlated with systolic and diastolic blood pressures are similar to other studies.35, 36 Most, but not all, studies find IOP rises with age as we did.2, 37, 38, 39 Both age and blood pressure are positively correlated with IOP, but are also correlated with each other (systolic blood pressure rises with increasing age); when both are included in regression analyses, the effect of age on IOP is diminished. Interestingly, when systolic and diastolic blood pressures were included in the regression analysis, it was found that age was no longer significantly correlated with GAT and IOPg, but still significantly positively correlated with IOPcc and DCT readings. Although the reasons are unclear as to the mechanism of this difference, we have observed that corneal hysteresis, a measure of corneal viscoelasticity, is significantly related to age, and both DCT and IOPcc are significantly correlated with hysteresis, whereas IOPg and GAT are not.13 Further research is needed to examine these differences.
In conclusion, we have found that the ORA and DCT generate IOP measurements that are significantly higher than those of GAT, but which are independent of CCT. All instruments had a significant but similar inter-measurement variation, although the coefficient of variation of DCT was lowest. It is worth pointing out that the ‘gold standard’ GAT has similar variation to the other machines, and as repeat measures are standard for non-contact tonometry, so clinicians should consider taking more than one reading for GAT to reduce measurement error. This study has added to the growing body of work on the ORA and DCT, which produce less corneal-dependent IOPs than GAT. We feel that, although not interchangeable with GAT, both these newer instruments have a place in the clinical setting, where it is clear that GAT readings are influenced by corneal factors.

References
Quigley HA . Number of people with glaucoma worldwide. Br J Ophthalmol 1996; 80: 389–393.
Rochtchina E, Mitchell P, Wang JJ . Relationship between age and intraocular pressure: the Blue Mountains Eye Study. Clin Experiment Ophthalmol 2002; 30: 173–175.
Chang TC, Congdon NG, Wojciechowski R, Muñoz B, Gilbert D, Chen P et al. Determinants and heritability of intraocular pressure and cup-to-disc ratio in a defined older population. Ophthalmology 2005; 112: 1186–1191.
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ . The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002; 86: 238–242.
Elmallah MK, Asrani SG . New ways to measure intraocular pressure. Curr Opin Ophthalmol 2008; 19: 122–126.
Brubaker RF . Tonometry and corneal thickness. Arch Ophthalmol 1999; 117: 104–105.
Ehlers N, Bramsen T, Sperling S . Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 1975; 53: 34–43.
Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 714–720.
Herndon LW . Measuring intraocular pressure-adjustments for corneal thickness and new technologies. Curr Opin Ophthalmol 2006; 17: 115–119.
Luce DA . Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg 2005; 31: 156–162.
Shah S, Laiquzzaman M, Cunliffe I, Mantry S . The use of the Reichert ocular response analyser to establish the relationship between ocular hysteresis, corneal resistance factor and central corneal thickness in normal eyes. Cont Lens Anterior Eye 2006; 29: 257–262.
Kanngiesser HE, Kniestedt C, Robert YC . Dynamic contour tonometry: presentation of a new tonometer. J Glaucoma 2005; 14: 344–350.
Carbonaro F, Andrew T, Mackey DA, Spector TD, Hammond CJ . The heritability of corneal hysteresis and ocular pulse amplitude: a twin study. Ophthalmology 2008; 115: 1545–1549.
Healey P, Carbonaro F, Taylor B, Spector TD, Mitchell P, Hammond CJ . The heritability of optic disc parameters: a classic twin study. Invest Ophthalmol Vis Sci 2008; 49: 77–80.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310.
Bland JM, Altman DG . Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135–160.
Lam A, Chen D, Chiu R, Chui WS . Comparison of IOP Measurements Between ORA and GAT in Normal Chinese. Optom Vis Sci 2007; 84: 909–914.
Pepose JS, Feigenbaum SK, Qazi MA, Sanderson JP, Roberts CJ . Changes in corneal biomechanics and intraocular pressure following LASIK using static, dynamic, and noncontact tonometry. Am J Ophthalmol 2007; 143: 39–47.
Martinez-de-la-Casa JM, Garcia-Feijoo J, Fernandez-Vidal A, Mendez-Hernandez C, Garcia-Sanchez J . Ocular response analyzer versus Goldmann applanation tonometry for intraocular pressure measurements. Invest Ophthalmol Vis Sci 2006; 47: 4410–4414.
Erdurmus M, Totan Y, Hepsen IF, Yagci R . Comparison of dynamic contour tonometry and noncontact tonometry in ocular hypertension and glaucoma. Eye 2008; 23 (3): 663–668.
Francis BA, Hsieh A, Lai MY, Chopra V, Pena F, Azen S et al. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology 2007; 114: 20–26.
Kotecha A, Elsheikh A, Roberts CR, Zhu H, Garway-Heath DF . Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Invest Ophthalmol Vis Sci 2006; 47: 5337–5347.
Medeiros FA, Weinreb RN . Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma 2006; 15: 364–370.
Klein BE, Klein R, Linton KL . Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 1992; 33: 2224–2228.
Martinez-de-la-Casa JM, Garcia-Feijoo J, Vico E, Fernandez-Vidal A, Benitez del Castillo JM, Wasfi M et al. Effect of corneal thickness on dynamic contour, rebound, and Goldmann tonometry. Ophthalmology 2006; 113: 2156–2162.
Pache M, Wilmsmeyer S, Lautebach S, Funk J . Dynamic contour tonometry versus Goldmann applanation tonometry: a comparative study. Graefes Arch Clin Exp Ophthalmol 2005; 243: 763–767.
Medeiros FA, Sample PA, Weinreb RN . Comparison of dynamic contour tonometry and goldmann applanation tonometry in African American subjects. Ophthalmology 2007; 114: 658–665.
Punjabi OS, Ho HK, Kniestedt C, Bostrom AG, Stamper RL, Lin SC . Intraocular pressure and ocular pulse amplitude comparisons in different types of glaucoma using dynamic contour tonometry. Curr Eye Res 2006; 31: 851–862.
Barleon L, Hoffmann EM, Berres M, Pfeiffer N, Grus FH . Comparison of dynamic contour tonometry and goldmann applanation tonometry in glaucoma patients and healthy subjects. Am J Ophthalmol 2006; 142: 583–590.
Duggal P, Klein AP, Lee KE, Iyengar SK, Klein R, Bailey-Wilson JE et al. A genetic contribution to intraocular pressure: the beaver dam eye study. Invest Ophthalmol Vis Sci 2005; 46: 555–560.
Schneider E, Grehn F . Intraocular pressure measurement-comparison of dynamic contour tonometry and Goldmann applanation tonometry. J Glaucoma 2006; 15: 2–6.
Boehm AG, Weber A, Pillunat LE, Koch R, Spoerl E . Dynamic contour tonometry in comparison to intracameral IOP measurements. Invest Ophthalmol Vis Sci 2008; 49 (6): 2472–2477.
Kniestedt C, Nee M, Stamper RL . Dynamic contour tonometry: a comparative study on human cadaver eyes. Arch Ophthalmol 2004; 122: 1287–1293.
Kniestedt C, Nee M, Stamper RL . Accuracy of dynamic contour tonometry compared with applanation tonometry in human cadaver eyes of different hydration states. Graefes Arch Clin Exp Ophthalmol 2005; 243: 359–366.
Healey PR, Mitchell P, Smith W, Wang JJ . The influence of age and intraocular pressure on the optic cup in a normal population. J Glaucoma 1997; 6: 274–278.
Klein BE, Klein R, Knudtson MD . Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. Br J Ophthalmol 2005; 89: 284–287.
Bonomi L, Marchini G, Marraffa M, Bernardi P, De Franco I, Perfetti S et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology 1998; 105: 209–215.
Wu SY, Leske MC . Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol 1997; 115: 1572–1576.
Shiose Y . Intraocular pressure: new perspectives. Surv Ophthalmol 1990; 34: 413–435.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Carbonaro, F., Andrew, T., Mackey, D. et al. Comparison of three methods of intraocular pressure measurement and their relation to central corneal thickness. Eye 24, 1165–1170 (2010). https://doi.org/10.1038/eye.2010.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.11
Keywords
This article is cited by
-
The alterations of corneal biomechanics in adult patients with corneal dystrophy
Eye (2023)
-
Comparison of Goldmann applanation and Ocular Response Analyser tonometry: intraocular pressure agreement and patient preference
Eye (2020)
-
Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma
Nature Genetics (2014)
-
Age variations in intraocular pressure in a cohort of healthy Austrian school children
Eye (2012)
-
The influence of soft contact lenses on the intraocular pressure measurement
Eye (2012)