Abstract
Objective:
To assess the efficacy of the heme oxygenase inhibitor, tin mesoporphyrin (SnMP), to reduce total bilirubin (TB) levels.
Study Design:
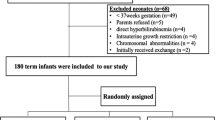
Masked, SnMP (4.5 mg kg−1), placebo-controlled, multicenter trial of single intramuscular injection to newborns ⩾35 weeks gestational age whose predischarge screening transcutaneous bilirubin (TcB) was >75th percentile.
Results:
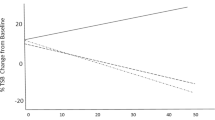
Two hundred and thirteen newborns (median age 30 h) were randomized to treatment with SnMP (n=87) or ‘sham’ (n=89). We found that the duration of phototherapy was halved. Within 12 h of SnMP administration, the natural TB trajectory was reversed. At age 3 to 5 days, TB in the SnMP-treated group was +8% but sixfold lower than the 47% increase in the sham-treated group (P<0.001). At age 7 to 10 days, mean TB declined 18% (P<0.001) compared with a 7.1% increase among controls. No short-term adverse events from SnMP treatment were noted other than photoreactivity due to inadvertent exposure to white light phototherapy.
Conclusion:
Early, predischarge SnMP administration decreased the duration of phototherapy, reversed TB trajectory and reduced the severity of subsequent hyperbilirubinemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Keren R, Tremont K, Luan X, Cnaan A . Visual assessment of jaundice in term and late preterm infants. Arch Dis Child Fetal Neonatal Ed 2009; 94: F317–F322.
Bhutani VK, Johnson LH, Maisels MJ, Newman TB, Phibbs C, Stark AR et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J Perinatol 2004; 24: 650–662.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004; 114: 297–316.
Dennery PA, Seidman DS, Stevenson DK . Neonatal hyperbilirubinemia. N Engl J Med 2001; 344: 581–590.
Johnson LH, Bhutani VK, Brown AK . System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr 2002; 140: 396–403.
Alexander D . A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics 2004; 113: 135.
American Academy of Pediatrics, Provisional Committee for Quality Improvement and Subcommittee on Hyperbilirubinemia. Practice parameter: management of hyperbilirubinemia in the healthy term newborn. Pediatrics 1994; 94: 558–565.
Maisels MJ, Kring E . The contribution of hemolysis to early jaundice in normal newborns. Pediatrics 2006; 118: 276–279.
Tidmarsh GF, Wong RJ, Stevenson DK . End-tidal carbon monoxide and hemolysis. J Perinatol 2014; 34: 577–581.
Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF . Hyperbilirubinemia in the newborn infant>or =35 weeks' gestation: an update with clarifications. Pediatrics 2009; 124: 1193–1198.
Bhutani VK, Zipursky A, Blencowe H, Khanna R, Sgro M, Ebbesen F et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res 2013; 74 (Suppl 1):86–100.
Stevenson DK, Rodgers PA, Vreman HJ . The use of metalloporphyrins for the chemoprevention of neonatal jaundice. Am J Dis Child 1989; 143: 353–356.
Galbraith RA, Drummond GS, Kappas A . Suppression of bilirubin production in the Crigler-Najjar type I syndrome: studies with the heme oxygenase inhibitor tin-mesoporphyrin. Pediatrics 1992; 89: 175–182.
Kappas A, Drummond GS, Henschke C, Valaes T . Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics 1995; 95: 468–474.
Valaes T, Drummond GS, Kappas A . Control of hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns using an inhibitor of bilirubin production, Sn-mesoporphyrin. Pediatrics 1998; 101: E1.
Martinez JC, Garcia HO, Otheguy LE, Drummond GS, Kappas A . Control of severe hyperbilirubinemia in full-term newborns with the inhibitor of bilirubin production Sn-mesoporphyrin. Pediatrics 1999; 103: 1–5.
Kappas A, Drummond GS, Valaes T . A single dose of Sn-mesoporphyrin prevents development of severe hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns. Pediatrics 2001; 108: 25–30.
Kappas A . A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics 2004; 113: 119–123.
U.S. Food and Drug Administration: Pediatric Advisory Subcommittee. 11–12 June 2003. Retrieved 10 July 2015, from http://www.fda.gov/ohrms/dockets/ac/03/briefing/3965B1_02_Cover%20memo.htm.
Bhutani VK, Johnson L, Sivieri EM . Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999; 103: 6–14.
Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH . Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000; 106: e17–e17.
Rubaltelli FF, Gourley GR, Loskamp N, Modi N, Roth-Kleiner M, Sender A et al. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics 2001; 107: 1264–1271.
Lo S, Doumas BT, Ashwood E . Performance of bilirubin determinations in US laboratories revisited. Clin Chem 2004; 50: 190–194.
Bhutani VK, Wong RJ, Vreman HJ, Stevenson DK . Bilirubin production and hour-specific bilirubin levels. J Perinatol. 2015; 35 (9): 735–738.
Kramer LI . Advancement of dermal icterus in the jaundiced newborn. Am J Dis Child 1969; 118: 454–458.
Stevenson DK, Wong RJ . Metalloporphyrins in the management of neonatal hyperbilirubinemia. Semin Fetal Neonatal Med 2010; 15: 164–168.
Kappas A. Development of heme oxygenase inhibitors for the prevention of severe jaundice in infants. In: Heme Oxygenase in Biology and Medicine. Springer: US, New York, 2002, pp 3–17.
Drummond GS, Kappas A . Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci 1981; 78: 6466–6470.
Delaney JK, Mauzerall D, Drummond GS, Kappas A . Photophysical properties of Sn-porphyrins: potential clinical implications. Pediatrics 1988; 81: 498–504.
Fort FL, Gold J . Phototoxicity of tin protoporphyrin, tin mesoporphyrin, and tin diiododeuteroporphyrin under neonatal phototherapy conditions. Pediatrics 1989; 84: 1031–1037.
Dennery PA, Vreman HJ, Rodgers PA, Stevenson DK . Role of lipid peroxidation in metalloporphyrin-mediated phototoxic reactions in neonatal rats. Pediatr Res 1993; 33: 87–91.
Keino H, Nagae H, Mimura S, Watanabe K, Kashiwamata S . Dangerous effects of tin-protoporphyrin plus photoirradiation on neonatal rats. Eur J Pediatr 1990; 149: 278–279.
Hintz S, Vreman H, Stevenson D . Mortality of metalloporphyrin-treated neonatal rats after light exposure. Dev Pharmacol Ther 1989; 14: 187–192.
Vreman H, Gillman M, Downum K, Stevenson D . In vitro generation of carbon monoxide from organic molecules and synthetic metalloporphyrins mediated by light. Dev Pharmacol Ther 1989; 15: 112–124.
Wong RJ, Bhutani VK, Vreman HJ, Stevenson DK . Pharmacology review tin mesoporphyrin for the prevention of severe neonatal hyperbilirubinemia. Neoreviews 2007; 8: e77–e84.
Morioka I, Wong RJ, Abate A, Vreman HJ, Contag CH, Stevenson DK . Systemic effects of orally-administered zinc and tin (IV) metalloporphyrins on heme oxygenase expression in mice. Pediatr Res 2006; 59: 667–672.
Fujioka K, Kalish F, Wong RJ, Stevenson DK . Inhibition of heme oxygenase activity using a microparticle formulation of zinc protoporphyrin in an acute hemolytic newborn mouse model. Pediatr Res 2016 (in press; doi:10.1038/pr.2015.207).
Moxon SG, Lawn JE, Dickson KE, Simen-Kapeu A, Gupta G, Deorari A et al. Inpatient care of small and sick newborns: a multi-country analysis of health system bottlenecks and potential solutions. BMC Pregnancy Childbirth 2015; 15 (Suppl 2):S7.
Acknowledgements
We thank Dr Lois Johnson for continued support and guidance for this research project. Dr Bhutani and Dr Johnson served as the Site PI and co-site PI, respectively, for the study at Pennsylvania Hospital, Philadelphia, PA, USA. In addition, we are thankful to Dr David K Stevenson and Ronald J Wong for their reviews of the manuscript, helpful critiques and for continued contributions to this area of research. We appreciate the assistance of Rosemary Dworanzyck and Emidio M Sivieri to attain the quality control of data collection. We thank the research assistants, nurses and nurse practitioners for helping with jaundice assessments and patient education during 2002 to 2003: Newborn Nurseries at Well Baby Nursery, Pennsylvania Hospital, Philadelphia; Mother-Baby Unit Newborn Nursery, University of New Mexico Hospital, New Mexico; Doernbecher Newborn Nursery of Virginia Commonwealth University Health System, Virginia; Rainbow Childrens’ Hospital, Robert Wood Johnson Hospital and William Beaumont Hospital. We also thank Martin E Castillo Cuadrado for his administrative support at the Stanford University. We appreciate the contributions of Dr A Kappas, Dr JF Lucey as well as Dr R Yukovich and Dr B Levinson (of WellSpring Pharmaceutical Corporation) for their individual guidance and support during the initial study design. The study was partially funded by WellSpring Pharmaceutical Corporation (Neptune, NJ) in 2002 to 2003 who provided the drug, technical supplies, technician and research assistant support and study coordination with the FDA. The drug, SnMP, was provided as a clinical formulary, Stanate, through an investigational drug exemption to the clinical study. WellSpring Pharmaceutical Corporation no longer has publication authority on this manuscript.
Author contributions
Vinod K Bhutani, MD, FAAP, wrote the first draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bhutani, V., Poland, R., Meloy, L. et al. Clinical trial of tin mesoporphyrin to prevent neonatal hyperbilirubinemia. J Perinatol 36, 533–539 (2016). https://doi.org/10.1038/jp.2016.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.22
This article is cited by
-
Chemoprevention of bilirubin encephalopathy with a nanoceutical agent
Pediatric Research (2023)
-
Stannsoporfin with phototherapy to treat hyperbilirubinemia in newborn hemolytic disease
Journal of Perinatology (2022)
-
Exchange transfusion for hemolytic hyperbilirubinemia: could some be averted by emergent administration of an inhibitor of bilirubin production?
Journal of Perinatology (2021)
-
Experimental models assessing bilirubin neurotoxicity
Pediatric Research (2020)
-
Care of Infants Born to Women with Diabetes
Current Diabetes Reports (2020)