Abstract
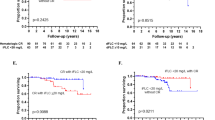
To improve the efficacy of risk-adapted melphalan (MEL) in patients with amyloidosis (AL), we conducted a phase II trial using bortezomib and dexamethasone (BD) as consolidation. Forty untreated patients with renal (70%), cardiac (65%), liver/gastrointestinal (15%) or nervous system (13%) AL were assigned MEL 100, 140 or 200 mg/m2 based on age, renal function and cardiac involvement. Hematological response was assessed at 3 months post stem cell transplant (SCT); patients with less than complete hematological response (CR) received BD consolidation. Four patients with advanced cardiac AL died within 100 days of SCT (10% treatment-related mortality). Survival at 12 and 24 months post treatment start was 88 and 82% overall and was 81 and 72% in patients with cardiac AL. At 3 months post SCT, 45% had ⩾ partial response (PR) including 27% CR. Twenty-three patients received consolidation and in 86% response improved; all patients responded in one cycle. At 12 and 24 months, 79 and 60% had ⩾ PR, 58 and 40% CR. Organ responses occurred in 55 and 70% at 12 and 24 months. Eight patients relapsed/progressed. One patient with serologic progression had organ impairment at time of progression. In newly diagnosed AL, BD following SCT rapidly and effectively improves responses resulting in high CR rates and maintained organ improvement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cohen AD, Comenzo RL . Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program 2010; 2010: 287–294.
Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med 2004; 140: 85–93.
Merlini G, Bellotti V . Molecular mechanisms of amyloidosis. N Engl J Med 2003; 349: 583–596.
Sanchorawala V, Seldin DC, Magnani B, Skinner M, Wright DG . Serum free light-chain responses after high-dose intravenous melphalan and autologous stem cell transplantation for AL (primary) amyloidosis. Bone Marrow Transplant 2005; 36: 597–600.
Comenzo RL, Gertz MA . Autologous stem cell transplantation for primary systemic amyloidosis. Blood 2002; 99: 4276–4282.
Gertz MA, Lacy MQ, Dispenzieri A, Ansell SM, Elliott MA, Gastineau DA et al. Risk-adjusted manipulation of melphalan dose before stem cell transplantation in patients with amyloidosis is associated with a lower response rate. Bone Marrow Transplant 2004; 34: 1025–1031.
Cohen AD, Zhou P, Chou J, Teruya-Feldstein J, Reich L, Hassoun H et al. Risk-adapted autologous stem cell transplantation with adjuvant dexamethasone +/− thalidomide for systemic light-chain amyloidosis: results of a phase II trial. Br J Haematol 2007; 139: 224–233.
Comenzo RL . How I treat amyloidosis. Blood 2009; 114: 3147–3157.
Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Blade J et al. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood 2011; 118: 865–873.
Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th international symposium on amyloid and amyloidosis, tours, france, 18-22 April 2004. Am J Hematol 2005; 79: 319–328.
Comenzo RL, Zhou P, Fleisher M, Clark B, Teruya-Feldstein J . Seeking confidence in the diagnosis of systemic AL (Ig light-chain) amyloidosis: patients can have both monoclonal gammopathies and hereditary amyloid proteins. Blood 2006; 107: 3489–3491.
Gertz M . MG. Definition of organ involvement and response to treatment in AL amyloidosis: An updated consensus opinion. Amyloid 2010; 17 (supplement 1): 48–49.
Palladini G, Dispenzieri A, Gertz MAA, Wechalekar A, Hawkins PN, Schonland SO et al. Validation of the criteria of response to treatment in AL amyloidosis. ASH Annual Meeting Abstracts 2010; 116: 1364.
Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain (AL) amyloidosis. Leukemia, (e-pub ahead of print 15 May 2012; doi:10.1038/leu.2012.100).
Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 2004; 22: 3751–3757.
Dispenzieri A, Merlini G, Comenzo RL . Amyloidosis: 2008 BMT Tandem Meetings (February 13–17, San Diego). Biol Blood Marrow Transplant 2008; 14 (1 Suppl 1): 6–11.
Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood 2003; 101: 2377–2380.
Lonial S, Kaufman J, Tighiouart M, Nooka A, Langston AA, Heffner LT et al. A phase I/II trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res 2010; 16: 5079–5086.
Sanchorawala V, Quillen K, Sloan JM, Andrea NT, Seldin DC . Bortezomib and high-dose melphalan conditioning for stem cell transplantation for AL amyloidosis: a pilot study. Haematologica 2011; 96: 1890–1892.
Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med 2007; 357: 1083–1093.
Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB et al. Cyclophosphamide-bortezomib-dexamethasone (CYBORD) produces rapid and complete hematological response in patients with AL amyloidosis. Blood 2012; 119: 4391–4394.
Venner CP, Lane T, Foard D, Rannigan L, Gibbs SD, Pinney JH et al. Cyclophosphamide, bortezomib and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression free survival. Blood 2012; 119: 4387–4390.
Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Buadi FK, Dingli D et al. Trends in day 100 and 2-year survival after auto-SCT for AL amyloidosis: outcomes before and after 2006. Bone Marrow Transplant 2011; 46: 970–975.
Wechalekar A, Schonland SO, Kastritis E, Hawkins PN, Dimopoulos MA, Russo P et al. European collaborative study of treatment outcomes in 347 patients with systemic AL amyloidosis with mayo stage III disease. ASH Annual Meeting Abstracts 2011; 118: 995.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012; 30: 989–995.
Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood 2010; 116: 4745–4753.
Richardson PG, Xie W, Mitsiades C, Chanan-Khan AA, Lonial S, Hassoun H et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol 2009; 27: 3518–3525.
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 2011; 12: 431–440.
O'Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res 2009; 15: 7085–7091.
Richardson PG, Baz R, Wang L, Jakubowiak AJ, Berg D, Liu G et al. Investigational agent MLN9708, An Oral proteasome inhibitor, in Patients (Pts) with relapsed and/or refractory multiple myeloma (MM): Results from the expansion cohorts of a phase 1 dose-escalation study. ASH Annual Meeting Abstracts 2011; 118: 301.
Solomon A, Weiss DT, Wall JS . Immunotherapy in systemic primary (AL) amyloidosis using amyloid-reactive monoclonal antibodies. Cancer Biother Radiopharm 2003; 18: 853–860.
Hrncic R, Wall J, Wolfenbarger DA, Murphy CL, Schell M, Weiss DT et al. Antibody-mediated resolution of light chain-associated amyloid deposits. Am J Pathol 2000; 157: 1239–1246.
Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 2010; 468: 93–97.
Acknowledgements
This study was supported by research funding from Millenium Pharmaceuticals (HL) and the Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation (RC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
HL and RLC received research support and served on the advisory board for Millenium Pharmaceuticals. HH and SG served on the advisory board for Millenium Pharmaceuticals.
Additional information
Author contributions
HL and RLC designed and performed research, collected data, analyzed and interpreted data, and wrote the paper; HH performed research, analyzed and interpreted data; MAR, MM, JL and CF performed research; CB and EH collected data; ER performed statistical analysis; SG edited the paper with critical review.
Rights and permissions
About this article
Cite this article
Landau, H., Hassoun, H., Rosenzweig, M. et al. Bortezomib and dexamethasone consolidation following risk-adapted melphalan and stem cell transplantation for patients with newly diagnosed light-chain amyloidosis. Leukemia 27, 823–828 (2013). https://doi.org/10.1038/leu.2012.274
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.274
Keywords
This article is cited by
-
Cutaneous manifestations of monoclonal gammopathy
Blood Cancer Journal (2022)
-
Depth of response prior to autologous stem cell transplantation predicts survival in light chain amyloidosis
Bone Marrow Transplantation (2021)
-
Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2021
Blood Cancer Journal (2021)
-
Updates in the Diagnosis and Management of AL Amyloidosis
Current Hematologic Malignancy Reports (2020)
-
High-dose melphalan and stem cell transplantation in systemic AL amyloidosis in the era of novel anti-plasma cell therapy: a comprehensive review
Bone Marrow Transplantation (2019)