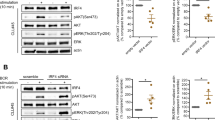
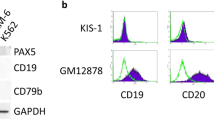
Abstract
The Ets family transcription factor PU.1 and the interferon regulatory factor (IRF)4 and IRF8 regulate gene expression by binding to composite DNA sequences known as Ets/interferon consensus elements. Although all three factors are expressed from the onset of B-cell development, single deficiency of these factors in B-cell progenitors only mildly impacts on bone marrow B lymphopoiesis. Here we tested whether PU.1 cooperates with IRF factors in regulating early B-cell development. Lack of PU.1 and IRF4 resulted in a partial block in development the pre-B-cell stage. The combined deletion of PU.1 and IRF8 reduced recirculating B-cell numbers. Strikingly, all PU.1/IRF4 and ~50% of PU.1/IRF8 double deficient mice developed pre-B-cell acute lymphoblastic leukemia (B-ALL) associated with reduced expression of the established B-lineage tumor suppressor genes, Ikaros and Spi-B. These genes are directly regulated by PU.1/IRF4/IRF8, and restoration of Ikaros or Spi-B expression inhibited leukemic cell growth. In summary, we demonstrate that PU.1, IRF4 and IRF8 cooperate to regulate early B-cell development and to prevent pre-B-ALL formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Pongubala JM, Nagulapalli S, Klemsz MJ, McKercher SR, Maki RA, Atchison ML . PU1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol 1992; 12: 368–378.
Kanno Y, Levi B-Z, Tamura T, Ozato K . Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J Interferon Cytokine Res 2005; 25: 770–779.
Eisenbeis CF, Singh H, Storb U . Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev 1995; 9: 1377–1387.
Eisenbeis CF, Singh H, Storb U . PU.1 is a component of a multiprotein complex which binds an essential site in the murine immunoglobulin lambda 2-4 enhancer. Mol Cell Biol 1993; 13: 6452–6461.
Brass AL, Zhu AQ, Singh H . Assembly requirements of PU.1-Pip (IRF-4) activator complexes: inhibiting function in vivo using fused dimers. EMBO J 1999; 18: 977–991.
Brass AL, Kehrli E, Eisenbeis CF, Storb U, Singh H . Pip, a lymphoid-restricted IRF, contains a regulatory domain that is important for autoinhibition and ternary complex formation with the Ets factor PU.1. Genes Dev 1996; 10: 2335–2347.
Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 2013; 38: 918–929.
Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 2010; 38: 576–589.
Scott EW, Simon MC, Anastasi J, Singh H . Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 1994; 265: 1573–1577.
Scott EW, Fisher RC, Olson MC, Kehrli EW, Simon MC, Singh H . PU.1 functions in a cell-autonomous manner to control the differentiation of multipotential lymphoid-myeloid progenitors. Immunity 1997; 6: 437–447.
Polli M, Dakic A, Light A, Wu L, Tarlinton D, Nutt S . The development of functional B lymphocytes in conditional PU.1 knock-out mice. Blood 2005; 106: 2083–2090.
Ye M, Ermakova O, Graf T . PU.1 is not strictly required for B cell development and its absence induces a B-2 to B-1 cell switch. J Exp Med 2005; 202: 1411–1422.
Sokalski KM, Li SKH, Welch I, Cadieux-Pitre H-AT, Gruca MR, Dekoter RP . Deletion of genes encoding PU.1 and Spi-B in B cells impairs differentiation and induces pre-B cell acute lymphoblastic leukemia. Blood 2011; 118: 1–33.
Johnson K, Hashimshony T, Sawai CM, Pongubala JM, Skok JA, Aifantis I et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity 2008; 28: 335–345.
Lu R, Medina KL, Lancki DW, Singh H . IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev 2003; 17: 1703–1708.
Ma S, Pathak S, Trinh L, Lu R . Interferon regulatory factors 4 and 8 induce the expression of Ikaros and Aiolos to down-regulate pre-B-cell receptor and promote cell-cycle withdrawal in pre-B-cell development. Blood 2008; 111: 1396–1403.
Ma S, Turetsky A, Trinh L, Lu R . IFN regulatory factor 4 and 8 promote Ig light chain kappa locus activation in pre-B cell development. J Immunol 2006; 177: 7898–7904.
Cook WD, McCaw BJ, Herring C, John DL, Foote SJ, Nutt SL et al. PU.1 is a suppressor of myeloid leukemia, inactivated in mice by gene deletion and mutation of its DNA binding domain. Blood 2004; 104: 3437–3444.
Holtschke T, Löhler J, Kanno Y, Fehr T, Giese N, Rosenbauer F et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 1996; 87: 307–317.
Metcalf D, Dakic A, Mifsud S, Di Rago L, Wu L, Nutt S . Inactivation of PU.1 in adult mice leads to the development of myeloid leukemia. Proc Natl Acad Sci USA 2006; 103: 1486–1491.
Rosenbauer F, Koschmieder S, Steidl U, Tenen DG . Effect of transcription-factor concentrations on leukemic stem cells. Blood 2005; 106: 1519–1524.
Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet 2004; 36: 624–630.
Scheller M, Schonheit J, Zimmermann K, Leser U, Rosenbauer F, Leutz A . Cross talk between Wnt/beta-catenin and Irf8 in leukemia progression and drug resistance. J Exp Med 2013; 210: 2239–2256.
Steidl U, Steidl C, Ebralidze A, Chapuy B, Han HJ, Will B et al. A distal single nucleotide polymorphism alters long-range regulation of the PU.1 gene in acute myeloid leukemia. J Clin Invest 2007; 117: 2611–2620.
Mullighan CG, Zhang J, Kasper LH, Lerach S, Payne-Turner D, Phillips LA et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 2011; 471: 235–239.
Zhang J, Mullighan CG, Harvey RC, Wu G, Chen X, Edmonson M et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 2011; 118: 3080–3087.
Bouamar H, Abbas S, Lin AP, Wang L, Jiang D, Holder KN et al. A capture-sequencing strategy identifies IRF8, EBF1, and APRIL as novel IGH fusion partners in B-cell lymphoma. Blood 2013; 122: 726–733.
Niebuhr B, Kriebitzsch N, Fischer M, Behrens K, Gunther T, Alawi M et al. Runx1 is essential at two stages of early murine B-cell development. Blood 2013; 122: 413–423.
Shukla V, Ma S, Hardy RR, Joshi SS, Lu R . A role for IRF4 in the development of CLL. Blood 2013; 122: 2848–2855.
Shaffer AL, Emre NC, Romesser PB, Staudt LM . IRF4: Immunity. Malignancy! Therapy? Clin Cancer Res 2009; 15: 2954–2961.
Adamaki M, Lambrou GI, Athanasiadou A, Tzanoudaki M, Vlahopoulos S, Moschovi M . Implication of IRF4 aberrant gene expression in the acute leukemias of childhood. PLoS One 2013; 8: e72326.
Acquaviva J, Chen X, Ren R . IRF-4 functions as a tumor suppressor in early B-cell development. Blood 2008; 112: 3798–3806.
Pathak S, Ma S, Trinh L, Eudy J, Wagner KU, Joshi SS et al. IRF4 is a suppressor of c-Myc induced B cell leukemia. PLoS One 2011; 6: e22628.
Jo SH, Schatz JH, Acquaviva J, Singh H, Ren R . Cooperation between deficiencies of IRF-4 and IRF-8 promotes both myeloid and lymphoid tumorigenesis. Blood 2010; 116: 2759–2767.
Xu LS, Sokalski KM, Hotke K, Christie DA, Zarnett O, Piskorz J et al. Regulation of B cell linker protein transcription by PU.1 and Spi-B in murine B cell acute lymphoblastic leukemia. J Immunol 2012; 189: 3347–3354.
Carotta S, Willis SN, Hasbold J, Inouye M, Pang SH, Emslie D et al. The transcription factors IRF8 and PU.1 negatively regulate plasma cell differentiation. J Exp Med 2014; 211: 2169–2181.
Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL . PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med 2005; 201: 1487–1502.
Mittrücker HW, Matsuyama T, Grossman A, Kündig TM, Potter J, Shahinian A et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science 1997; 275: 540–543.
Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA 2006; 103: 13789–13794.
DeKoter RP, Lee HJ, Singh H . PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity 2002; 16: 297–309.
Anderson KL, Nelson SL, Perkin HB, Smith KA, Klemsz MJ, Torbett BE . PU.1 is a lineage-specific regulator of tyrosine phosphatase CD45. J Biol Chem 2001; 276: 7637–7642.
Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007; 446: 758–764.
Decker T, Pasca di Magliano M, McManus S, Sun Q, Bonifer C, Tagoh H et al. Stepwise activation of enhancer and promoter regions of the B cell commitment gene Pax5 in early lymphopoiesis. Immunity 2009; 30: 508–520.
Minegishi Y, Rohrer J, Coustan-Smith E, Lederman HM, Pappu R, Campana D et al. An essential role for BLNK in human B cell development. Science 1999; 286: 1954–1957.
Pappu R, Cheng AM, Li B, Gong Q, Chiu C, Griffin N et al. Requirement for B cell linker protein (BLNK) in B cell development. Science 1999; 286: 1949–1954.
Jumaa H, Wollscheid B, Mitterer M, Wienands J, Reth M, Nielsen PJ . Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 1999; 11: 547–554.
Jumaa H, Bossaller L, Portugal K, Storch B, Lotz M, Flemming A et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature 2003; 423: 452–456.
Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 1996; 5: 537–549.
Ma S, Pathak S, Mandal M, Trinh L, Clark MR, Lu R . Ikaros and Aiolos inhibit pre-B-cell proliferation by directly suppressing c-Myc expression. Mol Cell Biol 2010; 30: 4149–4158.
Heizmann B, Kastner P, Chan S . Ikaros is absolutely required for pre-B cell differentiation by attenuating IL-7 signals. J Exp Med 2013; 210: 2823–2832.
Joshi I, Yoshida T, Jena N, Qi X, Zhang J, Van Etten RA et al. Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia. Nat Immunol 2014; 15: 294–304.
Schwickert TA, Tagoh H, Gultekin S, Dakic A, Axelsson E, Minnich M et al. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat Immunol 2014; 15: 283–293.
Mullighan C, Downing J . Ikaros and acute leukemia. Leuk Lymphoma 2008; 49: 847–849.
Mullighan CG, Su X, Zhang J, Radtke I, Phillips LAA, Miller CB et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 2009; 360: 470–480.
DeKoter R, Singh H . Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science 2000; 288: 1439–1441.
Rosenbauer F, Owens BM, Yu L, Tumang JR, Steidl U, Kutok JL et al. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat Genet 2006; 38: 27–37.
Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M . Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 2007; 27: 49–63.
Greig KT, de Graaf CA, Murphy JM, Carpinelli MR, Pang SH, Frampton J et al. Critical roles for c-Myb in lymphoid priming and early B-cell development. Blood 2010; 115: 2796–2805.
Ferreiros-Vidal I, Carroll T, Taylor B, Terry A, Liang Z, Bruno L et al. Genome-wide identification of Ikaros targets elucidates its contribution to mouse B-cell lineage specification and pre-B-cell differentiation. Blood 2013; 121: 1769–1782.
Acknowledgements
We thank M Reth and T Mak for mice, J Leahy for animal husbandry and the institute flow cytometry facility for excellent technical assistance. We thank Markus Jaritz for bioinformatic analysis. This work was supported by program and project grants (APP1054925 to SLN and 637345 to SC) and fellowships (APP1058238 to SLN) from the National Health and Medical Research Council (NHRMC) of Australia. SHMP was supported by the Leukaemia Foundation of Australia and SC by an NHMRC Career Development Fellowship. Research of the Busslinger group was supported by Boehringer Ingelheim and an ERC Advanced Grant (291740-LymphoControl). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIIS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Pang, S., Minnich, M., Gangatirkar, P. et al. PU.1 cooperates with IRF4 and IRF8 to suppress pre-B-cell leukemia. Leukemia 30, 1375–1387 (2016). https://doi.org/10.1038/leu.2016.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.27
This article is cited by
-
The role of CD180 in hematological malignancies and inflammatory disorders
Molecular Medicine (2023)
-
IRF4 drives clonal evolution and lineage choice in a zebrafish model of T-cell lymphoma
Nature Communications (2022)
-
The dynamic functions of IRF4 in B cell malignancies
Clinical and Experimental Medicine (2022)
-
Aberrant chromatin landscape following loss of the H3.3 chaperone Daxx in haematopoietic precursors leads to Pu.1-mediated neutrophilia and inflammation
Nature Cell Biology (2021)
-
Restoring PU.1 induces apoptosis and modulates viral transactivation via interferon-stimulated genes in primary effusion lymphoma
Oncogene (2017)