Abstract
The human EphA3 gene was discovered in a pre-B acute lymphoblastic leukemia (pre-B-ALL) using the EphA3-specific monoclonal antibody (mAb), IIIA4, which binds and activates both human and mouse EphA3. We use two models of human pre-B-ALL to examine EphA3 function, demonstrating effects on pre-B-cell receptor signaling. In therapeutic targeting studies, we demonstrated antitumor effects of the IIIA4 mAb in EphA3-expressing leukemic xenografts and no antitumor effect in the xenografts with no EphA3 expression providing evidence that EphA3 is a functional therapeutic target in pre-B-ALL. Here we show that the therapeutic effect of the anti-EphA3 antibody was greatly enhanced by adding an α-particle-emitting 213Bismuth payload.
Similar content being viewed by others
Introduction
Eph receptors, the largest family of receptor tyrosine kinases, interact with ephrin ligands to regulate cell growth, positioning and migration.1, 2 Eph and ephrin genes are highly expressed in various embryonic tissues.3, 4, 5 High expression of Eph genes were also observed in many cancers including leukemias, where both tumor-promoting and -inhibitory effects have been described.2, 6
EphA3 was identified in murine embryos and in human pre-B-ALL.7, 8 EphA3 expression is very low in adult tissues but high expression is described in cancers, including leukemia, sarcoma, melanoma and glioblastoma.2, 9 Expression was detected in stromal cells of the tumor microenvironment in several solid tumors.10 EphA3 copy number variation was observed in leukemias and EphA3 was identified as a cooperative response gene responsible for leukemia stem cell maintenance.11, 12
The EphA3-specific monoclonal antibody (mAb), IIIA4, binds and activates human and mouse EphA3,13 inducing internalization of receptor-antibody complexes.9 In glioblastoma cells, EphA3 knockdown reduced tumorigenicity in mice and treatment with radiolabeled IIIA4 antibody induced tumor regression of glioblastoma xenografts.14 In some tumors, IIIA4 antibody targeting of EphA3-positive stromal cells led to inhibition of tumor growth by disruption of architecture and function of vascular elements.10 These studies established EphA3 as a therapy target and KaloBios Pharmaceuticals Inc. (Brisbane, CA, USA) developed a Humaneered derivative of the original IIIA4 anti-EphA3 mAb (KB004) for which clinical trial is underway in hematological malignancies.
In this study, we show that IIIA4 antibody treatment of EphA3-positive human pre-B-ALL xenografts results in a direct antileukemic effect and an antibody-mediated effect on tumor cell spread to distant sites. We show that this effect is directed specifically at leukemic cells, and that payload delivery by IIIA4 is highly effective for antileukemia therapy.
Materials and methods
Cell culture and patient samples
The LK63 cell line was generated in our laboratory,15 and other cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI1640 supplemented with 10% fetal-calf-serum. LK63/A3KD (LK63 EphA3-knockdown) cells were derived as previously described.14 Reh/EphA3 (Reh EphA3-expressing) cells generated by infecting Reh cells with EphA3-encoding lentiviral particles (GeneCopoeia, Rockville, MD, USA; Supplementary Figure 1). Transfected cell lines (Reh/luciferase, LK63/luciferase, Reh/EphA3, LK63/A3KD) were maintained in RPMI1640 supplemented with 10% fetal-calf-serum with 0.5 μg/ml puromycin.
Patient samples were obtained from Queensland Children’s Tumor Bank in accordance with Human Ethics Committee-approved procedures for consent and sample collection.
Antibodies and Eph fusion proteins, flow cytometry and western blotting
In-house IgG1 mAbs and IIIA4 anti-EphA3 antibody were used. Mouse anti-human immunoglobulin M (IgM) antibody δADA4-4 was obtained from the ATCC. EphrinA5-Fc was used for activating EphA3. Alexa Fluor 488 goat anti-human IgG (A-11013; Life Technologies, Carlsbad, CA, USA) used as a secondary antibody.
Anti-human CD45-PE/Cy7 (304016; BioLegend, San Diego, CA, USA), anti-mouse CD45-APC (103112; Biolegend) and SYTOX-Blue (Life Technologies) viable cell markers were used to enumerate hematopoietic cells. Flow cytometric analysis was performed on a BD LSRFortessa (BD Biosciences, San Jose, CA, USA).
Antibodies used for western blot were as follows: EphA3 in-house antibody, FYN (4023S; Cell Signaling Technology, Inc., Danvers, MA, USA), p-FYN (SAB4301504; Sigma, St Louis, MO, USA) SRC (2108; Cell Signaling Technology, Inc.,), p-SRC (2101 Tyr416; Cell Signaling Technology, Inc.,) and β-actin (Sigma).
Amnis ImageStream
ImageStream was used for imaging flow cytometry (Amnis Corporation, Seattle, WA, USA).16 Cells were incubated with IIIA4 mAb or ephrinA5-FC preclustered with anti-human IgG and analyzed at time 0 and 20 min after incubation at 37 °C. Cell surface was stained with PKH26 (PKH26GL; Sigma). Cells were acquired on ImageStream using INSPIRE software and data were analyzed using IDEA software (Amnis Corporation). Internalization score is defined as the ratio of the intracellular signal intensity (intracellular mask) to the total cell intensity. Negative scores indicate predominant membrane expression and positive score indicates internalization. Colocalization is calculated as the correlation between the bright spots on or inside of the cells.
Gene expression microarrays and analysis
Reverse transcriptase-PCR performed as described previously14 and primers are listed in Supplementary Table 3.
Microarray gene expression profiling performed with a minimum of two experimental replicates by the Australian Genome Research Facility (AGRF, Melbourne, Victoria, Australia) using HumanHT-12 array chips (Illumina, San Diego, CA, USA). Data were analyzed using BRB-ArrayTools17 (V.4.5; Biometric Research Branch, Bethesda, MD, USA) as described.18 Additional details are provided in Supplementary Methods and summarized in Supplementary Tables 1 and 2 and Supplementary Figure 2. Ingenuity Pathway Analysis was described previously.18
Leukemic xenograft model
Mice were kept at QIMR-Berghofer Medical Research Institute (Brisbane, QLD, Australia) pathogen-free facility according to QIMR-Berghofer Medical Research Institute protocols. Xenografts established by tail vein injection of 5 × 106 LK63, Reh, LK63/A3KD and Reh/EphA3 cells into 6–8-week-old NOD/SCID mice. Xenografts were randomized to treatment groups (>3/per group) and received intraperitoneal injection of anti-EphA3 mAb (100 μg), control IgG antibody or vehicle (phosphate-buffered saline (PBS)), from day one and then every second day until the mice were euthanized due to disease progression. Mice were monitored for weight loss (scores 0–2), body posture (scores 0–2), activity level (scores 0–2) and grooming (scores 0–2). Mice were euthanized once the total score was >6.
213Bi-IIIA4 study
Bismuth-213 (213Bi) was used to radiolabel cIIIA4 and isotype control antibodies as described previously.19 To assess biodistribution of radioconjugates, mice were treated with 213Bi-cIIIA4 or 213Bi-isotype control at 6.0 μCi/28 μg in 0.1 ml saline 14 days post LK63 injection. Mice were euthanized at various time points and tissues were harvested for weighing and radioactive measurement in a dual gamma scintillation counter (Cobra II Auto Gamma, Packard Instruments, CT, USA).
The efficacy of 213Bi-cIIIA4 α-emitter radioimmunotherapy was assessed in leukemic xenografts generated with intravenous injection of 13 × 106 LK63 cells using two treatment regimens. In the first study, xenografts were injected with antibody intravenously at day 6 and in the second study xenografts received doses of treatment at days 2, 3, 5 and 6 after LK63 injection. In both studies, treatment groups comprised individual 213Bi-cIIIA4 doses of 12.5 μCi (115 μg protein) or 25 μCi (180 μg protein) or 213Bi-isotype control 12.5 μCi (75 μg protein) or 25 μCi (150 μg protein), delivered in 0.1–0.3 ml saline.
Statistics
Statistical analyses were performed using the GraphPad Prism Version 6.01 software (GraphPad Prism, San Diego, CA, USA). Data are shown as mean±s.e.m./s.d. of at least three replicates, unless stated otherwise. Significance was determined using an unpaired t-test or one-way analysis of variance with a minimal level of significance of P<0.05. Survival of the control and the treatment groups was compared using Kaplan–Meier analysis and statistical differences analyzed using the log-rank test.
Results
Expression of Eph receptors in leukemic cells
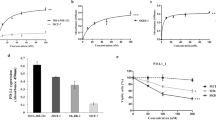
EphA expression in leukemia was assessed in a panel of leukemic cell lines and 30 clinical samples. Expression analysis in leukemic cells showed EphA3 as one of the highly expressed EphA transcripts (Figure 1a). Flow cytometric analysis showed high expression of EphA3 protein in LK63 and Jurkat, moderate expression in Molt-4 and no expression in Reh cells20, 21, 22, 23 (Figure 1b). Expression of EphA3 was then assessed in 30 acute leukemias with different karyotypes (t(1;19)E2A-PBX, t(12;21)ETV6-RUNX1, hyperdiploid>50 chromosomes, trisomy 4/10, MLL-rearranged, BCR-ABL1 and normal karyotype). Although none of the samples with MLL rearrangement and BCR-ABL1 showed EphA3 expression, modest and high expression of EphA3 was detected in one-third (10/30) of cases. EphA3 was most frequently expressed in pre-B-ALL with t(1;19)E2A-PBX karyotype (4/8) with two of the samples having expression levels similar to the t(1;19) bearing LK63 cell line (Figures 1c and d).15
Eph expression in leukemic cell lines. (a) Expression of EphA1, EphA2, EphA3 and EphA4 mRNA per 1000 β-actin using RT-PCR. (b) Flow cytometric expression analysis of EphA3 in leukemic cells. (c) Flow cytometric expression analysis of EphA3 using IIIA4 antibody in patient samples (n=30). (d) Representative overlay of flow cytometric analysis of EphA3 on patient samples.
Characterization of LK63 and Reh xenografts
Xenograft models included EphA3-positive (LK63), LK63/A3KD, EphA3-negative (Reh) and Reh EphA3-expressing (Reh/A3) cells. In the LK63 xenografts, cells grew in the bone marrow before infiltrating into the spleen and peripheral blood (Figure 2b). Splenic infiltration was identified as discrete foci of proliferating LK63 cells that became confluent (Figures 2c–e), consistent with the notion that leukemic cells were seeded into the spleen from the bone marrow. Engraftment of LK63/A3KD cells was slower than control LK63 cells, but the most striking difference was their considerably smaller spleen size (P<0.0001; Figure 2f). There was also a significant reduction in bone marrow engraftment (P=0.02) and no differences in spleen engraftment in LK63/A3KD xenografts compared with control LK63 xenografts (Figure 2g).
Leukemia progression in xenograft models. (a) Flow cytometry gating strategy: hCD45 and mCD45. (b) Leukemia progression in blood, spleen and bone marrow of LK63 xenograft (n=3). (c) Spleen weight of LK63 xenograft (n=3 per time-point) compared with normal mouse (n=1 per time-point). (d) Histology of normal spleen. (e) Histology of spleen from LK63 xenograft. (f) Reduced spleen size in LK63/A3KD compared with LK63 xenografts (P<0.0001; n=4 LK63, n=5 LK63/A3KD). (g) Significant reduction in the spleen and bone marrow engraftment of LK63/A3KD xenograft compared with LK63 xenograft (P=0.02) (n=3 LK63, n=5 LK63/A3KD). (h) Leukemia progression in blood, spleen and bone marrow of Reh xenograft. (i) Spleen weight of Reh xenograft (n=3 per time-point) compared with normal mice (n=1 per time-point). Data presented as mean percentage±s.e.m. Unpaired t-test performed for statistical analyses.
Reh engraftment was slower than LK63 xenografts. Cells preferentially grew in the bone marrow followed by the peripheral blood, evident once bone marrow engraftment was >50%. Unlike LK63 xenografts, Reh xenografts showed minimal splenic engraftment and no increase in spleen weight (Figures 2h and i).
Gene expression profiling of the LK63/A3KD cells compared with EphA3-high LK63 cells and the EphA3-negative Reh cells to identify differentially regulated genes (Supplementary Figure 2). B-cell activation was the biological process shared between these independent analyses (Supplementary Tables 1 and 2). We investigated 405 overlapping genes that were affected by EphA3 modulation in these cell lines (Supplementary Tables 1 and 2). As expected, EphA3 was among the overlapping genes, being downregulated in LK63/A3KD cells vs LK63 cells but upregulated in LK63 cells vs Reh cells. Network of interactions from the 405 deregulated genes (234 overexpressed (OE) and 171 underexpressed (UE) genes) was generated from the STRING database using evidence based on experiments/coexpression (Figure 3a). Notably, there was less CD79A and CD79B expression in LK63 compared with LK63/A3KD and Reh cells. As key elements of the B-cell receptor complex, we asked if increased CD79A correlated with an increase in BCR expression. We showed that surface IgM expression was indeed increased in LK63/A3KD compared with control LK63 cells (Figure 3b). Furthermore, as apparent from the network (Figure 3a), EphA3 acting on FYN is a likely mechanism driving gene expression changes in this network. The effect of EphA3 knockdown and activation on FYN phosphorylation showed that EphA3 activation in LK63 cells led to increased FYN phosphorylation. However less phosphorylated FYN was detected in LK63/A3KD, as expected, and IIIA4 induced less EphA3 activation and did not induce FYN phosphorylation in these cells (Supplementary Figure 3 and Figure 3c). FYN total protein was decreased in LK63/A3KD cells (Figure 3d).
Gene expression profiling after EphA3 modulation in LK63 and Reh cells. (a) Analysis of the deregulated genes in LK63 cells after EphA3 knockdown or in comparison with the EphA3-negative Reh cells, revealed a possibility that EphA3 affects CD79A/B and FYN expression to mediate the changes of expression of the genes in the network. (b) Flow cytometry of LK63/A3KD cells using IIIA4 and increased expression of CD79A/B demonstrated by increased IgM expression. (c) Western blots for FYN phosphorylation (Y530). (d) Western blot for total-FYN protein in LK63 and LK63/A3KD before or after EphA3 activation by ephrinA5-FC. The bands intensity was normalized to the Actin-control and the fold change compared with LK63 without EphA3 activation was calculated and shown under each lane.
Treatment of xenografted mice with IIIA4 mAb results in retardation of leukemic growth
The functional effects of EphA3 demonstrated in the LK63 model prompted us to test whether the IgG1 IIIA4 anti-EphA3 antibody could elicit a direct antitumor effect. As this isotype does not mediate effective Fc-mediated immune cell targeting, the variable region of the original IIIA4 antibody was engineered into both a murine-IgG2a backbone (IIIA4-IgG2a) and a human-IgG1 backbone (cIIIA4). The original IIIA4 antibody had a direct effect on growth, which minimally increased when the Fc was optimal for antibody-mediated cell cytotoxicity even in the NOD/SCID mice. Effective antibody-mediated cell cytotoxicity against LK63 was demonstrated using human effector cells and defucosylated, humaneered KB00424 (Supplementary Figure 4) where this optimized IIIA4 derivative showed significant killing of LK63 cells, whereas the cIIIA4 antibody was less effective.
As defucosylation does not affect murine ADCC, we used cIIIA4 in subsequent experiments. A dose–response experiment in LK63 xenografts demonstrated a maximum therapeutic response at 100 μg per dose (~4 mg/kg) of cIIIA4 (Figure 4a). In subsequent experiments, 100 μg of cIIIA4 antibody was administered on alternate days starting immediately after xenografting. Neither control human-IgG1 mAb nor vehicle showed an effect on engraftment (Figures 4b and c).
LK63 and Reh xenograft were treated with vehicle or cIIIA4 mAb. (a) Reduced spleen engraftment in LK63 xenograft at 30, 100, 250 and 1000 μg of cIIIA4 compared with vehicle treatment (P<0.0001). No significant difference between 100, 250 and 1000 μg cIIIA4 dosing (n⩾4). (b) Percentage of bone marrow engraftment in LK63 xenografts in response to PBS, IgG-control and cIIIA4 at 100 μg (n=8). (c) Reduced splenic engraftment in 100 μg cIIIA4-treated LK63 xenografts compared with PBS and IgG-control treatment (P<0.0002, n=8). (d) Significant reduction in cIIIA4-treated LK63 xenograft spleen weight (P=0.0006) and engraftment (P<0.0001) compared with PBS. There were no significant reduction in bone marrow engraftment (n=16 PBS, n=17 IIIA4, four experiments). (e) Representative Xenogen images of LK63 xenografts day 9, 14, 18 and 22. (f) No differences in spleen weight, spleen and bone marrow engraftments of Reh xenografts treated with cIIIA4 or PBS (n=15, three experiments). (g) Representative Xenogen images of Reh xenografts days 14, 18, 25 and 29. Point in graphs (d) and (e) corresponds to one mouse. Data presented as mean percentage±s.e.m. Unpaired t-tests or one-way analysis of variance (ANOVA) performed for statistical analyses.
As LK63 is positive for CD20, we also analyzed the effect of anti-CD20 (Mabthera) treatment on LK63 xenografts. The result showed no significant effect on leukemic progression (Supplementary Figures 4E–G).
Treatment of LK63 xenografts with cIIIA4 significantly reduced spleen engraftment (P<0.0001) as evident by the reduction in human CD45-positive cells in the spleen and reduced spleen weight (P=0.0006) compared with controls. Bone marrow engraftment was also reduced compared with controls (Figures 4d and e). Treatment of EphA3-negative Reh xenografts with cIIIA4 caused no significant changes in spleen size and spleen and bone marrow engraftment (Figures 4f and g).
To extend these results, cIIIA4 antibody treatment was also investigated using LK63/A3KD and Reh/A3 cells. Treatment of LK63/A3KD xenografts did not affect the spleen and bone marrow engraftment compared with controls (Figures 5a and b). Importantly, unlike Reh xenografts, treatment of Reh/A3 xenografts with cIIIA4 caused significant reduction in bone marrow (P=0.0072) and spleen (P=0.0464) engraftment compared with controls (Figures 5c and d).
LK63/A3KD and Reh/A3 xenografts treated with vehicle or cIIIA4 mAb. (a) No differences in spleen weight of LK63/A3KD xenografts treated with cIIIA4 or vehicle (b) No differences in spleen and bone marrow engraftment of LK63/A3KD xenograft treated with cIIIA4 or PBS (n=13 PBS, n=14 cIIIA4, three experiments). (c) No differences in spleen weight of Reh/A3 xenograft treated with cIIIA4 or vehicle (n=9 PBS, n=9 cIIIA4, three experiments). (d) Reh/A3 bone marrow (P=0.0072) and spleen (P=0.0464) engraftment were significantly reduced in cIIIA4-treated compared with PBS control(n=14 PBS, n=14 cIIIA4, four experiments). Each point in graphs (a) and (c) corresponds to one mouse. Data presented as mean percentage±s.e.m. Unpaired t-test performed for statistical analyses.
To simulate human leukemia where disease originates in bone marrow and then spreads to blood and other organs, Reh or LK63 cells were directly injected into the right femoral marrow cavity (Supplementary Methods). In both cases, leukemic deposits formed at the injection site followed by infiltration in other bones (left femoral), before spread to other organs. For LK63 cells, similar to the intravenous xenograft model extensive splenic infiltration was seen. Both models were treated with vehicle or cIIIA4 mAb and the response to cIIIA4 mimicked the intravenous model (Supplementary Figure 5).
EphrinA5 and cIIIA4 antibody induced internalization of EphA3
While the direct effect of cIIIA4 treatment in LK63 and Reh/A3 xenografts resulted in significant antitumor effect, this translated into a modest increase in survival. Therefore, to determine whether an antibody linked with a cytotoxic payload may be more efficacious, the degree of IIIA4 antibody internalization was explored.
LK63 and Reh cells were incubated with preclustered huIgG, IIIA4, preclustered IIIA4 or preclustered ephrinA5-Fc to induce receptor clustering and activation. As presented in Supplementary Figure 3, transient receptor phosphorylation was induced by these stimuli and preclustered antibody also induced phosphorylation of SRC, a known target of Eph signaling.25 Internalization of IIIA4 and ephrinA5-FC was analyzed in LK63 cells using AMNIS imaging flow cytometry at time 0 and 20 min after incubation at 37 °C where an increased internalization score upon treatment with IIIA4 antibody or ephrinA5-FC was observed. Internalization induced by ephrinA5 is in accordance with previous data26 (Figure 6).
Effect of EphA3 activation on signaling and internalization. (a) Representative images of treated cells at 0 and 20 min. (b) Increase in internalization score upon IIIA4 or ephrinA5-Fc treatment after 20min incubation at 37 °C in LK63 cells. (c) Median internalization score upon IIIA4 and ephrinA5-Fc treatment at 0 or 20 min after incubation at 37 °C in LK63 cells. (d) Percentage of cells that are internalized or colocalized at time 0 or 20 min after IIIA4 or ephrinA5-Fc treatment. In each AMNIS experiment, 5000 cells were collected and the experiment was repeated two times.
213Bi-IIIA4 therapy
Having demonstrated IIIA4-induced EphA3 internalization, we asked if a therapeutic cytotoxic payload could be delivered with minimal toxicity. 213Bi-cIIIA4 and control 213Bi-labeled antibodies were prepared at respective specific activities of 109 and 167 μCi/mg for in vitro and in vivo characterization.
Biodistribution of 213Bi-radiolabeled cIIIA4 and isotype control was determined in LK63 xenografts showing that 213Bi-cIIIA4 localized to the areas of leukemia involvement within 15 min of injection and were maintained over 3 h. Peak uptake of 20.8±3.9 and 20.9±3.8% ID/g was observed after 2 h in the liver and spleen, respectively. Minimal uptake (0.2–6% ID/g) was observed in other normal tissues at all time points (Figure 7a). The specificity of the 213Bi-cIIIA4 for EphA3-positive leukemia confirmed by low levels of tissue localization observed with the isotype control antibody (Figure 7b).
213Bi-labeled IIIA4 and control antibody treatments. (a) 213Bi-IIIA4 biodistribution in LK63 xenografts at 5 min, 1, 2 and 3 h after treatment. (b) Distribution of 213Bi-isotype controls in LK63 xenograft at 1 h after treatment (n=4). (c) No differences in single-dose 213Bi-IIIA4-treated (12.5 and 25 μCi) groups and the control groups (saline, 180 mg IIIA4, 213Bi-isotype control 12.5 and 25 μCi). (d) Multidose 213Bi-IIIA4 radioimmunotherapy. Increase in survival of IIIA4 and 213Bi-isotype control (P=0.001) compared with saline control. Significant increase in survival of 12.5 μCi 213Bi-IIIA4 (P=0.01) and 25 μCi 213Bi-IIIA4 (P<0.0001) observed compared with the IIIA4 control group. Significant increase in survival observed for 12.5 μCi 213Bi-IIIA4 compared to 12.5 μCi 213Bi-isotype (P=0.0011). Survival of the 25 μCi 213Bi-IIIA4 treated compared to 25 μCi 213Bi-isotype cohort was significantly improved (P=0.0001) (n=8). The results of radiolabeled antibody biodistribution over time calculated as percentage of injected dose per gram of blood or tissue (% ID/g) and expressed as mean±s.d. for each time point.
A single dose of 213Bi-cIIIA4 radioimmunotherapy in the LK63 xenografts 6 days after LK63 injection showed no significant differences in the median survival between the treatment groups (Figure 7c). However, four doses of 213Bi-cIIIA4 induced a highly significant increase in survival. Median survival of the control group was 21 days, whereas multidose treatment with unlabeled IIIA4 or 12.5 μCi 213Bi-isotype control caused an increase in median survival to 24 days. Radioimmunotherapy with 213Bi-cIIIA4 significantly increased survival in a dose-dependent manner with a median survival of 26.5 days at 12.5 μCi and 41.5 days at 25 μCi multidose treatment. The specificity of the cIIIA4 radioimmunotherapy is indicated by the significantly longer survival compared with the 213Bi-isotype control groups (Figure 7d).
Discussion
Expression of EphA3 in leukemia and the low expression on normal tissues led us to investigate the potential of EphA3 as a therapeutic target in ALL.27 We showed that EphA3 was expressed in ~1/3 of ALL cases and appears to be more significantly expressed in some subgroups, particularly cases having the t(1;19) translocation. Although only four cases with MLL translocations were tested, taken together with our previous data,28 it seems unlikely that EphA3 would be expressed in these leukemias as MLL transcriptionally induces expression of other Eph receptors with redundant function. Therapy with anti-EphA3 antibody was investigated using xenografts of the EphA3-positive (LK63, Reh/A3) and the EphA3-negative/low (Reh, LK63/A3KD) cell lines. In these models, leukemic cells preferentially grew in the bone marrow after intravenous xenotransplantation. However, in LK63, unlike Reh and Reh/A3 xenografts, secondary engraftment in the spleen preceded significant colonization of other organs and peripheral blood. When EphA3 expression was reduced in LK63/A3KD, the leukemic process was similar, except that splenic involvement was less marked. These patterns were confirmed by implanting cells directly into the bone marrow and showing that spread and growth in other bone marrow sites preceded systemic spread.
Microarray analysis of the LK63, LK63/A3KD and Reh cell lines identified elements of the B-cell receptor signaling complex (BCR), CD79A, CD79B and downstream effectors, particularly FYN, as differentially expressed in the presence of EphA3. Notably, IIIA4 antibody treatment resulted in an increase in phosphorylated FYN in LK63 cells but not in LK63/A3KD cells. However, increased CD79A levels in LK63/A3KD cells were correlated with increased surface IgM expression. As in normal pre-B cells, BCR signaling strength is important for survival of pre-B ALL cells.29, 30 In particular, excessive BCR signaling was shown to lead to cell death in leukemic cells, suggesting that EphA3 may act by reducing BCR signaling strength and thus promoting leukemic cell survival.
In LK63 xenografts, administration of either the mouse-IgG1 or the human-IgG1 cIIIA4 antibody resulted in inhibition of leukemia marrow infiltration, marked delay in tumor spread to spleen and other organs and increased in the latency of the disease. This is consistent with a direct effect of the mAb with some augmentation when Fc-mediated effects were present. In EphA3-negative Reh xenografts, no therapeutic effect was observed, suggesting that the effect of cIIIA4 mAb treatment was directed against leukemic cells expressing EphA3, rather than tumor microenvironment.10 In keeping with the lack of effect of cIIIA4 treatment on Reh xenografts, cIIIA4 treatment of LK63/A3KD xenografts showed considerably less effect on disease progression. However, treating Reh/A3 xenografts with cIIIA4 resulted in a significant reduction in bone marrow and spleen engraftment, comparable to the effect observed in LK63 xenografts. These results confirmed that in these leukemic models, in contrast to the solid tumor models reported by Vail et al.,10 the effect of cIIIA4 treatment was specifically directed against the leukemic cells with no detectable effect on tumor stromal elements.
Despite evidence for both a direct effect of the antibody on the leukemic cells and some augmentation when effective Fc-mediated function was present, there was only a limited effect on overall survival. The report of the first human trial of the ADCC-optimized KB004 antibody in AML patients by Swords et al.,31 whilst showing a good toxicity profile, did not demonstrate marked efficacy and fore-shadowed the investigation of ‘armed antibody’ as a potentially more effective therapy.31 Our previous studies showed radiolabeled targeting of EphA2 as a potential therapy target in AML,28 also in glioblastoma models using β-particle-emitting radioisotope coupled to cIIIA4 antibody resulted in a targeted antitumor effect in EphA3-expressing tumors.14 In leukemia to avoid bystander cell toxicity, we used an α-particle emitter, 213Bismuth, which depends on target internalization because of the high energy and short path length of the α-particle emission. Here we demonstrated that the IIIA4 antibody could induce EphA3 internalization, which makes this antibody an ideal candidate to target payloads including 213Bismuth to the leukemic cells. Using this form of radioimmunotherapy, we demonstrated markedly enhanced antitumor effect in mice without significant toxicity. These results strongly suggest that a therapeutic effect of cIIIA4 on EphA3-positive leukemia can be greatly enhanced by payloading with an isotope or potentially with cytotoxic drugs, as has been demonstrated in various hematological malignancies and solid tumors.31, 32
References
Pasquale EB . Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 2005; 6: 462–475.
Boyd AW, Bartlett PF, Lackmann M . Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov 2013; 13: 39–62.
Coulthard MG, Duffy S, Down M, Evans B, Power M, Smith F et al. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int J Dev Biol 2002; 46: 375–384.
Poliakov A, Cotrina M, Wilkinson DG . Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell 2004; 7: 465–480.
Adams RH, Eichmann A . Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2010; 2: a001875.
Pasquale EB . Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 2010; 10: 165–180.
Sajjadi FG, Pasquale EB, Subramani S . Identification of a new eph-related receptor tyrosine kinase gene from mouse and chicken that is developmentally regulated and encodes at least two forms of the receptor. New Biol 1991; 3: 769–778.
Wicks IP, Wilkinson D, Salvaris E, Boyd AW . Molecular cloning of HEK, the gene encoding a receptor tyrosine kinase expressed by human lymphoid tumor cell lines. Proc Natl Acad Sci USA 1992; 89: 1611–1615.
Boyd AW, Ward LD, Wicks IP, Simpson RJ, Salvaris E, Wilks A et al. Isolation and characterization of a novel receptor-type protein tyrosine kinase (hek) from a human pre-B cell line. J Biol Chem 1992; 267: 3262–3267.
Vail ME, Murone C, Tan A, Hii L, Abebe D, Janes PW et al. Targeting EphA3 inhibits cancer growth by disrupting the tumor stromal microenvironment. Cancer Res 2014; 74: 4470–4481.
Guan M, Liu L, Zhao X, Wu Q, Yu B, Shao Y et al. Copy number variations of EphA3 are associated with multiple types of hematologic malignancies. Clin Lymphoma Myeloma Leuk 2011; 11: 50–53.
Ashton JM, Balys M, Neering SJ, Hassane DC, Cowley G, Root DE et al. Gene sets identified with oncogene cooperativity analysis regulate in vivo growth and survival of leukemia stem cells. Cell Stem Cell 2012; 11: 359–372.
Vearing C, Lee FT, Wimmer-Kleikamp S, Spirkoska V, To C, Stylianou C et al. Concurrent binding of anti-EphA3 antibody and ephrin-A5 amplifies EphA3 signaling and downstream responses: potential as EphA3-specific tumor-targeting reagents. Cancer Res 2005; 65: 6745–6754.
Day BW, Stringer BW, Al-Ejeh F, Ting MJ, Wilson J, Ensbey KS et al. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell 2013; 23: 238–248.
Salvaris E, Novotny JR, Welch K, Campbell L, Boyd AW . Characterization of two novel pre-B-cell lines (LK63 and LiLa-1): potential models of pre-B-cell differentiation. Leuk Res 1992; 16: 655–663.
Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011; 333: 228–233.
Zhao Y, Simon R . BRB-ArrayTools Data Archive for human cancer gene expression: a unique and efficient data sharing resource. Cancer Inform 2008; 6: 9–15.
Al-Ejeh F, Miranda M, Shi W, Simpson PT, Song S, Vargas AC et al. Kinome profiling reveals breast cancer heterogeneity and identifies targeted therapeutic opportunities for triple negative breast cancer. Oncotarget 2014; 5: 3145–3158.
Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, Gansow OA et al. Stereochemical influence on the stability of radio-metal complexes in vivo. Synthesis and evaluation of the four stereoisomers of 2-(p-nitrobenzyl)-trans-CyDTPA. Bioorg Med Chem 1997; 5: 1925–1934.
Steube KG, Meyer C, Habig S, Uphoff CC, Drexler HG . Expression of receptor tyrosine kinase HTK (hepatoma transmembrane kinase) and HTK ligand by human leukemia-lymphoma cell lines. Leuk Lymphoma 1999; 33: 371–376.
Shimoyama M, Matsuoka H, Nagata A, Iwata N, Tamekane A, Okamura A et al. Developmental expression of EphB6 in the thymus: lessons from EphB6 knockout mice. Biochem Biophys Res Commun 2002; 298: 87–94.
Shimoyama M, Matsuoka H, Tamekane A, Ito M, Iwata N, Inoue R et al. T-cell-specific expression of kinase-defective Eph-family receptor protein, EphB6 in normal as well as transformed hematopoietic cells. Growth Factors 2000; 18: 63–78.
Dottori M, Down M, Huttmann A, Fitzpatrick DR, Boyd AW . Cloning and characterization of EphA3 (Hek) gene promoter: DNA methylation regulates expression in hematopoietic tumor cells. Blood 1999; 94: 2477–2486.
Tomasevic N, Luehrsen K, Baer M, Palath V, Martinez D, Williams J et al. A high affinity recombinant antibody to the human EphA3 receptor with enhanced ADCC activity. Growth Factors 2014; 32: 223–235.
Knoll B, Drescher U . Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci 2004; 24: 6248–6257.
Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PI, Lackmann M . Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol 2004; 164: 661–666.
Charmsaz S, Boyd AW . Expression and Function of the Eph Receptor Family in Leukemia and Hematopoietic Malignancies: Prospects for Targeted Therapies. J Leuk 2013; 1: 107.
Charmsaz S, Beckett K, Smith FM, Bruedigam C, Moore AS, Al-Ejeh F et al. EphA2 is a therapy target in EphA2-positive leukemias but is not essential for normal hematopoiesis or leukemia. PLoS One 2015; 10: e0130692.
Chen Z, Shojaee S, Buchner M, Geng H, Lee JW, Klemm L et al. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature 2015; 521: 357–361.
Kohrer S, Havranek O, Seyfried F, Hurtz C, Coffey GP, Kim E et al. Pre-BCR signaling in precursor B-cell acute lymphoblastic leukemia regulates PI3K/AKT, FOXO1 and MYC, and can be targeted by SYK inhibition. Leukemia 2016; 30: 1246–1254.
Swords RT, Greenberg PL, Wei AH, Durrant S, Advani AS, Hertzberg MS et al. KB004, a first in class monoclonal antibody targeting the receptor tyrosine kinase EphA3, in patients with advanced hematologic malignancies: results from a phase 1 study. Leuk Res 2016; 50: 123–131.
Parakh S, Parslow AC, Gan HK, Scott AM . Antibody-mediated delivery of therapeutics for cancer therapy. Expert Opin Drug Deliv 2016; 13: 401–419.
Acknowledgements
We acknowledge members of the QIMR-Berghofer flow cytometry and Animal facility. We acknowledge funding from Leukaemia Foundation and The Rio Tinto Ride To Conquer Cancer. We thank patients who contributed to the Queensland Children’s Tumor Bank. FA is supported by Future Fellowship from the ARC (ID:FT130101417). AMS is supported by an NHMRC fellowship and the Operational Infrastructure Support Scheme, Victorian Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
GTY and JW received commercial research support from and have ownership interest in KaloBios Pharmaceuticals Inc. This work was supported in part by a research grant from Kalobios Pharmaceuticals Inc.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Charmsaz, S., Al-Ejeh, F., Yeadon, T. et al. EphA3 as a target for antibody immunotherapy in acute lymphoblastic leukemia. Leukemia 31, 1779–1787 (2017). https://doi.org/10.1038/leu.2016.371
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.371
This article is cited by
-
The Hippo effector YAP1/TEAD1 regulates EPHA3 expression to control cell contact and motility
Scientific Reports (2022)
-
Critical role of EphA3 in cancer and current state of EphA3 drug therapeutics
Molecular Biology Reports (2020)
-
EphA3 targeting reduces in vitro adhesion and invasion and in vivo growth and angiogenesis of multiple myeloma cells
Cellular Oncology (2017)