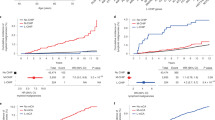
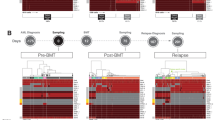
Abstract
Clonal hematopoiesis can be identified by the presence of somatic mutations in blood or bone marrow even in individuals without a myeloid malignancy. Advances in DNA sequencing have led to the discovery that clonal hematopoiesis is remarkably common and occurs in a wide variety of settings, each often described by unique acronym. These distinctions can be useful as the implications of clonal hematopoiesis depend almost entirely on the clinical context in which it is identified. However, some generalizations can be made. The prevalence of clonal hematopoiesis increases with age, particularly after the fifth decade of life. Clonal hematopoiesis in normal individuals with very small clones is typically benign, while patients with clinically abnormal hematopoiesis, larger clones and more driver gene mutations appear to be at much greater risk. Understanding the significance of clonal hematopoiesis in the various contexts in which it occurs can influence how physicians assess risk, select therapies and counsel their patients. This concise review examines the implications of clonal hematopoiesis in several settings, including normal aging, aplastic anemia, unexplained cytopenias and patients receiving cytotoxic chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Link DC, Walter MJ . 'CHIP'ping away at clonal hematopoiesis. Leukemia 2016; 30: 1633–1635.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371: 2488–2498.
Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014; 371: 2477–2487.
Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014; 20: 1472–1478.
Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet 2012; 44: 1179–1181.
Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 2017; e-pub ahead of print 8 may 2017 doi:10.1182/blood-2017-02-769869.
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015 Jul 02; 126: 9–16.
Takahashi K, Wang F, Kantarjian H, Doss D, Khanna K, Thompson E et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol 2016; 18: 100–111.
Gillis NK, Ball M, Zhang Q, Ma Z, Zhao Y, Yoder SJ et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol 2017; 18: 112–121.
Gibson CJ, Lindsley RC, Tchekmedyian V, Shi J, Mar BG, Jaiswal S et al. Clonal hematopoiesis associated with adverse outcomes following autologous stem cell transplantation for non-hodgkin lymphoma. Blood 2016; 128: 986–986.
Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015; 518: 552–555.
McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep 2015; 10: 1239–1245.
Young AL, Challen GA, Birmann BM, Druley TE . Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nature Commun 2016; 7: 12484.
Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med 2015; 373: 35–47.
Stanley N, Olson TS, Babushok DV . Recent advances in understanding clonal haematopoiesis in aplastic anaemia. Br J Haematol 2017; 177: 509–525.
Kulasekararaj AG, Jiang J, Smith AE, Mohamedali AM, Mian S, Gandhi S et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood 2014; 18: 2014–2005.
Ogawa S . Clonal hematopoiesis in acquired aplastic anemia. Blood 2016; 128: 337–347.
Valent P, Bain BJ, Bennett JM, Wimazal F, Sperr WR, Mufti G et al. Idiopathic cytopenia of undetermined significance (ICUS) and idiopathic dysplasia of uncertain significance (IDUS), and their distinction from low risk MDS. Leuk Res 2012; 36: 1–5.
Kwok B, Hall JM, Witte JS, Xu Y, Reddy P, Lin K et al. MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood 2015; 126: 2355–2361.
Cargo CA, Rowbotham N, Evans PA, Barrans SL, Bowen DT, Crouch S et al. Targeted sequencing identifies patients with preclinical MDS at high risk of disease progression. Blood 2015; 126: 2362–2365.
Kern W, Meggendorfer M, Haferlach C, Haferlach T . Integrated diagnostic approach for suspected myelodysplastic syndrome as a basis for advancement of diagnostic criteria. Blood 2016; 128: 299–299.
Malcovati L, Gallì A, Travaglino E, Ambaglio I, Rizzo E, Molteni E et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood 2017; e-pub ahead of print 19 April 2017 doi:10.1182/blood-2017-01-763425.
Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014; 506: 328–333.
Wong TN, Miller CA, Klco JM, Petti A, Demeter R, Helton NM et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood 2016; 127: 893–897.
Thol F, Klesse S, Kohler L, Gabdoulline R, Kloos A, Liebich A et al. Acute myeloid leukemia derived from lympho-myeloid clonal hematopoiesis. Leukemia 2017; 31: 1286–1295.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Bejar has received honoraria as a consultant and advisory member to Genoptix and Foundation Medicine. He has received royalties for IP licensed to Genoptix and has received research funding from Celgene.
Rights and permissions
About this article
Cite this article
Bejar, R. CHIP, ICUS, CCUS and other four-letter words. Leukemia 31, 1869–1871 (2017). https://doi.org/10.1038/leu.2017.181
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.181
This article is cited by
-
Pre-operative clonal hematopoiesis is related to adverse outcome in lung cancer after adjuvant therapy
Genome Medicine (2023)
-
Circulating microbial content in myeloid malignancy patients is associated with disease subtypes and patient outcomes
Nature Communications (2022)
-
Myelodysplastic syndromes
Nature Reviews Disease Primers (2022)
-
Myeloid neoplasms and clonal hematopoiesis from the RUNX1 perspective
Leukemia (2022)
-
Clonal hematopoiesis with JAK2V617F promotes pulmonary hypertension with ALK1 upregulation in lung neutrophils
Nature Communications (2021)