Abstract
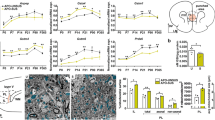
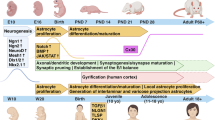
Schizophrenia pathophysiology implies both abnormal redox control and dysconnectivity of the prefrontal cortex, partly related to oligodendrocyte and myelin impairments. As oligodendrocytes are highly vulnerable to altered redox state, we investigated the interplay between glutathione and myelin. In control subjects, multimodal brain imaging revealed a positive association between medial prefrontal glutathione levels and both white matter integrity and resting-state functional connectivity along the cingulum bundle. In early psychosis patients, only white matter integrity was correlated with glutathione levels. On the other side, in the prefrontal cortex of peripubertal mice with genetically impaired glutathione synthesis, mature oligodendrocyte numbers, as well as myelin markers, were decreased. At the molecular levels, under glutathione-deficit conditions induced by short hairpin RNA targeting the key glutathione synthesis enzyme, oligodendrocyte progenitors showed a decreased proliferation mediated by an upregulation of Fyn kinase activity, reversed by either the antioxidant N-acetylcysteine or Fyn kinase inhibitors. In addition, oligodendrocyte maturation was impaired. Interestingly, the regulation of Fyn mRNA and protein expression was also impaired in fibroblasts of patients deficient in glutathione synthesis. Thus, glutathione and redox regulation have a critical role in myelination processes and white matter maturation in the prefrontal cortex of rodent and human, a mechanism potentially disrupted in schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 2003; 60: 443–456.
Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 2001; 98: 4746–4751.
Katsel P, Davis KL, Haroutunian V . Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res 2005; 79: 157–173.
Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD . Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res 2002; 27: 1193–1200.
Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B . Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry 2004; 161: 882–888.
Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD . The role of oligodendrocyte pathology in schizophrenia. Int J Neuropsychopharmacol 2007; 10: 537–545.
Du F, Cooper A, Cohen BM, Renshaw PF, Ongur D . Water and metabolite transverse T2 relaxation time abnormalities in the white matter in schizophrenia. Schizophr Res 2012; 137: 241–245.
Kubicki M, Westin CF, McCarley RW, Shenton ME . The application of DTI to investigate white matter abnormalities in schizophrenia. Ann NY Acad Sci 2005; 1064: 134–148.
Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S . Diffusion tensor imaging in schizophrenia. Eur Psychiatry 2008; 23: 255–273.
Jones DP . Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008; 295: C849–C868.
Bitanihirwe BK, Woo TU . Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev 2011; 35: 878–893.
Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M . Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol 2009; 19: 220–230.
Reddy R, Keshavan M, Yao JK . Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr Res 2003; 62: 205–212.
Yao JK, Keshavan MS . Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxidants Redox Signal 2011; 15: 2011–2035.
Meister A, Anderson ME . Glutathione. Annu Rev Biochem 1983; 52: 711–760.
Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci 2000; 12: 3721–3728.
Matsuzawa D, Hashimoto K . Magnetic resonance spectroscopy study of the antioxidant defense system in schizophrenia. Antioxidants Redox Signal 2011; 15: 2057–2065.
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT . Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 2011; 14: 123–130.
Gravina P, Spoletini I, Masini S, Valentini A, Vanni D, Paladini E, et al. Genetic polymorphisms of glutathione S-transferases GSTM1, GSTT1, GSTP1 and GSTA1 as risk factors for schizophrenia. Psychiatry Res 2011; 187: 454–456.
Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci USA 2007; 104: 16621–16626.
Rodriguez-Santiago B, Brunet A, Sobrino B, Serra-Juhe C, Flores R, Armengol L, et al. Association of common copy number variants at the glutathione S-transferase genes and rare novel genomic changes with schizophrenia. Mol Psychiatry 2010; 15: 1023–1033.
Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F et al. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. American journal of human genetics 2006; 79: 586–592.
Gokhale A, Larimore J, Werner E, So L, Moreno-De-Luca A, Lese-Martin C, et al. Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1. J Neurosci 2012; 32: 3697–3711.
Goldshmit Y, Erlich S, Pinkas-Kramarski R . Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2)O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem 2001; 276: 46379–46385.
Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, et al. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci USA 2013; 110: 12462–12467.
Krishnan N, Dickman MB, Becker DF . Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med 2008; 44: 671–681.
Otte DM, Sommersberg B, Kudin A, Guerrero C, Albayram O, Filiou MD, et al. N-acetyl cysteine treatment rescues cognitive deficits induced by mitochondrial dysfunction in G72/G30 transgenic mice. Neuropsychopharmacology 2011; 36: 2233–2243.
Gysin R, Kraftsik R, Boulat O, Bovet P, Conus P, Comte-Krieger E, et al. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. Antioxidants Redox Signal 2011; 15: 2003–2010.
Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci 2010; 30: 2547–2558.
Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ . Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry 2012; 73: 574–582.
Kulak A, Cuenod M, Do KQ . Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav Brain Res 2012; 226: 563–570.
Thorburne SK, Juurlink BH . Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 1996; 67: 1014–1022.
Jana M, Pahan K . Down-regulation of myelin gene expression in human oligodendrocytes by nitric oxide: implications for demyelination in multiple sclerosis. J Clin Cell Immunol 2013; 4.
Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ . Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci 1998; 18: 6241–6253.
Cavaliere F, Urra O, Alberdi E, Matute C . Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis 2012; 3: e268.
Smith J, Ladi E, Mayer-Proschel M, Noble M . Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad USA 2000; 97: 10032–10037.
Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust NZ J Psychiatry 2005; 39: 964–971.
Baumann PS, Crespi S, Marion-Veyron R, Solida A, Thonney J, Favrod J, et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): implementation of an early intervention programme for psychosis in Switzerland. Early Interv Psychiatry 2013; 7: 322–328.
Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of general psychiatry. 1994; 51: 849–859; discussion 63-4.
Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM . Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 2005; 54: 1377–1386.
Daducci A, Gerhard S, Griffa A, Lemkaddem A, Cammoun L, Gigandet X, et al. The connectome mapper: an open-source processing pipeline to map connectomes with MRI. PLoS One 2012; 7: e48121.
Richiardi J, Eryilmaz H, Schwartz S, Vuilleumier P, Van De Ville D . Decoding brain states from fMRI connectivity graphs. NeuroImage 2011; 56: 616–626.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006; 31: 968–980.
Greicius MD, Supekar K, Menon V, Dougherty RF . Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 2009; 19: 72–78.
Gruetter R . Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med 1993; 29: 804–811.
Mekle R, Mlynarik V, Gambarota G, Hergt M, Krueger G, Gruetter R . MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med 2009; 61: 1279–1285.
Mlynarik V, Gambarota G, Frenkel H, Gruetter R . Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med 2006; 56: 965–970.
Provencher SW . Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679.
Xin L, Gambarota G, Mlynarik V, Gruetter R . Proton T2 relaxation time of J-coupled cerebral metabolites in rat brain at 9.4T. NMR Biomed 2008; 21: 396–401.
Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP . Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem 2002; 277: 49446–49452.
McCarthy KD, de Vellis J . Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 1980; 85: 890–902.
Cabungcal JH, Nicolas D, Kraftsik R, Cuenod M, Do KQ, Hornung JP . Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: relevance to schizophrenia. Neurobiol Dis 2006; 22: 624–637.
Franklin BJ, Paxinos G . The Mouse Brain in Stereotaxic Coordinates. 3rd edn. New York, NY, USA: Academic Press, 2008.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Lowe MJ, Mock BJ, Sorenson JA . Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage 1998; 7: 119–132.
Lebel C, Beaulieu C . Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 2011; 31: 10937–10947.
Li Z, Dong T, Proschel C, Noble M . Chemically diverse toxicants converge on Fyn and c-Cbl to disrupt precursor cell function. PLoS Biol 2007; 5: e35.
Noble M, Smith J, Power J, Mayer-Proschel M . Redox state as a central modulator of precursor cell function. Ann NY Acad Sci 2003; 991: 251–271.
French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB . Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res 2009; 87: 3076–3087.
Beaulieu C . The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 2002; 15: 435–455.
Bartzokis G . Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology 2002; 27: 672–683.
Harris LW, Lockstone HE, Khaitovich P, Weickert CS, Webster MJ, Bahn S . Gene expression in the prefrontal cortex during adolescence: implications for the onset of schizophrenia. BMC Med Genom 2009; 2: 28.
Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R . Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA 2012; 109 (Suppl 2): 17186–17193.
Blakemore SJ, Choudhury S . Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry Allied Disc 2006; 47: 296–312.
Fields RD . White matter in learning, cognition and psychiatric disorders. Trends Neurosci 2008; 31: 361–370.
Nave KA . Myelination and support of axonal integrity by glia. Nature 2010; 468: 244–252.
Xin L, Mekle R, Ferrari C, Baumann PS, Alameda L, Moser H, et al. Genetic association with prefrontal glutathione deficit: a preliminary 3T1H MRS Study in Early Psychosis. Proceedings of the International Society of Magnetic Resonance in Medicine Annual Meeting and Exhibition, 10–16 May; Milan, Italy, 2014.
Abe J, Okuda M, Huang Q, Yoshizumi M, Berk BC . Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J Biol Chem 2000; 275: 1739–1748.
Sanguinetti AR, Cao H, Corley Mastick C . Fyn is required for oxidative- and hyperosmotic-stress-induced tyrosine phosphorylation of caveolin-1. Biochem J 2003; 376 (Part 1): 159–168.
Salmeen A, Barford D . Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal 2005; 7: 560–577.
Gao Y, Howard A, Ban K, Chandra J . Oxidative stress promotes transcriptional up-regulation of Fyn in BCR-ABL1-expressing cells. J Biol Chem 2009; 284: 7114–7125.
Ohnuma T, Kato H, Arai H, McKenna PJ, Emson PC . Expression of Fyn, a non-receptor tyrosine kinase in prefrontal cortex from patients with schizophrenia and its correlation with clinical onset. Brain Res Mol Brain Res 2003; 112: 90–94.
Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 2006; 117: 2093–2100.
Huang H, Gundapuneedi T, Rao U . White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology 2012; 37: 2693–2701.
Acknowledgements
This work was supported by the Swiss National Science Foundation (Grant Nos. 310030_135736/1 to KQD and PS, 320030_122419 to PC and KQD and 130090 to PH) and the National Center of Competence in Research (NCCR) ‘SYNAPSY—The Synaptic Bases of Mental Diseases’ (Grant No. 51AU40_125759). We thank the Brazilian Swiss Joint Research Program (BSJRP), the ‘Loterie Romande’, Damm-Etienne Foundation, Avina Foundation and Alamaya Foundation. PH was financially supported by Leenaards Foundation. We are grateful for technical assistance to Hélène Moser and Adeline Cottier. We also thank Dr Mehdi Gholam for advices on statistics, Dr Portia Vliet for the GCLC antibody and Dr Ibro Ambeskovic for advices on OPC culture and in vitro experiments. We extend thanks to Ying Chen for providing us with the GCLM-KO breeders. Most of all, we express our gratitude to all patients and healthy volunteers for their enduring participation.
Author Contributions
AM wrote the manuscript, designed and carried out the rodent experiments and performed human fibroblast culture. PSB wrote the manuscript and performed the patient recruitment and skin biopsy and analyzed DSI. AG performed DSI/fMRI acquisition and analyzed the data. LX and RM performed MRS acquisition and analyzed the data. MF wrote the manuscript, designed and performed human fibroblast culture and evaluated data from Stanley database. CB designed and prepared shRNA. MK established OPC culture. J-HC contributed to morphology analysis of GCLM-KO mice and editing the manuscript. PS designed and contributed to experiments in rodents and to the manuscript writing. CF recruited control subjects and early psychosis patients and performed psychiatric evaluations. MC contributed to the overall study concept and to the manuscript writing. RG supervised MRS study. J-PT supervised DSI/fMRI analysis. PH designed, analyzed and supervised DSI/fMRI study. PC contributed to the overall concept, and planned and coordinated the recruitment in human study. KQD conceived and directed the whole study, and contributed to the writing. All authors reviewed and edited the manuscript.
Author contributions
AM wrote the manuscript, designed and carried out the rodent experiments and performed human fibroblast culture. PSB wrote the manuscript and performed the patient recruitment and skin biopsy and analyzed DSI. AG performed DSI/fMRI acquisition and analyzed the data. LX and RM performed MRS acquisition and analyzed the data. MF wrote the manuscript, designed and performed human fibroblast culture and evaluated data from Stanley database. CB designed and prepared shRNA. MK established OPC culture. J-HC contributed to morphology analysis of GCLM-KO mice and editing the manuscript. PS designed and contributed to experiments in rodents and to the manuscript writing. CF recruited control subjects and early psychosis patients and performed psychiatric evaluations. MC contributed to the overall study concept and to the manuscript writing. RG supervised MRS study. J-PT supervised DSI/fMRI analysis. PH designed, analyzed and supervised DSI/fMRI study. PC contributed to the overall concept, and planned and coordinated the recruitment in human study. KQD conceived and directed the whole study, and contributed to the writing. All authors reviewed and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Monin, A., Baumann, P., Griffa, A. et al. Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. Mol Psychiatry 20, 827–838 (2015). https://doi.org/10.1038/mp.2014.88
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.88
This article is cited by
-
Sex-specific interactions between stress axis and redox balance are associated with internalizing symptoms and brain white matter microstructure in adolescents
Translational Psychiatry (2024)
-
Exploring the mediation of DNA methylation across the epigenome between childhood adversity and First Episode of Psychosis—findings from the EU-GEI study
Molecular Psychiatry (2023)
-
Introducing the brain erythropoietin circle to explain adaptive brain hardware upgrade and improved performance
Molecular Psychiatry (2022)
-
Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia
Molecular Psychiatry (2022)
-
Gene set enrichment analysis of pathophysiological pathways highlights oxidative stress in psychosis
Molecular Psychiatry (2022)