Abstract
Physical and cognitive exercise may prevent or delay dementia in later life but the neural mechanisms underlying these therapeutic benefits are largely unknown. We examined structural and functional magnetic resonance imaging (MRI) brain changes after 6 months of progressive resistance training (PRT), computerized cognitive training (CCT) or combined intervention. A total of 100 older individuals (68 females, average age=70.1, s.d.±6.7, 55–87 years) with dementia prodrome mild cognitive impairment were recruited in the SMART (Study of Mental Activity and Resistance Training) Trial. Participants were randomly assigned into four intervention groups: PRT+CCT, PRT+SHAM CCT, CCT+SHAM PRT and double SHAM. Multimodal MRI was conducted at baseline and at 6 months of follow-up (immediately after training) to measure structural and spontaneous functional changes in the brain, with a focus on the hippocampus and posterior cingulate regions. Participants’ cognitive changes were also assessed before and after training. We found that PRT but not CCT significantly improved global cognition (F(90)=4.1, P<0.05) as well as expanded gray matter in the posterior cingulate (Pcorrected <0.05), and these changes were related to each other (r=0.25, P=0.03). PRT also reversed progression of white matter hyperintensities, a biomarker of cerebrovascular disease, in several brain areas. In contrast, CCT but not PRT attenuated decline in overall memory performance (F(90)=5.7, P<0.02), mediated by enhanced functional connectivity between the hippocampus and superior frontal cortex. Our findings indicate that physical and cognitive training depend on discrete neuronal mechanisms for their therapeutic efficacy, information that may help develop targeted lifestyle-based preventative strategies.
Similar content being viewed by others
Introduction
Cognitive training1, 2 and physical exercise3, 4 can help support cognitive function in late life, stimulating interest in their potential for the delay or even prevention of dementia.5 Yet, how these lifestyle interventions work in humans is still not clear,6 despite a wealth of studies examining environmental enrichment7, 8 and voluntary running paradigms in rodents.9, 10 Human neuroimaging studies suggest the induction of some common activity-dependent mechanisms11 as well as biological processes that may be unique to each intervention,12, 13, 14 but head-to-head imaging studies are yet to be reported.
One major unresolved question is whether training antagonizes the degenerative effects of advancing age, cerebrovascular disease and Alzheimer’s disease (AD), or stimulates disease-independent mechanisms that cumulatively support cognition. Structurally, the natural history of AD begins with volume loss in entorhinal and hippocampal areas and then progresses to include the posterior cingulate, cortical temporal lobe and eventually most of the gray matter.15, 16 It is therefore interesting that physical activity has been linked to preserved hippocampal and frontal cortical volume in cross-sectional17, 18 and prospective studies,19 and more direct evidence of possible disease modification provided in a randomized controlled trial (RCT) where 1 year of moderate-intensity walking in healthy elders led to hippocampal expansion compared with atrophy in controls.20 However, the therapeutic relevance of this finding is not clear given the same trial reported equivalent memory change in walkers and controls.21 In contrast, computerized cognitive training is generally efficacious for memory in healthy elderly1 but does not lead to hippocampal structural plasticity. Rather, initial hippocampal volume can independently predict mnemonic improvements at the end of cognitive training.22
Resistance or strength training remains largely unstudied in animal models from a neuroscience perspective. In humans, resistance exercise produces complex systemic and metabolic changes23 and is effective for chronic age-related health issues such as sarcopenia,24, 25 osteoporosis26 and insulin resistance.27, 28 Research on the cognitive effects of resistance exercise in older adults is preliminary in comparison with aerobic exercise.29 One RCT found evidence of improved memory function following 6-month training,30 whereas two RCTs have found executive function improvements, but no memory effects, immediately following training.31, 32 To date, evidence for cerebral effects of resistance exercise in elders is mixed: one RCT initially reported that resistance exercise led to a small but significant reduction in whole-brain volume,32 but a subsequent subgroup reanalysis found attenuated white matter atrophy.33
Another relevant34, 35, 36 but poorly modeled mechanism in animals is chronic cerebrovascular disease, typically coexpressed alongside AD pathology in those with dementia37, 38 or dementia-precursor mild cognitive impairment (MCI).39 Ischemic and inflammatory brain tissue can be visualized on T2-weighted magnetic resonance imaging (MRI) as white matter hyperintensities (WMHs)40 and their severity is strongly linked to vascular risk factors.41, 42 Moreover, WMHs increase risk of cognitive impairment43 and independently predict transition from MCI to dementia.44, 45 It is therefore interesting that physical exercise embedded within a comprehensive vascular care program can slow progression of WMHs in older individuals with established dementia.46 Whether physical or cognitive exercise can produce similar effects on WMH burden in nondemented individuals has not been tested.
Frontal lobe functional connectivity is a candidate for a shared mechanism because it is responsive to both cognitive training12 and aerobic physical exercise.47, 48 Frontal lobe connectivity declines with age49, 50, 51 and has been further implicated in early AD,52, 53 but whether physical or cognitive exercise is more effective for network rescue is unknown. Another important connectivity pattern is the default mode network (DMN), a spatially distributed but temporally synchronized assembly of brain regions preferentially engaged when a person is internally focused and not attending to external stimuli.54 A common approach to defining the DMN relies on spontaneous activity in the posterior cingulate (PC);55 indeed, this brain region lies at the structural and functional core of the DMN.56 Whether physical or cognitive training produces functionally relevant changes to the DMN in older adults is not known.
There are hence several possible mechanisms by which cognitive training or physical exercise may achieve therapeutic efficacy but these have not been directly compared in a rigorous RCT setting. At least some of these mechanisms may be specific to physical exercise, and hence supported a prediction of additive therapeutic effects following combined intervention. The Study of Mental Activity and Resistance Training (SMART) Trial was therefore designed to determine whether resistance exercise, cognitive exercise or combined cognitive and resistance training can prevent or slow cognitive and functional decline in individuals with MCI.57, 58 Here, our aim was to identify therapeutically relevant brain mechanisms using multimodal MR imaging in the context of a fully factorial, active-controlled, double-blind RCT.
Materials and methods
Subjects and design
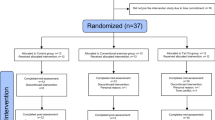
SMART trial volunteers (N=100; 68 females, average age=70.1 s.d. ±6.7 years) were nondemented, nondepressed individuals aged ⩾55 years who met Petersen’s original MCI criteria,59 having subjective memory complaints and a Mini-Mental State Examination score of 24–28 (29 was acceptable only if error noted in memory registration), a Clinical Dementia Rating scale 0 or 0.5 and independence in daily function. Individuals were randomized by computer-based sequence to one of four training groups (Figure 1a) in which they completed two types of supervised center-based training per session (physical and cognitive), twice per week, for a total of 26 weeks. Each session lasted 90 min and comprised either: (1) progressive resistance training (PRT)+computerized cognitive training (CCT), (2) PRT+SHAM computerized cognitive (that is, active cognitive control) training, (3) CCT+SHAM stretching and toning (active physical control) training and (4) SHAM physical+SHAM cognitive training. A battery of neuropsychological tests, physical and metabolic tests and multimodal MRI assessments were carried before and after the 6-month training period. Participants were blind to training group hypotheses and assessors were blinded to group allocation. There were no significant differences in dropout (8%), total training time (22.7 weeks), trained sessions per week (2.3 sessions) or absolute compliance (44.6 sessions) between the four interventions groups. Subjective appraisals were measured using the Memory Awareness Rating Scale60, 61 and Memory Complaint Score.62 All nonvoxel/vertex-based analyses (that is, cognitive and region-of-interest-based MR outcomes) were conducted using linear mixed models, and all analyses controlled for age, sex and education. Sample size calculations were based on the trial’s (clinical) primary outcome as reported previously.57, 58 All participants gave informed consent and the trial was approved by the human research ethics committee of the University of Sydney.
(a) Design and participant flow of the SMART trial. (b) Summary of factor-based interactions. (c) Participants in any PRT group significantly improved on ADAS-Cog (lower scores are better) compared with non-PRT groups (RES × TIME, F(90)=4.1, *P<0.05, Y axis reversed). (d) Participants in any CCT group attenuated the decline in Memory domain compared with non-CCT groups (COG × TIME, F(90)=5.7, *P<0.02). Error bars represent 95% confidence interval. Intention-to-treat analyses controlled for age, sex and education. ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive scale; CCT, computerized cognitive training; COG, cognitive training factor; MRI, magnetic resonance imaging; PRT, progressive resistance training; RES, progressive resistant training factor; SMART, Study of Mental Activity and Resistance Training.
The SMART Trial was prospectively registered with the Australian and New Zealand Clinical Trials Registry (Protocol No: X08-0064).
Interventions
Details about the structure, content and timing of the interventions can be found elsewhere.57 In brief, they are described as follows.
Cognitive training
CCT comprised the COGPACK program (http://www.cogpack.com/USA/frames.htm), a multidomain computer-based software package developed for neurorehabilitation. Sham CCT was also computer based: participants watched video clips of general interest documentary topics, followed by a set of simple questions regarding the presented material. CCT and sham cognitive training were matched for duration, setting and sensorimotor stimulation. All cognitive training was conducted in a dedicated study center under supervision. Supplementary Information contains the Manual of Procedures for implementing CCT as well as COGPACK definition files for identical replication on this software.
Physical exercise training
PRT was supervised by experienced research assistants (exercise physiologists and physiotherapists) in a physician-supervised clinic at the University of Sydney Exercise campus in a ratio of 1 trainer to 4–5 subjects. Pneumatic resistance machines (Keiser Sports Health Equipment, Fresno, CA, USA) were used for training at high intensity, 3 sets of 8 repetitions of each of 5–6 exercises/session for most major muscle groups (chest press, leg press, seated row, standing hip abduction, knee extension). Sham physical exercise included stretching and seated calisthenics, designed not to notably increase heart rate or aerobic capacity or improve balance or strength.
MRI scanning protocol
MRI data were acquired on a 3.0-Tesla Philips Achieva System (Amsterdam, The Netherlands). For each time point, brain structure was assessed using a T1-weighted whole-brain scan (sequence: T1TFE; TR/TE: 6.39/2.9 ms; slice thickness 1.0 mm without gap; field of view: 256 × 256; resolution 1 × 1 mm) and a T2-weighted FLAIR (FLuid-Attenuated Inversion Recovery) scan (resolution: 0.488 × 0.488 × 3.5 mm; TR/TE=10 000/110 ms). A resting-state functional MRI (fMRI) was conducted using T2* echo-planar BOLD sequence (TR/TE=2000/30 ms, 200 volumes, 6.5 min) with the subject’s eyes closed.
MRI preprocessing
T1 structure image preprocessing
Three MRI preprocessing methods were applied to examine the change of gray matter. (1) Expert hippocampal manual tracing was performed using our previous published protocol;63, 64 (2) Functional MRI of the brain (FMRIB)’s Integrated Registration and Segmentation Tool (FIRST v5.0.0 http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST) in FMRIB’s Software Library (FSL, v5.0, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) was used to measure hippocampal volume and morphometry; (3) the longitudinal pipeline in FreeSurfer (v5.1.0, http://freesurfer.net) was applied to measure the longitudinal training effect on cortical thickness on a vertex basis.65 Please refer to Supplementary Information for more details.
T2 fluid attenuated inversion recovery structure image
WMH volume is the main outcome of this MR modality. Regional WMH volumes were extracted using an in-house automatic pipeline that has been published elsewhere.66 The outputs included total WMH volume across whole brain, and regional WMH volume of 18 white matter regions.
Resting-state fMRI preprocessing
The fMRI data were preprocessed using the SPM8-based (Statistical Parametric Mapping) Data Processing Assistant for Resting-State fMRI (DPARSF) tool-box (www.restfmri.net/) based on published protocols.67 In brief, this involved: discarding the first 10 volumes of each participant, slice timing, normalization to standard MNI space, resampling into 2 × 2 × 2 mm3 isotropic voxels, smoothing using a 8-mm kernel, removal of global signal trends, bypass filtering of 0.01–0.08 Hz and finally regressing out nuisance signals related to white matter, whole-brain and cerebrospinal fluid signal as well as 6 coregistration factors.
Seed-wise functional connectivity maps were then generated individually using Resting-State fMRI Data Analysis Toolkit (REST, www.restfmri.net/).68 Bilateral hippocampal masks were selected as seeds from the Anatomical Automatic Labeling (AAL) template.69 The PC seed was obtained by thresholding a typical Default Mode Network generated using Independent Component Analysis (ICA) toolbox (Group ICA Toolbox GIFT, http://mialab.mrn.org/software/gift/) across whole baseline resting-state fMRI data. Individual functional connectivity (FC) maps for the hippocampal seed and PC seed were then generated based on correlations between the mean signal time course within each seed region and the rest of the brain.
MRI postprocessing
Voxel-based statistical analyses
The following analysis was carried out on seed-wise FC maps. For longitudinal general linear model (GLM) design, a flexible factorial design (Figure 1b) was used with four main factors: cognitive training factor (COG, yes/no two levels), progressive resistant training factor (RES, yes/no two levels), subjects and time. Three interactions factors were included in the model: COG × time, RES × time and COG × RES × time. Three covariates were also included in all models: age, sex and education years. Glm_flex, a postprocessing package based on SPM (http://nmr.mgh.harvard.edu/harvardagingbrain/People/AaronSchultz/Aarons_Scripts.html), was used to perform this model. Whole-brain cluster-level false discovery rate (FDR) correction issued with an initial threshold at Puncorrected<0.001 and cluster size k>100.
Hippocampal surface-based MR statistical analyses
We used this postprocessing method to test longitudinal effects on morphometry of hippocampal surfaces generated by FIRST. The GLM design is identical with the voxel-based statistical design above, but implemented on FSL. The correction method we used here is the permutation test (n=1000).
Vertex-based MR statistical analysis
Vertex-based analyses were used for cortical thickness (FreeSurfer) outputs. First, the change (rate) of cortical thickness that is defined by (thicknesstime2−thicknesstime1)/years between two scans. Second, GLMs were designed as per the voxel-based statistical design above, but without the factor of time (as thickness change was the dependent variable) and subject (this is redundant). The covariates were also age, sex and education years. Finally, the multiple comparison error was corrected using whole-brain vertex-based FDR correction or small-volume-based FDR correction. Two prespecified areas were chosen a priori for small-volume correction: posterior cingulate cortex and medial temporal lobe.
Statistical analyses
Clinical outcomes and all region-of-interest-based MR outcomes were analyzed using a linear mixed model in SPSS (IBM, Armonk, NY, USA. Release 2012). This model was designed with three main effects (time, COG and RES), three interactions (COG × time, RES × time and COG × RES × time) and three covariates (age, sex and education years). Mixed models are realistic and flexible with respect to possible variance differences between groups and over time and incorporate a model for missing values; all such analyses are therefore intention to treat. A compound symmetry model was used for repeat covariance. Pearson’s correlation was used to test the linkage between MR outcomes and cognitive outcomes across entire cohort. For mediation effect, Sobel tests were performed using the algorithm of Preacher and Hayes.70
Results
Cognitive outcomes
We first focus on therapeutic benefits on cognition immediately at the end of training (6 months). A thorough report of cognitive SMART outcomes at the end of 18-month longitudinal follow-up is available elsewhere.58 Participants in any resistance exercise group (RES factor, see Figure 1b) improved on our primary outcome, the Alzheimer’s Disease Assessment Scale-Cognitive scale (ADAS-Cog), compared with non-RES groups (RES × TIME intention-to-treat mixed linear model, F(90)=4.1, P<0.05 Figure 1c), but not on the composite Memory Domain score. Conversely, those in any CCT group (COG factor, see Figure 1b) experienced no decline in Memory Domain scores observed in non-CCT groups (COG × TIME intention-to-treat mixed linear model, F(90)=5.7, P<0.02, Figure 1d), but not on the ADAS-Cog. Contrary to our expectations, there was no triple interaction effect (RES × COG × TIME) on the primary outcome ADAS-Cog or Memory Domain score. There were no COG × Time or RES × Time effects for either the Executive Domain or Attention-Speed Domain.58 Furthermore, there were no significant COG × Time, RES × Time or COG × RES × Time effects on measures of subjective memory expectations (P-values >0.16) or memory concerns (P-values >0.47).
Given the unique condition of significant and discriminable cognitive effects based on RES and COG training factors unlikely to be confounded by differential mnemonic expectation or self-appraisal, we proceeded to test similar interactions for each brain imaging modality. For results that survived multiple-comparison correction, we then determined therapeutic relevance by testing for a relationship to change in ADAS-Cog or Memory Domain outcomes.
Structural mechanisms
Training-induced cortical thickness change was evaluated with Freesurfer without any findings surviving whole-brain correction. However, two prespecified regions were examined in more detail using region-of-interest analyses:57 hippocampus and posterior cingulate cortex. A significant RES × TIME interaction was found in the PC (Figure 2a, Psvc-corrected<0.05, T=3.24, kv=153, [−5 −21 42]). On the basis of extracted PC measures, it is clear that PRT protected individuals from atrophy: PC cortical thickness increased by an average of 0.01 mm per year (±s.d.=0.088) in PRT groups but decreased by an average of 0.05 mm per year (±0.085) in non-PRT groups (F(73)=11.1, P=0.0017, Figure 2b). When examined in more detail, combined PRT+CCT led to an expansion of cortical thickness and averted any atrophy in the PRT+SHAM group (Figure 2b). Therapeutic relevance was also supported given change in PC gray matter correlated with improvement in the trial’s primary outcome measure of global cognition, ADAS-Cog (Figure 2c, r=−0.25, P=0.030, N=75). A formal Sobel test with bootstrap estimation70 showed that change in PC thickness tended to mediate improvement in global cognition (indirect mediation mean estimated value −0.325, 95% confidence interval −0.914 to 0.013).
(a) Significant RES × TIME interaction was found in the posterior cingulate (PC). Green shows definition of our prespecified region of interest (ROI), and red shows suprathreshold voxels following small-volume correction (P<0.05, T=3.24, kv=153 [−5 −21 42]). (b) After extraction of individuals’ PC cortical thickness change (mm per year), participants in any PRT group displayed cortical expansion and less atrophy than those in non-PRT groups (RES × TIME, F(73)=11.1, *P=0.0017). (c) Enhancement of PC cortical thickness is correlated with improvement in global cognition (r=0.25, P=0.030, N=75, Y axis reversed). Error bars represent 95% confidence interval. All group-based analyses controlled for age, sex and education. ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive scale; CCT, computerized cognitive training; PRT, progressive resistance training; RES, progressive resistant training factor.
Given the importance of hippocampal atrophy in AD, we used two complementary methods to assess for possible structural plasticity: manual delineation (blinded to time point and group)63 (Figure 3a) and automated morphological shape analysis using the FSL-FIRST procedure as implemented in a prior report of aerobic exercise.20 No significant interactions were observed on hippocampal volume or shape using either of these methods (Figures 3b–d).
(a) Example of hippocampal manual tracing. (b) No significant FACTOR × TIME interactions were observed on hippocampal volume, although a trend was observed that PRT group exhibited less atrophy (F(75)=2.143, P=0.147). (c, d) Blue shows FIRST-generated hippocampal mesh. Brown shows areas of significant (P<0.05) RES × COG × TIME effects before multiple comparison correction, indicative of areas of relative spared atrophy in combined training in comparison with stand-alone training. No areas survived multiple-comparison correction. Error bars represent s.d. All analyses controlled for age, sex and education. CCT, computerized cognitive training; COG, cognitive training factor; FIRST, functional MRI of the brain (FMRIB)’s Integrated Registration and Segmentation Tool; PRT, progressive resistance training; RES, progressive resistant training factor.
Next, WMH volumes were analyzed at the whole-brain level as well as by major vascular territory as previously published by our group66 (Figure 4a). A near-significant trend was found for the RES × TIME interaction at the whole-brain level (log transformed, F(75)=2.8, P=0.09, Figure 4b), significant when analyzed at the regional level in the right periventricular zone (F(75)=4.3, P=0.042, Figure 4c) and right parietal zone (F(75)=4.1, P=0.046). In the right periventricular zone, WMHs regressed by 3.4% (±15.5%) over time in PRT groups but progressed by 3.0% (±15.9%) in non-PRT groups. Change in WMHs was not however linked to change in either ADAS-Cog or Memory Domain.
(a) Example of white matter hyperintensity (WMH) regional parcellation. (b) Near significant trend for RES × TIME interaction for whole-brain WMH volume (F(75)=2.8, P=0.09) and (c) significant RES × TIME interaction for right periventricular volume (F(75)=4.3, *P=0.042). (d) Example of an individual’s change in WMH burden following PRT+SHAM intervention (ID=10). Arrows highlight areas of diminished WMH intensity following training. Error bars represent s.d. All analyses controlled for age, sex and education. PRT, progressive resistance training; RES, progressive resistant training factor.
Functional mechanisms
Resting-state fMRI analysis focused on FC networks generated from two prespecified seeds: bilateral hippocampi and the PC (all results whole-brain corrected by false discovery rate). Complex training-induced changes were observed for each FC network (see Figure 5, as well as Supplementary Information).
(a) PC seed and PCFC map for cohort at baseline. (b) Spatial map of significant PCFC RES × TIME interaction anterior cingulate cluster (PFDR<0.05; see Supplementary Information for full set of results). (c) Graph of individual values shows that PRT training led to decreased PC–ACC functional connectivity compared with non-PRT training (F(67)=23.3, *P<0.001). (d) Scatterplot of individual change in PC–ACC functional connectivity was not related to change in memory domain performance (r=0.09, P=0.456, N=72) or ADAS-Cog (r=0.11, P=0.349; not shown). (e) HIP seed and HIPFC map for cohort at baseline. (f) Spatial map of significant HIPFC COG × TIME interaction in left superior frontal lobe cluster (sFTL; PFDR<0.05; see Supplementary Information for full set of results). (g) Plot of individual values shows that any CCT training led to increased HIP–sFTL functional connectivity compared with non-CCT training (F(65)=6.1, *P=0.012). (h) Scatterplot shows that increased HIP–sFTL functional connectivity is positively correlated with improved overall memory performance (r=0.33, P=0.005, N=72). All group-based analyses controlled for age, sex and education. ACC, anterior cingulate cortex; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive scale; CCT, computerized cognitive training; COG, cognitive training factor; FC, functional connectivity; FDR, false discovery rate; HIP, hippocampus; PC, posterior cingulate; PRT, progressive resistance training; RES, progressive resistant training factor.
For PCFC, RES × TIME effects were found indicative of decreased connectivity with the left inferior temporal lobe (F(67)=14.8, P<0.001, Supplementary Information) as well as the anterior cingulate cortex (F(67)=23.3, P<0.001, Figures 5a–c). COG × TIME analysis also indicated decreased connectivity with the left superior frontal lobe (F(67)=31.7, P<0.001; Supplementary Information) and anterior cingulate cortex (F(67)=13.9, P<0.001; Supplementary Information). Furthermore, a unique RES × COG × TIME interaction was found for the combined intervention in comparison with either stand-alone training, characterized by strongly decreased connectivity between the PC and anterior cingulate cortex (F(65)=5.3, P=0.017, see further results in Supplementary Information).
Analysis of the HIPFC network found RES × TIME effects suggestive of increased connectivity with the right middle frontal lobe (F(67)=13.0, P=0.001, Supplementary Information), but decreased connectivity with the right inferior temporal lobe (F(67)=18, P<0.001, Supplementary Information). COG × TIME analysis found evidence for increased connectivity between the hippocampus and left superior frontal lobe (P=0.012, Figures 5e–g). In terms of therapeutic relevance, of all FC changes noted, it was only this strengthening of hippocampal functional connectivity that correlated with improved memory domain performance (r=0.33, P=0.005, N=72 and compare Figure 5h with Figure 5d). Furthermore, Sobel test found that strengthening of hippocampal-superior frontal connectivity mediated improvement in overall memory ability (indirect mediation mean estimated value 0.093, 95% confidence interval 0.006–0.215).
Finally, a unique RES × COG × TIME interaction was found for increased hippocampal–anterior cingulate cortex connectivity (F(66)=4.6, P=0.005, Supplementary Information) as well as increased hippocampal–right superior frontal lobe connectivity (F(65)=7.0, P<0.001, Supplementary Information) in the combined training condition compared with stand-alone training.
Discussion
Here we report for the first time that resistance training can conserve and even increase cortical thickness in the posterior cingulate. This mechanism may be salient to long-term protection from further cognitive decline and impairment because loss of PC gray matter is a biomarker of AD,71, 72 most likely because of neuronal loss in specific cortical laminae.73, 74 Furthermore, we found that individual variability in PC plasticity was correlated to improvement on the trial’s primary outcome, the ADAS-Cog. Previously, we have shown that resistance training improves the chances of categorical improvement on ADAS-Cog from impaired to nonimpaired,58 and hence this newly described mechanism is therapeutically relevant and unique to PRT as it is not induced by CCT. Accordingly, our data suggest that PRT can help improve global cognition in older individuals at risk for dementia by attenuating and perhaps reversing a salient AD process.
No evidence was found for structural plasticity in the hippocampus subsequent to either type of training. This is consistent with null MRI findings following memory training alone75 but is inconsistent with a previous report following long-term aerobic exercise.20 Because we replicated that study’s image-processing pipeline, technical differences do not readily explain this discrepancy. Rather, it is possible that aerobic and resistance training produces distinct patterns of structural plasticity in older individuals. Evidence for structural plasticity in the rodent hippocampus76 almost invariably derives from aerobic voluntary wheel running—to date, there are no comparable animal data about the structural brain effects of resistance exercise. Exercise dose is another possible explanation. Hippocampal enlargement was found after 1 year of moderate-intensity walking (~150 sessions, total dose ~80 h),20 whereas in the SMART Trial exercise groups received an average of 42 sessions of high-intensity PRT (total dose ~38 h).
A novel potential disease-modifying mechanism was suggested by modest regression of WMHs in those who underwent PRT compared with progression in non-PRT groups. WMHs are very common in the elderly40 and strongly implicated in the pathogenesis of cognitive impairment of both AD and vascular origin.43 Our data suggest that resistance exercise may have a positive role in combating this marker of cerebral small vessel disease, but caution is required because positive results in the periventricular and parietal zones did not survive whole-brain correction. Interestingly, current general health guidelines recommend a combination of resistance and aerobic exercise for older adults77 but the relative importance of resistance versus aerobic exercise to long-term brain health is not well understood. Given the emerging importance of metabolic derangements to the development of AD,78, 79 further research is required to understand the molecular and cellular mechanisms linking resistance training to cerebrovascular health and disease.
Breakdown of the DMN is also an early event in AD,80, 81, 82, 83 possibly related to build-up of neuropathology in the posterior cingulate.84 It is hence notable that combined resistance and cognitive training produced a strong and distinctive alteration to the DMN, weakening of posterior-to-anterior cingulate synchronization (among other changes). It is however unlikely that this network change was deleterious for three reasons. First, positive structural plasticity in the posterior cingulate was observed that is in the opposite direction to that seen in early AD. Second, there was no connection between weakening of the DMN in our patients and cognitive outcomes. Third, weakening of the DMN following 8-month aerobic exercise has been reported in a different setting (overweight children85), suggesting these changes are not necessarily related to AD pathology but a general consequence of physical exercise. That said, the functional significance of DMN modification by cognitive and physical exercise in our trial remains unclear.
In contrast, hippocampal functional networks were unequivocally strengthened by CCT. This form of network change was not only correlated to improvement in overall memory performance, but was a formal mediator of mnemonic benefits. Prior reports of hippocampal–superior frontal lobe connectivity indicate that it is degraded in MCI,53, 81, 86 and therefore strengthening of this resting-state network may represent a unique CCT-inducible mechanism that counteracts age- or AD-related network dysfunction.
Finally, against our expectations we found no additional therapeutic benefit from combining resistance and cognitive training compared with single intervention. Given that we have described distinct inducible mechanisms stimulated by each kind of training, saturation of a common biological process is therefore not persuasive by way of explanation. On the other hand, overdosing of our subjects in the combined intervention and consequent mental exhaustion is possible. For example, it is now clear that CCT more than three times a week neutralizes the efficacy of this intervention.1 In our combined training group, participants were asked to undertake 90 min of intense training during each session; in comparison, those in the stand-alone groups carried out 45 min of intense training followed by 45 min of a more relaxing sham exercise (either a stretching and toning class or watching short documentaries on computer). Future studies may prefer to carry out cognitive and physical exercise training on separate days rather than during the same session, similar to recommendations for rest days between intensive physical exercise sessions.87
Overall, the SMART trial shows that resistance and cognitive training can be used to target different cognitive domains based on distinct brain mechanisms relevant to aging, cerebrovascular disease and AD. Given the alarming disease burden predicted for dementia and neurocognitive disorders over the coming decades, this information should help design more effective dementia prevention trials as well as contribute to their clinical and community implementation.
References
Lampit A, Hallock H, Valenzuela MJ . Computerized cognitive training in healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med 2014; 11: e1001756.
Gates N, Fiatarone Singh MA, Sachdev PS, Valenzuela M . The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry 2013; 21: 1086–1097.
Hillman CH, Erickson KI, Kramer AF . Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 2008; 9: 58–65.
Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 2010; 72: 239–252.
Barnes DE, Yaffe K . The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011; 10: 819–828.
Lampit A, Duffy S, Valenzuela M . Physical Exercise for brain health in later life: How does it work? In: Physical Exercise Interventions for Mental Health, Profs Linda Lam & Michelle Riba (eds), Cambridge University Press: Cambridge, UK, 2016.
Nithianantharajah J, Hannan A . Enriched environments, experience-dependent plasticity and disorders of the nervous system 2248. Nat Rev Neurosci 2006; 7: 697–709.
Valenzuela M, Breakspear M, Sachdev P . Complex mental activity and the ageing brain: molecular, cellular and cortical network mechanisms. Brain Res Rev 2007; 56: 198–213.
Cotman CW, Berchtold N, Christie L . Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007; 30: 465–472.
Siette J, Westbrook RF, Cotman C, Sidhu K, Zhu W, Sachdev P et al. Age-specific effects of voluntary exercise on memory and the older brain. Biol Psychiatry 2013; 73: 435.
Lustig C, Shah P, Seidler R, Reuter-Lorenz PA . Aging, training, and the brain: a review and future directions. Neuropsychol Rev 2009; 19: 504–522.
Suo C, Valenzuela MJ . Neuroimaging outcomes of brain training trials. In: Neuroimaging - Cognitive and Clinical Neuroscience, Prof. Peter Bright (ed), InTech: Rijeka, Croatia, 2012; 417–430.
Zatorre RJ, Fields RD, Johansen-Berg H . Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 2012; 15: 528–536.
Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC . Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 2011; 86: 876–884.
Braak H, Braak E . Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–259.
Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM . The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 2010; 6: 67–77.
Erickson KI, Gildengers AG, Butters MA . Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci 2013; 15: 99–108.
Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 2003; 58: 176–180.
Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Hazlett KE et al. Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease. Front Aging Neurosci 2014; 6: 61.
Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–3022.
Coen RF, Lawlor BA, Kenny R . Failure to demonstrate that memory improvement is due either to aerobic exercise or increased hippocampal volume. Proc Natl Acad Sci USA 2011; 108: E89; author reply E90.
Engvig A, Fjell AM, Westlye LT, Skaane NV, Sundseth O, Walhovd KB . Hippocampal subfield volumes correlate with memory training benefit in subjective memory impairment. Neuroimage 2012; 61: 188–194.
Strasser B, Pesta D . Resistance training for diabetes prevention and therapy: experimental findings and molecular mechanisms. Biomed Res Int 2013; 2013: 8.
Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 2000; 101: 828–833.
American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009; 41: 1510–1530.
Layne JE, Nelson ME . The effects of progressive resistance training on bone density: a review. Med Sci Sports Exerc 1999; 31: 25–30.
Yardley JE, Kenny GP, Perkins BA, Riddell MC, Balaa N, Malcolm J et al. Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care 2013; 36: 537–542.
Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002; 25: 2335–2341.
Liu-Ambrose T, Donaldson MG . Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med 2009; 43: 25–27.
Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 2007; 39: 1401–1407.
Liu-Ambrose T, Donaldson MG, Ahamed Y, Graf P, Cook WL, Close J et al. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J Am Geriatr Soc 2008; 56: 1821–1830.
Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC . Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med 2010; 170: 170–178.
Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T . Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc 2015; 21: 745–756.
Breteler MM . Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Ann NY Acad Sci 2000; 903: 457–465.
Valenzuela M, Esler M, Ritchie K, Brodaty H . Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry 2012; 2: e107.
Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR . Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997; 277: 813–817.
Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001; 357: 169–175.
Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA . The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 2009; 18: 691–701.
Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA . The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009; 66: 200–208.
Pantoni L . Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701.
Debette S, Markus HS . The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341: c3666.
Gattringer T, Enzinger C, Ropele S, Gorani F, Petrovic KE, Schmidt R et al. Vascular risk factors, white matter hyperintensities and hippocampal volume in normal elderly individuals. Dement Geriatr Cogn Disord 2012; 33: 29–34.
Wallin A, Fladby T . Do white matter hyperintensities on MRI matter clinically? BMJ 2010; 341: c3400.
Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol 2008; 65: 94–100.
van Straaten EC, Harvey D, Scheltens P, Barkhof F, Petersen RC, Thal LJ et al. Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol 2008; 255: 1302–1308.
Richard E, Gouw AA, Scheltens P, van Gool WA . Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer's disease (EVA) study. Stroke 2010; 41: 554–556.
Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2010; 2: 32.
Voss MW, Vivar C, Kramer AF, van Praag H . Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 2013; 17: 525–544.
Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA 2007; 104: 18760–18765.
Allen G, Barnard H, McColl R, Hester AL, Fields JA, Weiner MF et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol 2007; 64: 1482–1487.
Wang Z, Liang P, Jia X, Jin G, Song H, Han Y et al. The baseline and longitudinal changes of PCC connectivity in mild cognitive impairment: a combined structure and resting-state fMRI study. PLoS One 2012; 7: e36838.
Liu Y, Wang K, Yu C, He Y, Zhou Y, Liang M et al. Regional homogeneity, functional connectivity and imaging markers of Alzheimer's disease: a review of resting-state fMRI studies. Neuropsychologia 2008; 46: 1648–1656.
Wang Z, Liang P, Jia X, Qi Z, Yu L, Yang Y et al. Baseline and longitudinal patterns of hippocampal connectivity in mild cognitive impairment: evidence from resting state fMRI. J Neurol Sci 2011; 309: 79–85.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL . A default mode of brain function. Proc Natl Acad Sci USA 2001; 98: 676–682.
Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods 2008; 169: 249–254.
Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 2009; 106: 2035–2040.
Gates NJ, Valenzuela M, Sachdev PS, Singh NA, Baune BT, Brodaty H et al. Study of Mental Activity and Regular Training (SMART) in at risk individuals: a randomised double blind, sham controlled, longitudinal trial. BMC Geriatr 2011; 11: 19.
Fiatarone Singh MA, Gates N, Saigal N, Wilson GC, Meiklejohn J, Brodaty H et al. The Study of Mental and Resistance Training (SMART) Study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc 2014; 15: 873–880.
Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E . Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56: 303–308.
Clare L, Wilson BA, Carter G, Roth I, Hodges JR . Assessing awareness in early-stage Alzheimer’s disease: development and piloting of the Memory Awareness Rating Scale. Neuropsychol Rehabil 2002; 12: 341–362.
Clare L, Whitaker CJ, Nelis SM . Appraisal of memory functioning and memory performance in healthy ageing and early-stage Alzheimer's disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2010; 17: 462–491.
Gates N, Valenzuela M, Sachdev PS, Singh MA . Psychological well-being in individuals with mild cognitive impairment. Clin Interv Aging 2014; 9: 779–792.
Suo C, Leon I, Brodaty H, Trollor J, Wen W, Sachdev P et al. Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. Neuroimage 2012; 63: 1542–1551.
Valenzuela MJ, Sachdev P, Wen W, Chen X, Brodaty H . Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One 2008; 3: e2598.
Reuter M, Schmansky NJ, Rosas HD, Fischl B . Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012; 61: 1402–1418.
Wen W, Sachdev P . The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage 2004; 22: 144–154.
Yan G, Sun H, Sun W, Zhao L, Meng X, Wang X . Rapid and global detection and characterization of aconitum alkaloids in Yin Chen Si Ni Tang, a traditional Chinese medical formula, by ultra performance liquid chromatography-high resolution mass spectrometry and automated data analysis. J Pharm Biomed Anal 2010; 53: 421–431.
Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011; 6: e25031.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289.
Preacher KJ, Hayes AF . SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comp 2004; 36: 717–731.
Barnes J, Godbolt AK, Frost C, Boyes RG, Jones BF, Scahill RI et al. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiol Aging 2007; 28: 20–28.
Lehmann M, Crutch SJ, Ridgway GR, Ridha BH, Barnes J, Warrington EK et al. Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer's disease. Neurobiol Aging 2011; 32: 1466–1476.
Vogt BA, Van Hoesen GW, Vogt LJ . Laminar distribution of neuron degeneration in posterior cingulate cortex in Alzheimer's disease. Acta Neuropathol 1990; 80: 581–589.
Vogt BA, Vogt LJ, Vrana KE, Gioia L, Meadows RS, Challa VR et al. Multivariate analysis of laminar patterns of neurodegeneration in posterior cingulate cortex in Alzheimer's disease. Exp Neurol 1998; 153: 8–22.
Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA et al. Effects of memory training on cortical thickness in the elderly. Neuroimage 2010; 52: 1667–1676.
Cotman CW, Berchtold NC . Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002; 25: 295–301.
Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007; 39: 1435–1445.
Luchsinger JA . Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur J Pharmacol 2008; 585: 119–129.
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease—is this type 3 diabetes? J Alzheimers Dis 2005; 7: 63–80.
Greicius MD, Srivastava G, Reiss AL, Menon V . Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004; 101: 4637–4642.
Bai F, Watson DR, Yu H, Shi Y, Yuan Y, Zhang Z . Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Res 2009; 1302: 167–174.
Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ et al. Reduced resting-state brain activity in the "default network" in normal aging. Cereb Cortex 2008; 18: 1856–1864.
Damoiseaux JS . Resting-state fMRI as a biomarker for Alzheimer's disease? Alzheimers Res Ther 2012; 4: 8.
Giannakopoulos P, Hof PR, Giannakopoulos AS, Herrmann FR, Michel JP, Bouras C . Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of very old patients. Arch Neurol 1995; 52: 1150–1159.
Krafft CE, Pierce JE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ et al. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience 2014; 256: 445–455.
Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage 2006; 31: 496–504.
United States Department of Health and Human Services. Physical Activity Guidelines for Americans. 2008; http://health.gov/paguidelines/guidelines/.
Acknowledgements
This study was funded by the National Health and Medical Research Council (NHMRC) of Australia Dementia Research Grant, Project Grant ID No. 512672 from 2008 to 2011. Additional funding for a research assistant position was sourced from the NHMRC Program Grant ID No. 568969 and the project was supported by the University of Sydney and University of New South Wales. MJV was supported by the University of New South Wales Vice Chancellor’s Fellowship and a NHMRC Clinical Career Development Fellowship (1004156) and the Sydney Medical School Foundation. YM, GCW and JM are supported by the CRN for Advancing Exercise and Sport Science as a postdoctoral research associate, PhD scholarship and as a research assistant respectively. This work fulfilled a portion of the degree requirements for PhD for Nicola Gates and Chao Suo. We also thank Brooke McMahon for assistance with recruitment and training, Dominique Williamson, Dr Shelley Kay and Dr Angela Wright for assistance with assessments, Brad Lloyd and Dr Angela Wright for their assistance with Manual of Procedures preparation, Kate Bradshaw for her assistance with recruitment and Diana Marcela Martinez Piamba for hippocampal tracing. Donations for participant rewards were received from Gregory and Carr Funerals. We also thank the extraordinary generosity and commitment of the participants and their families who devoted their time to this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest. MJV has previously received honoraria for speaking at Pfizer and The Brain Department LLC-sponsored events, has received research funding from The Brain Department LLC for unrelated work and currently receives in-kind research support in the form of no-cost software from BrainTrain Inc (USA) and in-kind research support from NeuroNation for unrelated projects. HB has been an investigator for Pfizer, Novartis, Janssen, Lilly, Medivation, Sanofi and Servier; a sponsored speaker for Pfizer, Novartis, Janssen and Lundbeck; and is on advisory Boards for Pfizer, Novartis, Janssen, Lundbeck, Merck and Baxter. BTB is a member of advisory boards and/or gave presentations for the following companies, for which he received honoraria: AstraZeneca, Lundbeck, Pfizer, Servier and Wyeth. NG holds shares in HeadStrong Brain LLC New York although no dividends, gifts or royalties have ever been received and no work has been conducted for the company since 2007.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Suo, C., Singh, M., Gates, N. et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry 21, 1633–1642 (2016). https://doi.org/10.1038/mp.2016.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.19
This article is cited by
-
Alzheimer’s disease risk reduction in clinical practice: a priority in the emerging field of preventive neurology
Nature Mental Health (2024)
-
Effects of a physical exercise or motor activity protocol on cognitive function, lipid profile, and BDNF levels in older adults with mild cognitive impairment
Molecular and Cellular Biochemistry (2023)
-
Combining exercise with cognitive training and vitamin D3 to improve functional brain connectivity (FBC) in older adults with mild cognitive impairment (MCI). Results from the SYNERGIC trial
GeroScience (2023)
-
Increasing participation in habitual intellectual activities on modulating functional connectivity of default mode network among older adults at risk of dementia: study protocol of a randomized controlled trial
Trials (2022)
-
Associations between grip strength, brain structure, and mental health in > 40,000 participants from the UK Biobank
BMC Medicine (2022)