Abstract
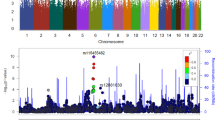
Despite moderate heritability, only one study has identified genome-wide significant loci for cannabis-related phenotypes. We conducted meta-analyses of genome-wide association study data on 2080 cannabis-dependent cases and 6435 cannabis-exposed controls of European descent. A cluster of correlated single-nucleotide polymorphisms (SNPs) in a novel region on chromosome 10 was genome-wide significant (lowest P=1.3E−8). Among the SNPs, rs1409568 showed enrichment for H3K4me1 and H3K427ac marks, suggesting its role as an enhancer in addiction-relevant brain regions, such as the dorsolateral prefrontal cortex and the angular and cingulate gyri. This SNP is also predicted to modify binding scores for several transcription factors. We found modest evidence for replication for rs1409568 in an independent cohort of African American (896 cases and 1591 controls; P=0.03) but not European American (EA; 781 cases and 1905 controls) participants. The combined meta-analysis (3757 cases and 9931 controls) indicated trend-level significance for rs1409568 (P=2.85E−7). No genome-wide significant loci emerged for cannabis dependence criterion count (n=8050). There was also evidence that the minor allele of rs1409568 was associated with a 2.1% increase in right hippocampal volume in an independent sample of 430 EA college students (fwe-P=0.008). The identification and characterization of genome-wide significant loci for cannabis dependence is among the first steps toward understanding the biological contributions to the etiology of this psychiatric disorder, which appears to be rising in some developed nations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
United Nations Office on Drugs and Crime World Drug Report 2015. United Nations: Vienna, Austria, 2015 Report no. Sales No. E.15.XI.6.
Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 Study. PLoS ONE 2013; 8: e76635.
Chen CY, O'Brien MS, Anthony JC . Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend 2005; 79: 11–22.
Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC . Cannabis dependence in young adults: an Australian population study. Addiction 2002; 97: 187–194.
Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS et al. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med 2002; 32: 195–207.
Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry 2015; 72: 1235–1242.
Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction 2010; 105: 417–430.
Sherva R, Wang Q, Kranzler H, Zhao H, Koesterer R, Herman A et al. Genome-wide association study of cannabis dependence severity, novel risk variants, and shared genetic risks. JAMA Psychiatry 2016; 30: 10.
Agrawal A, Lynskey MT, Hinrichs A, Grucza R, Saccone SF, Krueger R et al. A genome-wide association study of DSM-IV cannabis dependence. Addict Biol 2011; 16: 514–518.
Agrawal A, Lynskey MT, Bucholz KK, Kapoor M, Almasy L, Dick DM et al. DSM-5 cannabis use disorder: a phenotypic and genomic perspective. Drug Alcohol Depend 2014; 134: 362–369.
Verweij KJ, Vinkhuyzen AA, Benyamin B, Lynskey MT, Quaye L, Agrawal A et al. The genetic aetiology of cannabis use initiation: a meta-analysis of genome-wide association studies and a SNP-based heritability estimation. Addict Biol 2012; 18: 846–850.
Minica CC, Dolan CV, Hottenga JJ, Pool R, Fedko IO, Mbarek H et al. Heritability, SNP- and gene-based analyses of cannabis use initiation and age at onset. Behav Genet 2015; 45: 503–513.
Stringer S, Minica CC, Verweij KJ, Mbarek H, Bernard M, Derringer J. et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry 2016; 6: e769.
Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLOS ONE 2013; 8: e55821.
Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M . Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse 2010; 45: 1787–1808.
Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci 2014; 34: 5529–5538.
Pagliaccio D, Barch DM, Bogdan R, Wood PK, Lynskey MT, Heath AC et al. Shared predisposition in the association between cannabis use and subcortical brain structure. JAMA Psychiatry 2015; 72: 994–1001.
Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res 2010; 34: 840–852.
Wetherill L, Agrawal A, Kapoor M, Bertelsen S, Bierut LJ, Brooks A et al. Association of substance dependence phenotypes in the COGA sample. Addict Biol 2015; 20: 617–627.
Wang JC, Foroud T, Hinrichs AL, Le NX, Bertelsen S, Budde JP et al. A genome-wide association study of alcohol-dependence symptom counts in extended pedigrees identifies C15orf53. Mol Psychiatry 2013; 18: 1218–1224.
Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E et al. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci USA 2010; 107: 5082–5087.
Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA et al. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry 2011; 70: 513–518.
Saccone SF, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC et al. Genetic linkage to chromosome 22q12 for a heavy smoking quantitative trait in two independent samples. Am J Hum Genet 2007; 80: 856–866.
Kristjansson S, McCutcheon VV, Agrawal A, Lynskey MT, Conroy E, Statham DJ et al. The variance shared across forms of childhood trauma is strongly associated with liability for psychiatric and substance use disorders. Brain Behav 2016; 6: e00432.
Nelson EC, Agrawal A, Heath AC, Bogdan R, Sherva R, Zhang B et al. Evidence of CNIH3 involvement in opioid dependence. Mol Psychiatry 2015; 21: 608–614, 10.
Howie BN, Donnelly P, Marchini J . A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009; 5: e1000529.
Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR . MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 2010; 34: 816–834.
O'Connell J, Gurdasani D, Delaneau O, Pirastu N, Ulivi S, Cocca M et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet 2014; 10: e1004234.
Browning BL, Browning SR . A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet 2009; 84: 210–223.
American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th edn revised. American Psychiatric Association: Washington, DC, 1994.
Wetherill L, Agrawal A, Kapoor M, Bertelsen S, Bierut LJ, Brooks A et al. Association of substance dependence phenotypes in the COGA sample. Addict Biol 2014; 20: 617–627, 10.
Bierut L, Agrawal A, Bucholz K, Doheny KF, Laurie CC, Pugh EW et al. A Genome-wide Association Study of Alcohol Dependence. Proc Natl Acad Sci USA 2010; 107: 5082–5087.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Chen MH, Yang Q . GWAF: an R package for genome-wide association analyses with family data. Bioinformatics 2010; 26: 580–581.
Chen WM, Abecasis GR . Family-based association tests for genomewide association scans. Am J Hum Genet 2007; 81: 913–926.
Willer CJ, Li Y, Abecasis GR . METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010; 26: 2190–2191.
de Leeuw CA, Mooij JM, Heskes T, Posthuma D . MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol 2015; 11: e1004219.
Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry 2014; 19: 41–49.
Nikolova YS, Knodt AR, Radtke SR, Hariri AR . Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol Psychiatry 2016; 21: 348–356.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH . An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19: 1233–1239.
Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010; 26: 2336–2337.
GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013; 45: 580–585.
Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci 2016; 19: 1442–1453.
Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 2012; 22: 1790–1797.
Zhou X, Maricque B, Xie M, Li D, Sundaram V, Martin EA et al. The Human Epigenome Browser at Washington University. Nat Methods 2011; 8: 989–990.
Koob GF, Volkow ND . Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35: 217–238.
Gibbs JR, van der Brug MP, Hernandez DG, Traynor BJ, Nalls MA, Lai SL et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet 2010; 6: e1000952.
Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N et al. Common genetic variants influence human subcortical brain structures. Nature 2015; 520: 224–229.
Machiela MJ, Chanock SJ . LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015; 31: 3555–3557.
Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res 2012; 22: 1760–1774.
Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA 2010; 107: 21931–21936.
Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh PR et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet 2015; 47: 1228–1235.
Gusev A, Lee SH, Trynka G, Finucane H, Vilhjalmsson BJ, Xu H et al. Partitioning heritability of regulatory and cell-type-specific variants across 11 common diseases. Am J Hum Genet 2014; 95: 535–552.
Corradin O, Scacheri PC . Enhancer variants: evaluating functions in common disease. Genome Med 2014; 6: 85.
Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 2012; 337: 1190–1195.
Goldstein RZ, Volkow ND . Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 2011; 12: 652–669.
Laughon A . DNA binding specificity of homeodomains. Biochemistry 1991; 30: 11357–11367.
Erkman L, Yates PA, McLaughlin T, McEvilly RJ, Whisenhunt T, O'Connell SM et al. A POU domain transcription factor-dependent program regulates axon pathfinding in the vertebrate visual system. Neuron 2000; 28: 779–792.
McEvilly RJ, de Diaz MO, Schonemann MD, Hooshmand F, Rosenfeld MG . Transcriptional regulation of cortical neuron migration by POU domain factors. Science 2002; 295: 1528–1532.
McEvilly RJ, Rosenfeld MG . The role of POU domain proteins in the regulation of mammalian pituitary and nervous system development. Prog Nucleic Acid Res Mol Biol 1999; 63: 223–255.
Sato H, Miyamoto T, Yogev L, Namiki M, Koh E, Hayashi H et al. Polymorphic alleles of the human MEI1 gene are associated with human azoospermia by meiotic arrest. J Hum Genet 2006; 51: 533–540.
Gundersen TD, Jorgensen N, Andersson AM, Bang AK, Nordkap L, Skakkebaek NE et al. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am J Epidemiol 2015; 182: 473–481.
Fasano S, Meccariello R, Cobellis G, Chianese R, Cacciola G, Chioccarelli T et al. The endocannabinoid system: an ancient signaling involved in the control of male fertility. Ann N Y Acad Sci 2009; 1163: 112–124.
Grimaldi P, Orlando P, Di SS, Lolicato F, Petrosino S, Bisogno T et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci USA 2009; 106: 11131–11136.
Szutorisz H, Egervari G, Sperry J, Carter JM, Hurd YL . Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicol Teratol 2016; 58: 107–114.
Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 2010; 42: 441–447.
Acknowledgements
AA acknowledges support from the National Institute on Drug Abuse (NIDA) for the core research pertaining to this study, via K02DA032573 and R01DA023668. CEC received support from the National Science Foundation (DGE-1143954) and the Mr and Mrs Spencer T Olin Fellowship Program. DAAB acknowledges NIMH (T32-GM008151) and NSF (DGE-1745038). JLM acknowledges K01DA037914. LD is supported by an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellowship (1041472). LJB acknowledges R01DA036583. RB receives additional support from the National Institutes of Health (R01-AG045231, R01-HD083614 and U01-AG052564).
COGA: The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B Porjesz, V Hesselbrock, H Edenberg, L Bierut, includes 11 different centers: University of Connecticut (V Hesselbrock); Indiana University (HJ Edenberg, J Nurnberger Jr and T Foroud); University of Iowa (S Kuperman and J Kramer); SUNY Downstate (B Porjesz); Washington University in St Louis (L Bierut, J Rice, K Bucholz and A Agrawal); University of California at San Diego (M Schuckit); Rutgers University (J Tischfield and A Brooks); Department of Biomedical and Health Informatics, The Children’s Hospital of Philadelphia; Department of Genetics, Perelman School of Medicine, University of Pennsylvania, Philadelphia PA (L Almasy), Virginia Commonwealth University (D Dick), Icahn School of Medicine at Mount Sinai (A Goate) and Howard University (R Taylor). Other COGA collaborators include: L Bauer (University of Connecticut); J McClintick, L Wetherill, X Xuei, Y Liu, D Lai, S O’Connor, M Plawecki, S Lourens (Indiana University); G Chan (University of Iowa; University of Connecticut); J Meyers, D Chorlian, C Kamarajan, A Pandey, J Zhang (SUNY Downstate); J-C Wang, M Kapoor, S Bertelsen (Icahn School of Medicine at Mount Sinai); A Anokhin, V McCutcheon, S Saccone (Washington University); J Salvatore, F Aliev, B Cho (Virginia Commonwealth University); and Mark Kos (University of Texas Rio Grande Valley). A Parsian and M Reilly are the NIAAA Staff Collaborators.
Funding support for GWAS genotyping performed at the Johns Hopkins University Center for Inherited Disease Research was provided by the National Institute on Alcohol Abuse and Alcoholism, the NIH GEI (U01HG004438) and the NIH contract ‘High throughput genotyping for studying the genetic contributions to human disease’ (HHSN268200782096C). GWAS genotyping was also performed at the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine, which is partially supported by NCI Cancer Center Support Grant P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant UL1RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. COGA-f genotypic data are available via dbGaP: phs000763.v1.p1 and COGA-c genotypic data are available via phs000125.v1.p1
We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, P Michael Conneally, Raymond Crowe and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
CATS (dbGaP: phs000277.v1.p1): Funding support for the Comorbidity and Trauma Study (CATS) was provided by the National Institute on Drug Abuse (R01 DA17305); GWAS genotyping services at the Center for Inherited Disease Research (CIDR) at The Johns Hopkins University were supported by the National Institutes of Health (contract N01-HG-65403). The National Drug and Alcohol Research Centre at the University of New South Wales is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grants Fund.
SAGE (dbGaP: phs000092.v1.p1): Funding support for the Study of Addiction: Genetics and Environment (SAGE) was provided through the NIH Genes, Environment and Health Initiative (GEI) (U01 HG004422). SAGE is one of the GWASs funded as part of the Gene Environment Association Studies (GENEVA) under GEI. Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center (U01 HG004446). Assistance with data cleaning was provided by the National Center for Biotechnology Information. Support for collection of datasets and samples was provided by the Collaborative Study on the Genetics of Alcoholism (COGA; U10 AA008401), the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392) and the Family Study of Cocaine Dependence (FSCD; R01 DA013423, R01 DA019963). Funding support for genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the NIH GEI (U01HG004438), the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NIH contract ‘High throughput genotyping for studying the genetic contributions to human disease’(HHSN268200782096C).
OZALC+ (dbGaP: phs000181.v1.p1): Supported by NIH grants AA07535, AA07728, AA13320, AA13321, AA14041, AA11998, AA17688, DA012854, DA019951; by grants from the Australian National Health and Medical Research Council (241944, 339462, 389927, 389875, 389891, 389892, 389938, 442915, 442981, 496739, 552485, 552498); by grants from the Australian Research Council (A7960034, A79906588, A79801419, DP0770096, DP0212016, DP0343921); and by the FP-5 GenomEUtwin Project (QLG2-CT-2002–01254). GWAS genotyping at CIDR was supported by a grant to the late Richard Todd, PhD, MD, former PI of grant AA13320 and a key contributor to research described in this manuscript. Project 7 data collection was also supported by AA011998_5978.
Yale-Penn: This study was supported by grants RC2 DA028909, R01 DA12690, R01 DA12849, R01 DA18432, R01 AA11330 and R01 AA017535 from the National Institutes of Health (NIH), the Veterans Affairs Connecticut Healthcare Center and the Philadelphia Veterans Affairs Mental Illness Research, Education and Clinical Center. A portion of the data are available via dbGaP (phs000277.v1.p1).
DNS: The Duke Neurogenetics Study is supported by Duke University and National Institute on Drug Abuse grant DA033369.
The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health. Additional funds were provided by the NCI, NHGRI, NHLBI, NIDA, NIMH and NINDS. Donors were enrolled at Biospecimen Source Sites funded by NCI\SAIC-Frederick, Inc. (SAIC-F) subcontracts to the National Disease Research Interchange (10XS170), Roswell Park Cancer Institute (10XS171) and Science Care, Inc. (X10S172). The Laboratory, Data Analysis and Coordinating Center (LDACC) was funded through a contract (HHSN268201000029C) to The Broad Institute, Inc. Biorepository operations were funded through an SAIC-F subcontract to Van Andel Institute (10ST1035). Additional data repository and project management were provided by SAIC-F (HHSN261200800001E). The Brain Bank was supported by a supplements to University of Miami grants DA006227 and DA033684, and to contract N01MH000028. Statistical Methods development grants were made to the University of Geneva (MH090941 and MH101814), the University of Chicago (MH090951, MH090937, MH101820 and MH101825), the University of North Carolina—Chapel Hill (MH090936 and MH101819), Harvard University (MH090948), Stanford University (MH101782), Washington University St Louis (MH101810) and the University of Pennsylvania (MH101822). The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 09/24/2016.
Data were generated as part of the CommonMind Consortium supported by funding from Takeda Pharmaceuticals Company Limited, F Hoffman-La Roche Ltd and NIH grants R01MH085542, R01MH093725, P50MH066392, P50MH080405, R01MH097276, RO1-MH-075916, P50M096891, P50MH084053S1, R37MH057881 and R37MH057881S1, HHSN271201300031C, AG02219, AG05138 and MH06692. Brain tissue for the study was obtained from the following brain bank collections: the Mount Sinai NIH Brain and Tissue Repository, the University of Pennsylvania Alzheimer’s Disease Core Center, the University of Pittsburgh NeuroBioBank and Brain and Tissue Repositories and the NIMH Human Brain Collection Core. CMC Leadership: Pamela Sklar, Joseph Buxbaum (Icahn School of Medicine at Mount Sinai), Bernie Devlin, David Lewis (University of Pittsburgh), Raquel Gur, Chang-Gyu Hahn (University of Pennsylvania), Keisuke Hirai, Hiroyoshi Toyoshiba (Takeda Pharmaceuticals Company Limited), Enrico Domenici, Laurent Essioux (F. Hoffman-La Roche Ltd), Lara Mangravite, Mette Peters (Sage Bionetworks), Thomas Lehner, Barbara Lipska (NIMH).
Expression and covariate data for the methylation eQTL analysis was derived from https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE15745. Genotypes were derived via authorized access to MK, JCW and AG from dbGaP (phs000249.v2.p1). Funding support for the ‘Brain eQTL (expression data) Study’ was provided through the Division of Aging Biology and the Division of Geriatrics and Clinical Gerontology, NIA. The Brain eQTL (expression data) Study includes a GWAS funded as part of the Intramural Research Program, NIA. Funding sources: Z01 AG000949-02 and Z01 AG000015-49.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
We disclose that Drs LJ Bierut, JP Rice, J-C Wang and AM Goate are listed as inventors on the patent ‘Markers for Addiction’ (US 20070258898) covering the use of certain SNPs in determining the diagnosis, prognosis and treatment of addiction. Dr Kranzler has been a consultant, advisory board member or CME speaker for Lundbeck, and Indivior. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative (ACTIVE), which was supported in the last three years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor and Amygdala Neurosciences. Dr Nurnberger is an investigator for Assurex and a consultant for Janssen. All other authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Agrawal, A., Chou, YL., Carey, C. et al. Genome-wide association study identifies a novel locus for cannabis dependence. Mol Psychiatry 23, 1293–1302 (2018). https://doi.org/10.1038/mp.2017.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.200
This article is cited by
-
The genetic aetiology of cannabis use: from twin models to genome-wide association studies and beyond
Translational Psychiatry (2022)
-
Genetic basis of cannabis use: a systematic review
BMC Medical Genomics (2021)
-
Genetics of substance use disorders in the era of big data
Nature Reviews Genetics (2021)
-
Cannabis use and cannabis use disorder
Nature Reviews Disease Primers (2021)
-
Genetic determinants of cannabis use: a systematic review protocol
Systematic Reviews (2020)